Abstract
Background
In HIV-infected women, urine concentrations of novel tubulointerstitial injury markers, interleukin-18 (IL-18) and kidney injury marker-1 (KIM-1) are associated with kidney function decline and all-cause mortality. We hypothesized that HIV-infected individuals with preserved kidney filtration function would have more extensive kidney injury, as determined by urine injury markers, compared to the uninfected controls, and that risk factors for tubulointerstitial injury would differ from risk factors for albuminuria.
Methods
In this cross-sectional study, we compared urine concentrations of IL-18, KIM-1, and ACR in 908 HIV-infected and 289 HIV-uninfected women enrolled in the Women’s Interagency HIV Study, utilizing stored urine specimens from visits between 1999 and 2000.
Results
After multivariate-adjusted linear regression analysis, mean urine concentrations were higher in HIV-infected individuals by 38% for IL-18 (p<0.0001), 12% for KIM-1 (p=0.081), and 47% for ACR (p<0.0001). Higher HIV RNA level (15% per 10-fold increase, p<0.0001), lower CD4 count (8% per doubling, p=0.0025), HCV infection (30%, p=0.00018), and lower HDL (5% per 10 mg/dL, p=0.0024) were each associated with higher IL-18 concentrations. In contrast, hypertension (81%, p<0.0001) and diabetes (47%, p=0.018) were among the strongest predictors of higher ACR, though HIV RNA level (15% per 10-fold increase, p=0.0004) was also associated with higher ACR.
Conclusions
HIV-infected women had more extensive tubulointerstitial and glomerular injury than uninfected women, but the associated factors differed among the urine biomarkers. Combinations of urinary biomarkers should be investigated to further characterize early kidney injury in HIV-infected women.
Introduction
With dramatic improvements in survival with HIV infection, kidney disease has become an increasingly common co-morbidity. Prevalence estimates for chronic kidney disease (CKD) range from 7-33% among persons with HIV.[1-5] Compared with uninfected individuals, HIV-infected persons have a 10-fold risk of end-stage renal disease (ESRD), a 5-fold prevalence of microalbuminuria, and a 10-fold prevalence of elevated serum cystatin C levels, a marker of reduced kidney function.[6-8] Furthermore, in a contemporary cohort study of HIV-infected individuals on antiretroviral therapy, both cystatin C-based estimated glomerular filtration rate (eGFRCys) <60 ml/min/1.73m2 and albuminuria were independently associated with a doubling of all-cause mortality.[9] These findings demonstrate the importance of kidney disease to prognosis in the current era of HIV infection and treatment.
A major challenge in the prevention of kidney disease in HIV infection is the reliance on serum creatinine to detect impaired kidney function. Serum cystatin C levels appear to be more sensitive for detecting early impairments of kidney filtration function that have prognostic significance in the setting of HIV infection.[7, 10] However, HIV-infected individuals may be susceptible to significant kidney injury prior to the loss of filtration function. Albuminuria has traditionally been utilized as a measure of glomerular injury. One study found that HIV-infected individuals had a 5-fold odds of albuminuria compared with HIV-uninfected individuals;[8] most of these persons had an eGFR in the normal range. Conversely, only 14-44% of HIV-infected persons with eGFR <60 mL/min/1.73m2 have demonstrable albuminuria. This finding suggests that albuminuria does not adequately capture the full spectrum of kidney injury in the setting of HIV infection.[11-14]
Novel urinary biomarkers have been developed that are more specific to tubulointerstitial injury; these were originally investigated as tools to detect early acute kidney injury. However, studies in ambulatory subjects without HIV infection have found that these novel injury biomarkers are also associated with longitudinal decline in kidney function.[15, 16] In the Women’s Interagency HIV Study (WIHS), we recently found that urine interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), and albuminuria had independent and complementary associations with longitudinal kidney function decline.[17] Furthermore, IL-18 and ACR were associated with higher mortality risk among HIV-infected participants, independent of cystatin C, creatinine, and other risk factors in adjusted analyses.[18]
Despite these longitudinal associations, we do not know whether HIV-infected individuals excrete higher levels of these urine biomarkers compared to the general population. We hypothesized that HIV-infected individuals would have more extensive kidney injury compared with HIV-uninfected individuals, as determined by three tubulointerstitial injury markers: urine IL-18, KIM-1, and NGAL. In addition, we hypothesized that the risk factors for glomerular injury in HIV-infected persons, as manifest by the albumin-to-creatinine ratio (ACR), would differ from the risk factors for tubulointerstitial injury. To investigate these hypotheses, we conducted a cross-sectional study nested within the nationally representative cohort of ethnically diverse, HIV-infected and HIV-uninfected women enrolled in the WIHS. We compared urine concentrations of IL-18, KIM-1, NGAL, and ACR between HIV-infected and HIV-uninfected participants, adjusting for differences in traditional risk factors for kidney disease. Then, we described the determinants of the four urine biomarkers to investigate whether each captures a distinct pattern of injury.
Methods
Study Population
The WIHS is a multicenter, prospective cohort study of HIV-infected and HIV-uninfected women enrolled at six U.S. locations: Bronx, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington, DC. Details of the study design, data collection methods, and baseline characteristics are published elsewhere.[19] The institutional review boards of participating institutions approved the study protocol, and informed consent was obtained from all study participants. Briefly, participants undergo semiannual visits that include an interviewer administered questionnaire, a physical examination, and collection of laboratory specimens.
The WIHS Kidney Aging study was designed as a nested cohort study to investigate the onset of kidney disease in the setting of HIV, utilizing stored urine and serum specimens. For this cross-sectional study, we included all 908 HIV-infected and 289 HIV-uninfected women in whom urine samples were collected between October 1999 and March 2000. WIHS was approved by the relevant institutional review boards at all study sites. This study of kidney injury was also approved by the University of California, San Francisco, San Francisco VA Medical Center, and Yale committees on human research.
Urine Biomarkers
The outcomes of this study were the urine concentrations of four kidney injury biomarkers: IL-18, KIM-1, NGAL, and ACR. All four markers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory. Urine IL-18 was measured using a commercially available ELISA kit (Medical & Biological Laboratories Co., Nagoya, Japan). The urine KIM-1 ELISA was constructed using commercially available reagents (R & D Systems, Inc., Minneapolis, MN).[20] Urine NGAL was assayed using a human-specific commercially available ELISA (AntibodyShop, Grusbakken, Denmark).[21] Urine albumin and creatinine were measured by immunoturbidimetry and colorimetric enzyme assay, respectively, using a Siemens Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany. Coefficients of variation for the urine measurements were: IL-18, 7.2%; KIM-1, 5.2%; NGAL, 5.4%; albumin, 5.9%; and creatinine, 4.1%. All urine specimens were in continuous storage at −80°C until biomarker measurement without prior freeze-thaw. Laboratory personnel performing the biomarker assays were blinded to clinical information regarding WIHS participants, including their HIV status, and the samples from the HIV-infected and uninfected participants were interspersed.
Covariates
Candidate covariates included demographic characteristics, traditional risk factors for kidney disease, and HIV-specific risk factors which were assessed at each WIHS semiannual visit. The following characteristics were tested as candidate covariates in all multivariate models: age and race/ethnicity; antihypertensive use, diabetes (fasting glucose ≥126mg/dL, self-reported diabetes, self-reported diabetes medication use, or HbA1c ≥6.5%), cigarette smoking status (current, former, never), and menopause status; systolic and diastolic blood pressure, LDL and HDL cholesterol, triglycerides, body mass index (BMI), waist circumference, hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA following a positive HCV antibody result), and current heroin use. We also tested the following HIV-related characteristics: current CD4 lymphocyte count, nadir CD4 lymphocyte count, history of AIDS diagnosis, current HIV viral load, current highly active antiretroviral therapy (HAART) use, current nucleoside reverse transcriptase inhibitor (NRTI) use, current non-nucleoside reverse transcriptase inhibitor (NNRTI) use, and current protease inhibitor (PI) use. There was minimal use of tenofovir at the baseline of this study. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 5 imputations to yield ~95% relative efficiency.[22] The percentage of missing observations for each covariate ranged from less than 1% to 15%.
Glomerular filtration rate was estimated using the CKD-EPI equations for creatinine (eGFRCr) and cystatin C (eGFRCys), respectively.[23, 24]
Statistical Analysis
We compared demographic and clinical characteristics of HIV-infected and uninfected participants using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Spearman coefficients were used to evaluate correlations between biomarkers.
We used multivariable linear regression to evaluate the associations of HIV infection and other factors with the four urine biomarkers (IL-18, KIM-1, NGAL, and ACR), in separate models for each outcome. In sensitivity analyses, we utilized urine biomarker-to-creatinine ratio as the outcome, analogous to ACR used in clinical practice. We used Huber-White standard errors which are designed to be robust to non-normally distributed residuals.[25, 26] Because the biomarkers were right-skewed, each outcome was log-transformed for analysis; results were back-transformed to produce estimated percentage differences. To determine whether HIV infection was independently associated with each biomarker, multivariable models were sequentially adjusted for: 1) HIV status, 2) demographics, 3) kidney disease risk factors (hypertension, diabetes, and HCV infection), 4) ACR, and 5) eGFRCys. We also performed a nested analysis restricted to the 679 HIV-infected and 256 uninfected women with eGFRCys > 60 ml/min/1.73m2 and ACR < 30 to determine whether urine IL-18, KIM-1, and NGAL levels are higher in HIV infection in the absence of clinically detectable chronic kidney disease.
As an alternate approach, we dichotomized each biomarker as elevated or normal, using the highest tertile cutpoint (“T3”) in uninfected women to define those with elevated biomarker levels. We used Poisson regression with a robust variance estimator to assess the relative risk of having “elevated” urine biomarker levels (defined as urine biomarker concentration > T3) in HIV-infected participants as compared with uninfected controls after multivariate adjustment.[27]
Next, we examined differences in associated risk factors by fitting separate linear regression models for each biomarker outcome in HIV-infected women. We used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that were not associated with the outcome. Due to cosegregation of HCV infection, current smoking, and heroin use, we preferentially included HCV infection in each model if it reached a significance level of p<0.05. As an alternative model building approach, we used Bayesian model averaging and retained predictors with posterior probabilities >35%.[28] Models constructed using the two approaches were very similar.
Bayesian model averaging was performed using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
The median age was 41 among the 908 HIV-infected participants, and 40 among the 289 HIV-uninfected women from WIHS (Table 1). Over half of the participants were African-American, and three-fourths were current or former smokers. Diabetes and hypertension were prevalent in one-tenth and one-fourth of women, respectively, and did not differ by HIV status. HIV-infected women were more likely to be postmenopausal and infected with HCV than HIV-uninfected women. Compared with uninfected women, HIV-infected women had higher triglycerides and lower HDL, BMI, and waist circumference. Among HIV-infected women, the median CD4 lymphocyte count was 397 cells/mm3, and nearly one-third had an undetectable HIV viral load. Among both groups of women, fewer than 10% had an eGFR <60ml/min/1.73m2, as calculated by serum cystatin C or creatinine.
Table 1. Baseline characteristics of HIV-infected and uninfected women in WIHS.
| HIV+ (N = 908) |
Control (N = 289) |
P Value | |
|---|---|---|---|
| Age (y) | 41 (36-46) | 40 (34-45) | 0.0086 |
| <30 | 55 (6%) | 38 (13%) | |
| 30-40 | 350 (39%) | 109 (38%) | |
| 40-50 | 410 (45%) | 111 (38%) | |
| >50 | 93 (10%) | 31 (11%) | |
| Race | 0.0058 | ||
| African American | 524 (58%) | 178 (62%) | |
| Caucasian | 175 (19%) | 33 (11%) | |
| Other | 209 (23%) | 78 (27%) | |
| Cigarette Smoking | 0.071 | ||
| Current | 464 (51%) | 170 (59%) | |
| Past | 224 (25%) | 62 (21%) | |
| Never | 220 (24%) | 57 (20%) | |
| Diabetes Mellitus | 86 (9%) | 26 (9%) | 0.91 |
| Hypertension | 228 (25%) | 80 (28%) | 0.40 |
| Antihypertensive Use | 98 (11%) | 35 (12%) | 0.52 |
| Menopause | 185 (21%) | 40 (14%) | 0.0076 |
| Hepatitis C | 281 (31%) | 62 (22%) | 0.0021 |
| LDL (mg/dL) | 103 (80-132) | 104 (86-129) | 0.30 |
| HDL (mg/dL) | 44 (36-56) | 51 (42-62) | <0.0001 |
| TG (mg/dL) | 133 (93-196) | 101 (73-150) | <0.0001 |
| Body Mass Index (kg/m2) | 27 (23-31) | 29 (24-34) | <0.0001 |
| Waist Circumference (cm) | 88 (80-99) | 93 (80-104) | 0.0064 |
| Current Heroin Use | 43 (5%) | 23 (8%) | 0.053 |
| Current HAART Use | 533 (59%) | ||
| Current NRTI Use | 606 (67%) | ||
| Current NNRTI Use | 246 (27%) | ||
| Current PI Use | 381 (42%) | ||
| Current CD4 Count (cells/mm3) | 397 (245-576) | ||
| Nadir CD4 Count (cells/mm3) | 212 (109-326) | ||
| History of AIDS | 445 (49%) | ||
| HIV Viral Load (copies/mL) | |||
| ≤80 | 276 (31%) | ||
| 81-1999 | 204 (23%) | ||
| 2000-9999 | 147 (16%) | ||
| >10000 | 275 (30%) | ||
| eGFR Cys <60ml/min/1.73m2 | 84 (9%) | 6 (2%) | <.0001 |
| eGFR Cr <60ml/min/1.73m2 | 64 (7%) | 14 (5%) | 0.22 |
Data are presented as Median (IQR) or numbers (percent).
Abbreviations: IQR, interquartile range; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor
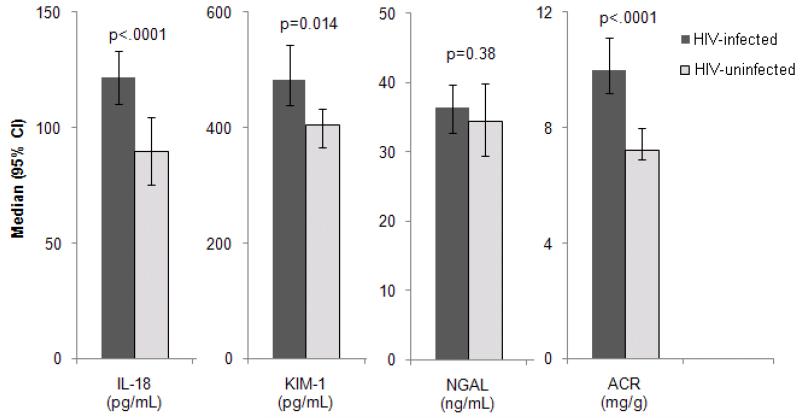
HIV-infected women had more extensive kidney injury than uninfected women, manifested by higher median urine levels of IL-18, KIM-1, NGAL, and ACR (Figure 1). Urinary IL-18 concentrations and ACR levels were approximately 40% higher in HIV-infected women (p<0.0001), with minimal attenuation after controlling for demographics and traditional kidney risk factors (Table 2). After additional adjustment for ACR and eGFRCys, HIV infection was associated with 28% higher IL-18 (p=0.0007) and 33% higher ACR (p=0.0003). HIV infection had weaker associations with urine KIM-1 and NGAL concentrations in multivariate analyses; the HIV effect was mostly attenuated by adjustment for eGFRCys rather than ACR.
Figure 1. Association of HIV infection with urine biomarkers among HIV-infected (N=908) and uninfected (N=289) WIHS participants.
Note: Bars represent median urine biomarker concentrations with 95% confidence intervals. P-values from Mann-Whitney U test.
Abbreviations: ACR, albumin-to-creatinine ratio; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin.
Table 2. Association of HIV infection with urine biomarkers using linear regression analysis.
| IL-18 (pg/mL) |
KIM-1 (pg/mL) |
NGAL (ng/mL) |
ACR (mg/g) |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Models | % Estimate1 (95% CI) |
P Value | % Estimate (95% CI) |
P Value | % Estimate (95% CI) |
P Value | % Estimate (95% CI) |
P Value |
| Unadjusted | 38 (19, 59) | <0.0001 | 16 (1.6, 32) | 0.028 | 9.8 (−5.5, 28) | 0.22 | 40 (21, 62) | <0.0001 |
|
Demographic- Adjusted2 |
43 (24, 65) | <0.0001 | 14 (0.32, 30) | 0.045 | 14 (−2.0, 33) | 0.090 | 40 (20, 62) | <0.0001 |
|
Multivariate- Adjusted3 |
38 (20, 59) | <0.0001 | 12 (−1.4, 28) | 0.081 | 17 (0.30, 35) | 0.046 | 47 (26, 71) | <0.0001 |
|
Multivariate- Adjusted4+ACR |
39 (20, 60) | <0.0001 | 12 (−1.8, 28) | 0.090 | 13 (−3.2, 31) | 0.12 | - | - |
|
Multivariate- Adjusted+ACR +eGFRCys5 |
28 (11, 48) | 0.0007 | 1.9 (−11, 16) | 0.78 | 2.4 (−12, 19) | 0.76 | 33 (14, 55) | 0.0003 |
Percent estimate represents estimated percentage difference in biomarker for HIV-infected participants vs. HIV-uninfected participants using mean urine biomarker levels. All analyses were performed using log-transformed mean urine values; results are back-transformed to produce % estimate.
Adjusted for age and race.
Adjusted for age, race, and traditional kidney disease risk factors. Factors forced into full model included hypertension, diabetes mellitus, and HCV infection.
Adjusted for all covariates listed above with addition of albumin-creatinine ratio.
Adjusted for all covariates listed with addition of eGFRCys.
Abbreviations: ACR, albumin-creatinine ratio; CI, confidence interval; eGFRCys, estimated glomerular filtration rate calculated using cystatin C; HCV, hepatitis C virus; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin.
Findings were similar when the biomarkers were standardized to urine creatinine. After adjustment for demographics and traditional risk factors for kidney disease, HIV infection was associated with 42% (28-58%) higher urine IL-18/Cr, 16% (6-27%) higher urine KIM-1/Cr, and 19% (4-36%) higher NGAL/Cr. With sequential adjustment for ACR and eGFRCys, HIV infection remained strongly associated with urine IL-18/Cr (32%, p<0.0001), but was no longer significantly associated with urine KIM-1/Cr (6%, p=0.25) or NGAL/Cr (5%, p=0.46). When we dichotomized each tubulointerstitial biomarker as elevated or normal, HIV-infected women had a 30% higher risk of having “elevated” urine IL-18 as compared with uninfected participants (95% CI 1.05-1.67), after multivariate adjustment including ACR and eGFRCys. There were no statistically significant associations of HIV infection with “elevated” levels of urine KIM-1 (RR 1.04; 95% CI 0.82-1.32) or NGAL (RR 1.06, 95% CI 0.83-1.34).
When we restricted our linear regression analysis to the 679 HIV-infected and 256 uninfected women with eGFRCys > 60 ml/min/1.73m2 and ACR < 30, mean urine IL-18 levels remained 38% higher (p<0.0001) in HIV- infected women after multivariate adjustment. Urine KIM-1 and NGAL levels were not statistically associated with HIV infection after multivariate analysis (7.4% (p=0.32) and 9.0% (p=0.29), respectively).
In both HIV-infected and uninfected women, the three tubulointerstitial injury biomarkers (IL-18, KIM-1, and NGAL) showed moderately strong positive inter-correlations (r = 0.3 to 0.5; p<.0001). By contrast, in HIV-infected women, ACR was weakly correlated with NGAL (r=0.12, p=0.0003) and showed little association with IL-18 (r=−0.018, p=0.59) or KIM-1 (r=0.014, p=0.68). In HIV-uninfected women, ACR was weakly negatively associated with KIM-1 (r=−0.19, p=0.001) and IL-18 (r=−0.14, p=0.019), and had little association with NGAL (r=−0.062, p=0.29).
We then examined factors associated with kidney injury markers in HIV-infected women (Table 3). Among the demographic factors, older age was consistently associated with lower levels of urine injury markers; the effect was more pronounced in all models after adjustment for eGFRCys. African-American race was strongly associated with higher urine IL-18, NGAL, and ACR. HIV-infected women who were not of Caucasian or African-American race also had substantially higher urine IL-18 levels.
Table 3. Factors independently associated with urine biomarker concentrations among HIV-infected WIHS participants (N=908).
| IL-18 | KIM-15 | NGAL | ACR | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| % Estimate (95% CI)1 | % Estimate (95% CI) | % Estimate (95% CI) | % Estimate (95% CI) |
||||
|
| |||||||
| Full Model2 | Full Model +ACR+eGFRCys3 |
Full Model2 | Full Model +ACR+eGFRCys3 |
Full Model2 | Full Model +ACR+eGFRCys3 |
Full Model +eGFRCys4 |
|
| Demographics | |||||||
| Age (per decade) | −6 (−14, 3) | −11 (−19, −3) + | −8 (−18, 3) | −13 (−22, −3)* | −11 (−20, −2)* | −19 (−27, −9) ▲ | −9 (−20, 4) |
| African-American6 | 59 (34, 88)▲ | 63 (38, 92) ▲ | −5 (−22, 16) | −1 (−18, 20) | 58 (29, 94) ▲ | 60 (31, 96) ▲ | 43 (12, 83) + |
| Other Race6 | 48 (20, 81) + | 51 (23, 84) ▲ | 4 (−18, 30) | 7 (−14, 34) | 24 (−2, 58) | 32 (5, 67)* | 7 (−20, 44) |
| Traditional | |||||||
| Hypertension | - | - | 31 (12, 54) ▲ | 23 (5, 44) + | 25 (2, 52)* | 8(−11, 31) | 69 (31, 118) ▲ |
| Diabetes | - | - | - | - | - | - | 43 (5, 95)* |
| LDL (per 10 mg/dL) | - | - | - | - | 2 (0, 5)* | 3 (1, 6)* | - |
| HDL (per 10 mg/dL) | −5 (−9, −2) + | −5 (−8, −1)* | - | - | - | - | - |
| Current Smoking | - | - | 15 (0, 32)* | 7 (−7, 22) | 23 (6, 43) + | 19 (1, 40)* | −28 (−40, −12) + |
| HIV-related | |||||||
| CD4 Count (per doubling) |
−8 (−13, −3) + | −8 (−13, −3) + | −8 (−13, −2) + | −5 (−11, 0) | −7 (−12, 0)* | −5 (−10, 1) | |
| Viral Load7 | 15 (8, 22) ▲ | 12 (6, 20) ▲ | - | - | - | - | 10 (1, 19)* |
| NNRTI Use | - | - | - | - | - | - | 57 (29, 91) ▲ |
| Other | |||||||
| HCV Infection | 30 (13, 49)+ | 20 (4, 39)* | 20 (3, 40)* | 11 (−5, 29) | - | - | - |
| Heroin Use | - | 39 (2, 90)* | 32 (−2, 78) | - | - | - | |
Results for each model displayed as percent estimate (95% confidence interval). Percent estimate represents estimated percentage difference in biomarker attributable to each factor, after performing stepwise multivariable linear regression. All analyses were performed using log-transformed mean urine values; results are back-transformed to produce % estimates.
Adjusted for demographics and the factors displayed under each respective biomarker.
Adjusted for demographics, the factors displayed under each biomarker, ACR and eGFRCys.
Multivariate model for ACR adjusts for eGFRCys in addition to listed covariates.
Multivariate model for KIM-1 adjusts for menopause in addition to listed covariates.
Versus Caucasian
per 10-fold increase
denote P-value <.05, <.01, <.001, respectively
Abbreviations: ACR, albumin-to-creatinine ratio; HCV, hepatitis C virus; HDL, high-density lipoprotein; eGFRCys, estimated glomerular filtration rate calculated using cystatin C; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin. NNRTI, non-nucleoside reverse transcriptase inhibitor.
HIV-related characteristics were the strongest clinical factors associated with urinary IL-18. Lower CD4 lymphocyte count, higher HIV viral load, HCV infection, and lower HDL were each independently associated with higher urine IL-18 concentrations. In contrast, hypertension diabetes, and NNRTI use were the strongest predictors of higher ACR, although higher HIV viral load was also associated with ACR. Of the different antiretroviral medications in the NNRTI class, only efavirenz was independently associated with higher ACR (64% higher ACR in individuals reporting efavirenz use, p=0.0012), but the prevalence of this agent was only 13% in this study.
We also examined the associations of individual antiretroviral medications with each urine biomarker (Supplementary Table 1). Thirteen antiretroviral medications were reported in use with a prevalence ≥ 1% at the time of this study. There were no class-specific patterns of association with the biomarkers, nor was any one biomarker consistently associated with multiple antiretroviral medications. Reported prevalence of use for each antiretroviral medication ranged from 1-48%.
Discussion
In this cross-sectional analysis of WIHS participants, we found that HIV-infected women had higher urine concentrations of four kidney injury markers, IL-18, KIM-1, NGAL, and ACR, as compared with HIV-uninfected participants. Factors associated with the three novel tubulointerstitial injury markers – IL-18, KIM-1, and NGAL – differed from factors associated with albuminuria, a clinical marker of glomerular injury. While albuminuria was most strongly associated with the traditional kidney risk factors of hypertension and diabetes, the tubulointerstitial injury markers, particularly IL-18, had strong associations with HIV-related and other factors, such as CD4 lymphocyte count, HIV viral load, and HCV infection. In combination with our earlier work demonstrating associations of these urine biomarkers with kidney function decline and mortality risk, this study demonstrates the potential of tubulointerstitial injury biomarkers to capture a unique dimension of kidney disease.
To our knowledge, this is the first study to compare urine levels of IL-18, KIM-1, and NGAL in a large cohort of HIV-infected and uninfected individuals. Urinary IL-18 and KIM-1 are specific to the proximal tubule, and have been implicated in ischemia-reperfusion injury to the kidney.[29-32] Urinary IL-18 also predicts the duration of acute kidney injury (AKI),[33] in-hospital mortality,[34] and severity of kidney disease in patients with nephrotic syndrome,[35, 36] while urine KIM-1 levels have been associated with the severity of pathology in CKD.[37, 38] In contrast to IL-18 and KIM-1, NGAL is expressed by epithelial cells in the distal tubule. Urinary NGAL concentrations have been shown to predict AKI in children and adults,[39-41] and are also elevated in individuals with polycystic kidney disease,[42] IgA nephropathy,[43] and lupus nephritis.[44]
Our finding that HIV-infected women have higher urine concentrations of IL-18, KIM-1 and NGAL suggests that HIV infection or HIV-specific therapies promote renal tubulointerstitial injury. This hypothesis is supported by prior kidney biopsy studies implicating the proximal and distal tubules as early targets of HIV infection.[13, 45-47] One recent kidney biopsy series of 25 HIV-infected individuals with proteinuric glomerulopathies found that urine NGAL excretion was 4-fold higher in individuals with HIVAN as compared with other etiologies of HIV-related kidney disease.[48] Though the study was small, it suggests that urine kidney injury markers may be linked to specific types of kidney pathology. Additional studies are needed to correlate the concentrations of tubulointerstitial and glomerular injury markers with specific pathologic findings on kidney biopsy.
The relatively low prevalence of eGFR <60ml/min/1.73m2 in our cohort implies that HIV-infected individuals experience significant kidney injury prior to the clinician’s ability to detect an appreciable loss of filtration function. The measurement of tubulointerstitial biomarkers, in addition to eGFR and albuminuria, could constitute a novel and more comprehensive method of screening for and quantifying kidney injury in HIV-infected individuals. The ability to detect early kidney injury is particularly important in the HIV-infected population because HIV-related muscle loss makes creatinine-based eGFR less accurate and HIV-related therapies can be nephrotoxic. This study identified an association between NNRTI use and higher ACR, but these results must be interpreted cautiously given the cross-sectional design and low prevalence of use of individual NNRTIs. The associations of specific antiretroviral medications with kidney injury and decline, particularly tenofovir-based regimens, should be further evaluated in rigorous longitudinal cohort studies with measurement of urine biomarkers in patients with well-controlled HIV infection. For instance, one could prospectively measure urine tubulointerstitial injury markers in individuals being initiated on tenofovir-based antiretroviral regimens to assess their ability to detect kidney injury prior to the onset of Fanconi’s syndrome, albuminuria, or CKD.
An important finding of this study is the independent association of African-American race with higher levels of several kidney injury markers in HIV infection. Prior cohort studies of HIV-infected individuals have demonstrated that African-Americans have a markedly higher incidence of ESRD as compared with Caucasians, and experience faster progression from CKD to ESRD.[49-51] Additionally, HIV-associated nephropathy (HIVAN) was historically observed almost exclusively in African-American individuals. Our finding of elevated urine IL-18, NGAL, and ACR in African-Americans with HIV infection implicates tubulointerstitial and glomerular injury in the pathogenesis of this racial predilection for HIV-related kidney disease. Recent literature suggests there may be genetic polymorphisms that predispose African-Americans to kidney disease to a greater degree than Caucasians.[52] Future studies should investigate potential mechanisms linking these specific genetic polymorphisms to susceptibility to tubulointerstitial and glomerular injury.
There are several limitations to this study. First, as a cross-sectional study, we have identified associations between the urine biomarkers, HIV infection, and related comorbidities, but we cannot make conclusions regarding causality. Second, the urine specimens were collected and stored over a decade prior to biomarker measurement. However, protein degradation over time would be expected to bias our study towards null findings. Third, because we exclusively studied women, we cannot generalize these results to men. Fourth, the analyses correcting urine IL-18, KIM-1 and NGAL for urine creatinine concentration must be interpreted cautiously, as creatinine excretion may be unpredictable in HIV-infected women who may experience muscle loss. Fifth, we did not have access to serum concentrations of the tubulointerstitial injury markers, so we cannot exclude the possibility that higher serum levels contributed to the observed elevations in urine. Finally, although we adjusted for multiple potential confounders, the possibility of residual confounding exists for our associations of HIV infection with the kidney injury biomarkers.
In summary, we found that HIV-infected women have more extensive kidney injury as compared with HIV-uninfected women, manifested by elevations in four kidney injury markers: urine IL-18, KIM-1, NGAL, and ACR. Future studies should correlate these biomarkers with pathology from kidney biopsies and evaluate the clinical utilities of using urine biomarkers to detect early kidney injury related to HIV infection or its treatments.
Supplementary Material
Acknowledgments
Dr. Parikh is a co-inventor on IL-18 patent issued to University of Colorado. Dr. Devarajan is a co-inventor on patents related to the use of NGAL as a marker of acute and chronic kidney injury.
Funding Sources
The WIHS Kidney Aging Study is funded by grant 1 R01 AG034853-01A2 (PI, Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, Anastos K, Klassen PS, Svetkey LP. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Szczech LA, Hoover DR, Feldman JG, Cohen MH, Gange SJ, Gooze L, Rubin NR, Young MA, Cai X, Shi Q, Gao W, Anastos K. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199–1206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 3.Krawczyk CS, Holmberg SD, Moorman AC, Gardner LI, McGwin GJ. Factors associated with chronic renal failure in HIV-infected ambulatory patients. Aids. 2004;18:2171–2178. doi: 10.1097/00002030-200411050-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dube MP. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 5.Gardner LI, Holmberg SD, Williamson JM, Szczech LA, Carpenter CC, Rompalo AM, Schuman P, Klein RS. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. Acquir Immune Defic Syndr. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 6.Eggers PW, Kimmel PL. Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol. 2004;15:2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 7.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld G, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczech LA, Grunfeld G, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG. Microalbuminuria in HIV infection. Aids. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, Szczech LA, Grunfeld C, Shlipak MG. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driver TH, Scherzer R, Peralta CA, Tien PC, Estrella MM, Parikh CR, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Abraham A, Shlipak MG. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS. 2013 doi: 10.1097/QAD.0b013e328362e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wools-Kaloustian K, Gupta SK, Muloma E, Owino-Ong’or W, Sidle J, Aubrey RW, Shen J, Kipruto K, Zwickl BE, Goldman M. Renal disease in an antiretroviral-naive HIV-infected outpatient population in Western Kenya. Nephrol Dial Transplant. 2007;22:2208–2212. doi: 10.1093/ndt/gfm223. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, Wong KM, Lee MP, Liu YL, Kwok H, Chung R, Chau KF, Li CK, Li CS. Prevalence of chronic kidney disease in Chinese HIV-infected patients. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm350. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, D’Agati VD. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2008 doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyatt CM, Winston JA, Malvestutto CD, Fishbein DA, Barash I, Cohen AJ, Klotman ME, Klotman PE. Chronic kidney disease in HIV infection: an urban epidemic. Aids. 2007;21:2101–2103. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 15.Astor BC, Kottgen A, Hwang SJ, Bhavsar N, Fox CS, Coresh J. Trefoil Factor 3 Predicts Incident Chronic Kidney Disease: A Case-Control Study Nested within the Atherosclerosis Risk in Communities (ARIC) Study. Am J Nephrol. 2011;34:291–297. doi: 10.1159/000330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Seaghdha CM, Hwang SJ, Bhavsar NA, Kottgen A, Coresh J, Astor BC, Fox CS. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis. 2011;57:841–849. doi: 10.1053/j.ajkd.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women: Biomarkers and Kidney Decline. J Acquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peralta CA, Scherzer R, Grunfeld C, Abraham A, Tien P, Bennett M, Butch AW, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C, Shlipak M. Urinary Biomarkers of Kidney Injury Are Associated with All-Cause Mortality in the Women’s Interagency HIV Study. 2012 doi: 10.1111/hiv.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J, WIHS Collaborative Study Group The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 20.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilks WR, Richardson S, Spiegehalter DJ. Markov chain Monte Carlo in practice. Chapman & Hall; London: 1996. [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber P. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967;1:221–233. [Google Scholar]
- 26.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 27.Lumley T, Kronmal R. M S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators, and Algorithms. UW Biostatistics Working Paper Series. 2006 Working Paper 293. [Google Scholar]
- 28.Hoeting JMD, Raftery A, Volinsky C. Bayesian Model Averaging: A Tutorial. Statistical Science. 1999;14:382–401. [Google Scholar]
- 29.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 31.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 32.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 33.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 34.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 35.Kilis-Pstrusinska K, Medynska A, Zwolinska D, Wawro A. Interleukin-18 in urine and serum of children with idiopathic nephrotic syndrome. Kidney Blood Press Res. 2008;31:122–126. doi: 10.1159/000124284. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Kanmatsuse K. Elevated interleukin-18 levels in the urine of nephrotic patients. Nephron. 2001;88:334–339. doi: 10.1159/000046017. [DOI] [PubMed] [Google Scholar]
- 37.Nepal M, Bock GH, Sehic AM, Schultz MF, Zhang PL. Kidney injury molecule-1 expression identifies proximal tubular injury in urate nephropathy. Ann Clin Lab Sci. 2008;38:210–214. [PubMed] [Google Scholar]
- 38.van Timmerren MM, ven den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007:212. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 39.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 40.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 41.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–378. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 43.Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol. 2007;123:227–234. doi: 10.1016/j.clim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 45.Ross MJ, Klotman PE. Recent progress in HIV-associated nephropathy. J Am Soc Nephrol. 2002;13:2997–3004. doi: 10.1097/01.asn.0000040750.40907.99. [DOI] [PubMed] [Google Scholar]
- 46.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D’Agati VD, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 47.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 48.Sola-Del Valle DA, Mohan S, Cheng JT, Paragas NA, Sise ME, D’Agati VD, Barasch J. Urinary NGAL is a useful clinical biomarker of HIV-associated nephropathy. Nephrol Dial Transplant. 2011;26:2387–2390. doi: 10.1093/ndt/gfr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O’Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007;72:1380–1387. doi: 10.1038/sj.ki.5002541. [DOI] [PubMed] [Google Scholar]
- 50.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak M. Risk factors for end-stage renal disease in HIV-infected individuals: traditional and HIV-related factors. AJKD. 2012 doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosset S, Tzur S, Behar DM, Wasser WG, Skorecki K. The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat Rev Nephrol. 7:313–326. doi: 10.1038/nrneph.2011.52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.