Abstract
The conventional model of language‐related brain structure describing the arcuate fasciculus as a key white matter tract providing a direct connection between Wernicke's region and Broca's area has been called into question. Specifically, the inferior precentral gyrus, possessing both primary motor (Brodmann Area [BA] 4) and premotor cortex (BA 6), has been identified as a potential alternative termination. The authors initially localized cortical sites involved in language using measurement of event‐related gamma‐activity on electrocorticography (ECoG). The authors then determined whether language‐related sites of the temporal lobe were connected, via white matter structures, to the inferior frontal gyrus more tightly than to the precentral gyrus. The authors found that language‐related sites of the temporal lobe were far more likely to be directly connected to the inferior precentral gyrus through the arcuate fasciculus. Furthermore, tractography was a significant predictor of frontal language‐related ECoG findings. Analysis of an interaction between anatomy and tractography in this model revealed tractrography to have the highest predictive value for language‐related ECoG findings of the precentral gyrus. This study failed to support the conventional model of language‐related brain structure. More feasible models should include the inferior precentral gyrus as a termination of the arcuate fasciculus. The exact functional significance of direct connectivity between temporal language‐related sites and the precentral gyrus requires further study. Hum Brain Mapp 35:2333–2347, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: high‐frequency oscillations (HFOs), ripples, event‐related augmentation of gamma‐activity, DTI, tractography, speech, naming, connectivity
INTRODUCTION
The Wernicke–Lichtheim model, formulated by 1885, was the first to suggest a direct anatomical connection between Wernicke's region of the posterior left temporal lobe and Broca's area of the left inferior frontal gyrus [Graves, 1997]. This model laid out the origins of our modern understanding of language in a diagrammatic form that was both easy to understand and predictive of what type of lesion might lead to what type of symptom [Graves, 1997; Lichtheim, 1885]. Geschwind's work in the 20th century is well known for advancing the idea that the arcuate fasciculus represented the physical manifestation of the concept of a direct connection between Wernicke's region and Broca's area [Geschwind, 1970]. Even after recent reconsideration of the model [Catani and Mesulam, 2008; Friederici, 2012; Poeppel et al., 2012], these two cortical regions and the associated white matter tract have constituted our understanding of language‐related structure and localization in the left hemisphere.
It is interesting that a review of the literature back to the 19th century does not yield a single study that clearly demonstrates the arcuate fasciculus' assumed termination within Broca's area [Martino et al., 2013], with Broca's area strictly defined as the lateral surface of the combination of pars triangularis (Brodmann Area [BA] 45) and pars opercularis (BA 44) of the left inferior frontal gyrus [Dronkers et al., 2007; Strotzer, 2009]. In many studies using diffusion‐weighted imaging tractography, a termination of the arcuate fasciculus within Broca's area is assumed, often by seeding fibers directly from Broca's area [Brauer et al., 2011; Ellmore et al., 2009]. A lack of fibers associated with portions of Broca's area, pars triangularis in particular, is often interpreted as an imaging shortfall rather than a reflection of actual connectivity [Diehl et al., 2010]. Indeed, several studies have provided new evidence that the anterior terminations of the arcuate fasciculus, the cytoarchitecture traditionally associated with Broca's area, and the functional correlates of language often extend outside the anatomically defined boundaries of Broca's area [Dronkers et al., 2007], with a notable extension into the inferior precentral gyrus [Amunts and Zilles, 2012; Amunts et al., 2003; Bizzi et al., 2012; Catani and Mesulam, 2008]; the new term “Broca's territory” [Catani et al., 2005] has even been proposed to encompass cortex adjacent to the historically relevant Broca's area. Functional data generated by a study of patients with gliomas located in the inferior precentral gyrus and the inferior frontal gyrus of the left hemisphere suggest that essential language function can be located within the inferior precentral gyrus [Bizzi et al., 2012]; of 19 patients, the only case of Broca (expressive) aphasia was associated with a glioma in the inferior precentral gyrus that compressed but spared pars opercularis of the inferior frontal gyrus.
The signals detected and routinely analyzed by electrocorticography (ECoG) methods fall within a class of electrophysiological activities known as local field potentials, which have been shown to correlate well with the blood oxygen level‐dependent signal detected by functional magnetic resonance imaging (MRI) [Logothetis, 2003]. Task‐related amplitude augmentation of broadband gamma‐range signals in excess of 50 Hz has been shown to accurately localize cortical function [Crone et al., 2011; Jerbi et al., 2009; Miller et al., 2008]. We use an auditory descriptive naming task to elicit gamma (50–150 Hz)‐augmentation in cerebral regions mediating language; an approach with promising evidence of validity against electrical brain stimulation and other methodologies [Brown et al., 2008, 2012; Cervenka et al., 2013; Kojima et al., 2012, 2013].
The aim of this study involved the utilization of language‐related ECoG results to generate temporal lobe seed and supra‐Sylvian target regions for tractographic analysis. Such an approach provides the ability to avoid specific anatomical assumptions about the localization of Wernicke's region. While seeding tractrography from language‐related gamma‐sites of the temporal lobe, indicated by ECoG analysis to be involved in language‐related processing, we hypothesized that fibers would track predominantly to the inferior frontal gyrus rather than the precentral gyrus. Furthermore, we hypothesized that fibers would track more frequently to frontal lobe sites showing gamma‐augmentation during a language task when compared with those that do not. We expected that testing these hypotheses would provide additional evidence to validate the aforementioned model of language‐related brain structure [Graves, 1997].
MATERIALS AND METHODS
Study Patients
Patients were selected by using the following inclusion criteria: (i) a history of left‐hemispheric focal epilepsy scheduled for extraoperative subdural ECoG recording as part of presurgical evaluation at Children's Hospital of Michigan or Harper University Hospital, Detroit; (ii) age of 5 years or older; (iii) measurement of ECoG amplitude augmentations driven by a language task described in “Auditory Naming Task” section; and (iv) preoperative MRI, including 55‐direction diffusion‐weighted imaging. Our recent ECoG study demonstrated that language‐related gamma‐sites were identified in patients aged 4 years and older and that resection of such sites predicted postoperative language deficits with statistical significance [Kojima et al., 2013]. Exclusion criteria consisted of (i) the presence of physical deformities of the brain, including tumor or malformation, that disrupt the normal structure of the left superior temporal region, inferior parietal region, postcentral gyrus, precentral gyrus, or inferior frontal gyrus, (ii) history of hearing impairment, (iii) right language dominance as determined by Wada testing (i.e., intracarotid sodium amobarbital procedure) or left‐ or ambiguous‐handedness when Wada test results are not available [Knecht et al., 2000; Moddel et al., 2009], (iv) multiple seizure foci involving both hemispheres, (v) documented history of language delay represented by Verbal Comprehension Index or Verbal Intelligence Quotient less than 70, and (vi) history of previous neurological surgery. We studied a series of five patients satisfying all criteria (age range: 6–21 years; four females; Table 1). This study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from all patients or their legal parent or guardian.
Table 1.
Patient data
| Patient | Gender | Age at surgery (years) | Dominant hand | Age at epilepsy diagnosed | Antiepileptic mediations | VCIa | VIQa | Schooling | Wada testa (language) | Seizure type | ECoG electrode placement | Seizure onset zone | ECoG contacts (total) | Histology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 17 | Rt | 5 | LEV, OXC | 109 | 104 | Above average, normal 12th grade | Lt | Focal Sz w/sGTC | Lt FPTO | Lt medial T | 112 | Gliosis and microdysgenesis |
| 2 | Female | 21 | Rt | 21 | LEV | N/A | N/A | High school diploma | N/A | Focal Sz w/sGTC | Lt PTO | Lt O | 68 | Low‐grade tumor |
| 3 | Female | 15 | Rt | 13 | LEV | N/A | N/A | Above average, normal 10th grade | N/A | Focal Sz w/sGTC | Lt FPTO | Lt medial and anterior T | 100 | Low‐grade tumor |
| 4 | Female | 6 | Rt | 1 | VPA, LMG | N/A | 74 | Normal kindergarten | N/A | Focal Sz | Lt FPTO | Lt medial T | 118 | Hippocampal sclerosis |
| 5 | Female | 12 | Rt | 9 | LMG, LAC | 97 | N/A | Normal 6th grade | N/A | Focal Sz | Lt FPTO | Lt medial T | 110 | Hippocampal sclerosis |
Abbreviations: LEV, levetiracetam; LMG, lamotrigine; LAC, lacosamide; OXC, oxcarbazepine; VPA, valproate; RT, right; Lt, left; Sz, seizure; sGTC, secondarily generalized tonic‐clonic Sz; F, frontal; T, temporal; O, occipital; P, parietal.
VCI, Verbal Comprehension Index; VIQ, Verbal Intelligence Quotient. Neuropsychological testing was performed based on clinical necessity. WADA testing (i.e., intracarotid sodium amobarbital procedure) results are provided to indentify the language‐dominant hemisphere. Because of the use of an auditory language task, we include the measures VIQ and VCI, when available.
Subdural platinum grid electrode (10‐mm intercontact distance; 4‐mm diameter) placement was performed as described previously by our team [Asano et al., 2009a; Wu et al., 2011]. Electrode plates were stitched to adjacent plates and/or the edge of dura mater to avoid movement of subdural electrodes after placement. Extraoperative video‐ECoG recordings were obtained for 3–5 days using a 192‐channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America, Foothill Ranch, CA) at a sampling frequency of 1,000 Hz, as previously described [Wu et al., 2011]. Total electrode contact number ranged from 68 to 118 (Table 1).
Coregistration of Electrodes on Individual Three‐Dimensional MRI
MRI, including a volumetric‐T1‐weighted spoiled gradient echo image as well as a fluid‐attenuated inversion recovery image of the entire head, was obtained preoperatively using a previously described protocol [Nagasawa et al., 2010a]. Planar X‐ray images (lateral and anteroposterior) were acquired with subdural electrodes in place for localization on the brain surface [Dalal et al., 2008; Miller et al., 2007; von Stockhausen et al., 1997]; three metallic fiducial markers at anatomically well‐defined locations aided coregistration with MRI. A three‐dimensional MRI brain surface image was created with electrode sites delineated [Alkonyi et al., 2009; Muzik et al., 2007; von Stockhausen et al., 1997]. Accuracy was confirmed by intraoperative digital photographs showing in situ electrode locations [Asano et al., 2005; Nagasawa et al., 2010a; Wu et al., 2011]. Figure 1 shows typical electrode coverage.
Figure 1.
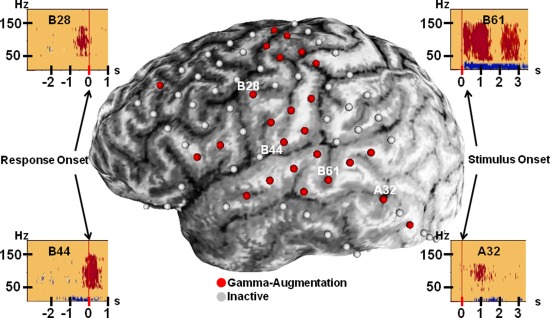
Electrode coverage. Typical electrode coverage is demonstrated in Patient 1. In four of the five patients, with Patient 2 as the exception, an 8 × 8 grid of electrodes covered portions of the lateral frontal, parietal, and temporal lobes. Variably, electrode strips extended coverage, as seen here in the inferior temporal region. Electrodes associated with epileptiform activity or artifacts were excluded from study, as they were from this image. Electrodes with “red” color represent those with significant gamma‐augmentation between 50 and 150 Hz. Four such electrodes were selected as examples to depict frequency–time plots. Results are similar to those reported previously [Brown et al., 2008]. Results from stimulus‐onset analysis are shown for electrodes A32 and B61; B61 exhibits auditory activity, as previously described [Brown et al., 2012]. These two temporal lobe electrode sites were among those used to generate tractography seed regions. Electrodes B28 and B44 are over the inferior portion of the precentral gyrus, and results from response‐onset analysis are displayed. Note that B28 exhibits augmentation that is largely occurring prior to response onset. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Auditory Naming Task
Language mapping by measurement of auditory naming‐related gamma‐activity was performed using an auditory naming task similar to that previously reported [Brown et al., 2008; Kojima et al., 2012]; more specifically described as an auditory descriptive naming task [Cervenka et al., 2013]. None of the patients had a seizure within 2 h prior to or during task performance. While awake and comfortably seated on a bed in a room with unwanted noises minimized, patients received up to 100 question‐and‐answer trials. Question stimuli ranged from 1 to 2.5 s in duration. All questions were delivered via playback of an audio recording of the author's (E.C.B.) voice using Presentation version 9.81 software (Neurobehavioral Systems, Albany, CA) and were designed to elicit 1 or 2 word answers with nouns, e.g., “What flies in the sky?”
The audible session was recorded and integrated with ECoG, as previously described [Brown et al., 2008]. Subsequently, the onset and offset of auditory stimuli as well as the onset of the patient's vocalization of the response were marked for each trial. Cool Edit Pro version 2.00 (Syntrillium Software, Phoenix, AZ) was used to visually and audibly aid in the manual determination of these time points. The response time was defined as the period between offset of stimulus presentation and onset of the respective overt response. Patients were instructed to answer “I don't know” when they did not know the answer to or did not understand a stimulus.
Evaluation of ECoG Amplitude Changes
Each ECoG trace was transformed into the time–frequency domain, and we determined “when” and “where” gamma‐activity was augmented. The time–frequency analysis used in the current study was previously validated [Brown et al., 2008; Hoechstetter et al., 2004; Nagasawa et al., 2010a; Wu et al., 2011]. In short, the “primary” measures of interest were the percent change in amplitude of gamma‐activity relative to that during the reference period (i.e., the resting baseline) as well as statistical significance of task‐related augmentation of gamma‐activity. The details of the analytic methods are described below.
Stimulus onset, stimulus offset, and response onset analyses were carried out across all suitable trials, as previously described [Brown et al., 2012]. Briefly, inclusion criteria ensure that a period of silence serving as a reference period of a minimum of 400‐ms duration prior to the onset of stimulus presentation was present for each trial. For response‐onset analysis, a maximum variation in reaction time of 1 s was imposed across included trials. Exclusion criteria removed EMG/EOG and movement artifacts as well as data associated with runs of repetitive spike‐wave discharges lasting longer than 3 s from analysis. Trials in which the stimulus was not heard were excluded from all analyses. Trials with incorrect answers were excluded from stimulus offset and response onset analyses.
Time–frequency analysis was performed using BESA® EEG V.5.1.8 software (MEGIS Software GmbH, Gräfelfing, Germany). Each suitable ECoG trial was transformed into the time–frequency domain using a previously described complex demodulation technique [Hoechstetter et al., 2004; Papp and Ktonas, 1977; Wu et al., 2011]. A given ECoG channel was assigned amplitude values as a function of frequency and time. For evaluation of gamma‐activity, time–frequency transformation was performed for frequencies between 10 and 200 Hz and latencies between −600 and +4,000 ms relative to the onset of stimulus presentation in steps of 5 Hz and 10 ms, as previously reported [Brown et al., 2008]. The time–frequency transformation was obtained by multiplication of the time‐domain signal with a complex exponential, followed by a band‐pass filter. The band‐pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. The filter had a full width at half maximum of 2 × 15.8 ms in the temporal domain and 2 × 7.1 Hz in the frequency domain. The corresponding time–frequency resolution was ±15.8 ms and ±7.1 Hz (defined as the 50% power drop of the finite impulse response filter). At each time–frequency bin, we analyzed the percent change in amplitude (averaged across trials) relative to the grand mean amplitude of the reference period for each frequency epoch. Results are referred to as “event‐related synchronization and desynchronization” [Pfurtscheller and Lopes da Silva, 1999] or “temporal spectral evolution” (TSE) [Salmelin and Hari, 1994].
To test for statistical significance in obtained TSE values, a two‐step statistical analysis was performed using BESA software [Brown et al., 2008; Nagasawa et al., 2010a; Wu et al., 2011]. Initially, a studentized bootstrap statistic [Davidson and Hinkley, 1999] was applied to obtain an uncorrected P‐value independently for each time–frequency bin. In the second step, correction for multiple testing was performed, accounting for the partial correlation between neighboring TSE values. The following modified Bonferroni correction was used [Auranen, 2002; Simes, 1986]: P‐values derived for a particular channel were sorted in ascending order (p i, i = 1, … , N, where N is the number of bins), and the maximum index, m, for which p i < α × i/N was determined. The corrected significance level, α, was set to 0.05. All TSE values corresponding to indices i < m were considered statistically significant. This is less conservative than the classical Bonferroni correction but well suited for multiple correlated items [Simes, 1986].
As described previously [Asano et al., 2009b; Brown et al., 2012; Fukuda et al., 2010; Nagasawa et al., 2010a,b; Wu et al., 2011], an additional manual correction was used. A given cortical site was declared to be a “language‐related gamma‐site” only if, after the modified Bonferroni correction [Simes, 1986], a minimum of eight time–frequency bins contained within the gamma‐site ranging from 50 to 150 Hz were arranged in a continuous array spanning (i) at least 20 Hz in width and (ii) at least 20 ms in duration. We previously reported that electrical stimulation of sites showing such gamma‐augmentation resulted in relevant sensory, motor, or language symptoms with significant accuracy [Kojima et al., 2012; Nagasawa et al., 2010a,b]. We also found that resection of language‐related gamma‐sites predicted a postoperative language impairment [Kojima et al., 2013]. All electrodes identified herein as “activated” have exhibited significant gamma‐augmentation by this method; selected examples can be seen in Figure 1. The precise anatomical location of each language‐related gamma‐site was defined as described in “Image processing and analysis” section.
Diffusion Tensor Imaging
Image acquisition
MRI scans were performed with a 3‐T Twin‐Speed GE Scanner using an eight‐channel head coil; Patient 3 was instead scanned on a 1.5‐T GE Scanner. Diffusion‐weighted images were acquired with multislice single‐shot diffusion‐weighted echo‐planar imaging (EPI) sequence at TR = 12,500 ms, TE = 88.7 ms, FOV = 24 cm, 128 × 128 acquisition matrix (nominal resolution = 1.89 mm), contiguous 3‐mm thickness in order to obtain axial slices of the whole brain using 55 isotropic, noncollinear gradient directions with b = 1,000 s/mm2, one b = 0 acquisition, and number of excitations = 1. Approximate scanning time for the acquisition was 12 min using double refocusing pulse sequence and parallel imaging capability (ASSET factor of 2) to reduce eddy current artifacts and geometric distortion artifacts derived from EPI. Because the scans were from clinical MRI studies, sedation was used as necessary by the sedation team at Children's Hospital of Michigan according to standard clinical protocols. However, younger children were scanned while sleeping, and all patients were monitored for movement during scanning.
Image processing and analysis
SPM 8 (Wellcome Trust Centre for Neuroimaging, London, UK) was used for eddy current correction of diffusion‐weighted images. Freesurfer (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA) was used to reconstruct all T1 images. ECoG electrodes were represented in our processing stream by spherical masks of 8‐mm radius that were placed on the T1 MRI surface using in‐house software following detailed manual correction of deskulling procedures. Electrode spheres were transferred into Freesurfer to generate corresponding white matter surface labels including those voxels of the white matter surface that coincide with any portion of a sphere; an example can be seen in Figure 2B. A white matter mask, generated by Freesurfer reconstruction, was grown by 1 mm and inverted using in‐house software to generate an exclusion mask for tractographic analysis in each subject.
Figure 2.
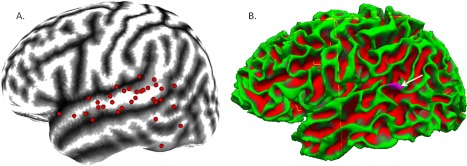
Language‐related temporal electrodes contributing seed masks. Lateral temporal seed masks were generated from electrodes exhibiting language‐related gamma‐augmentation on ECoG. A: By using an in‐house conformal cortical mapping technique [Brown et al., 2012; Muzik et al., 2007], we mapped language‐related temporal lobe electrode sites used in the generation of tractography seed regions in individual patients to the surface of an MNI‐152 brain atlas. Sites shown here to lie over the Sylvian fissure were required to have either 50% extension of the mask into the temporal lobe white matter or to exhibit an auditory type of activity [Brown et al., 2012] with any amount of mask extension into the temporal lobe white matter. It can be seen that most language‐related sites of the lateral temporal lobe were along the surface of the superior temporal gyrus; indeed, this is our temporal region of best electrode coverage across patients. There appears to be some clustering over the posterior superior temporal region. A few sites over the posterior middle and inferior temporal gyri were also found to exhibit language‐related gamma‐augmentation and their associated seed masks were included. B: A seed mask (purple region identified by white arrow) associated with one language‐related temporal lobe ECoG site is displayed on the white matter surface of Patient 1, as generated by Freesurfer reconstruction; superficial white matter in green with deeper white matter in red. Only voxels associated with the white matter surface within 8 mm of the electrode center are included in seed and target masks of electrode sites. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In this study, anatomical regions were defined with standardized, reproducible methodology. Broca's area was defined as the combination of the pars opercularis and pars triangularis [Dronkers et al., 2007] of the left inferior frontal gyrus with boundaries determined by Freesurfer's parcellation algorithm; widely used and available. Precentral and postcentral gyri were defined similarly. Wernicke's region, which lacks an anatomical definition [Bogen and Bogen, 1976], was not limited to any specific cortical location. Instead, we used the location of language‐related gamma‐sites of the lateral temporal lobe to define our posterior language sites, which is used here as an approximation of Wernicke's region. The specific anatomical location associated with each ECoG electrode was defined by the Freesurfer cortical parcellation containing at least 50% of the voxels associated with a particular electrode's white matter surface label. The only exception to this rule occurred when an ECoG electrode was resting on the Sylvian fissure, as it is possible that such an electrode may detect neural activities derived from either infra‐ or supra‐Sylvian structures. Individual white matter surface labels were not allowed to include areas on both sides of the Sylvian fissure. If such an electrode showed “auditory” activity, that which has an onset of gamma‐augmentation within 300 ms of stimulus onset and an offset of gamma‐augmentation prior to 300 ms after stimulus offset [Brown et al., 2012], only the voxels of the temporal lobe were chosen to remain within the label. Otherwise, the side containing at least 50% of the voxels associated with the label was kept.
Tractography
Probabilistic tractography was based on constant solid angle orientation distribution functions [Aganj et al., 2010] as implemented in FSL (FMRIB Centre, Oxford, UK). White matter surface labels associated with language‐related gamma‐sites of the lateral temporal lobe were used as seed regions to initiate fiber tracking into the white matter. Fibers were restricted by probabilistic correction for fractional anisotropy and allowed to make angles of up to 90°. To ensure convergence in the tracking algorithm, 5,000 fibers were generated from each seed voxel. With specific attention to the pars opercularis, pars triangularis, precentral gyrus, and postcentral gyrus, tractographic analysis was performed in three stages: (1) we determined whether anatomical white matter surface labels parcellated by Freesurfer revealed the favored anatomical termination of fibers from language‐related gamma‐sites in the temporal lobe; (2) we determined whether tractography from temporal language‐related gamma‐sites could predict language‐related gamma‐sites within these supra‐Sylvian regions; and (3) tractography was performed in the reverse direction for qualitative verification of findings.
Statistical Analysis
All statistical tests were conducted using IBM SPSS Statistics version 20 software (SPSS, Chicago, IL). The Generalized Estimating Equations framework was used to account for repeated measures within individuals. The first tractographic analysis compared the degree of white matter connectivity with language‐related gamma‐sites of the lateral temporal lobe between the supra‐Sylvian target regions delineated by Freesurfer parcellation: pars opercularis, pars triangularis, precentral gyrus, and postcentral gyrus. Here, after accounting for repeated measures within patients, the aim was to determine whether supra‐Sylvian target region could predict tractography fiber counts from seeds in the language‐related gamma‐sites of the lateral temporal lobe; this serves to compare the level of connectivity between the language‐related temporal lobe sites and each supra‐Sylvian anatomical target. A negative binomial distribution link was used to test this while assuming independent correlations between the regions. Parameter estimates for each region were then compared with that of the postcentral gyrus (negative control) to determine pairwise differences.
In the second analysis, we determined whether or not the occurrence of a fiber termination associated with a supra‐Sylvian target electrode could predict the occurrence of significant language‐related gamma‐augmentation during the language task at that electrode, even after accounting for anatomical region and within‐patient repeated measures. Additionally, we assessed for an interaction between anatomical region and diffusion‐weighted tractography fiber termination. With ECoG and tractography variables expressed in binary form, a binomial logistic model was used with an exchangeable correlation matrix.
RESULTS
Supra‐Sylvian Anatomical Targets
All five subjects were included in the analysis of anatomical targets for language‐related temporal seeds. The pars triangularis of the inferior frontal gyrus did not receive diffusion‐weighted tractography fibers in any subject. Therefore, the pars triangularis and pars opercularis were combined into one region, referred to here as Broca's area, to facilitate statistical comparison. Across 33 temporal lobe seeds showing language‐related gamma‐augmentation in these five patients (Fig. 2), supra‐Sylvian anatomical target region was found to be a significant predictor of tractography fiber counts (P‐value < 0.001). Comparisons of the parameter estimates revealed that the precentral gyrus received a larger tractography fiber count than the postcentral gyrus (P‐value < 0.001); fiber counts to Broca's area were not significantly different from those to the postcentral gyrus (P‐value = 0.424). Figure 3 depicts the dominance of the precentral gyrus termination in each subject. All fibers terminating in any region followed a counterclockwise path involving portions of the temporal, parietal, and frontal lobes; reminiscent of the traditional arcuate fasciculus.
Figure 3.
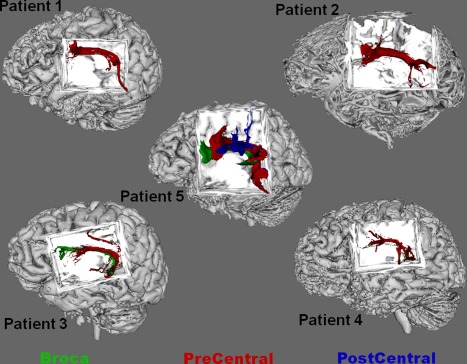
Composite fiber tracts across all patients. The connectivity distribution of all fibers from each temporal seed is shown in composite for each patient. The distribution for fibers terminating within the precentral gyrus is shown in red, that within Broca's area shown in green, and that within the postcentral gyrus is shown in blue. In each patient, the dominant fiber pathway resembles that of the traditional arcuate fasciculus, and its primary termination in all subjects is within the precentral gyrus. Some fibers are seen to go to Broca's area in a few patients. In each of these cases showing a termination within Broca's area, all such fibers are terminating within the pars opercularis of the inferior frontal gyrus; no fibers were found to terminate within pars triangularis in any patient. Across patients and seeds, fiber counts to Broca's area were not significantly larger than those to the postcentral gyrus. Fiber counts to the precentral gyrus were larger than fiber counts to the postcentral gyrus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Region Specific Association With Gamma‐Activity on ECoG
Of the five subjects, only four of them received ECoG electrode coverage that included the precentral gyrus or Broca's area. In these four subjects, we evaluated whether the occurrence of a diffusion‐weighted tractography fiber termination predicted the occurrence of significant language‐related gamma‐augmentation on ECoG. We found that even after accounting for both patient and anatomy, the presence of a fiber termination was a significant predictor of language‐related gamma‐sites (P‐value = 0.027). Furthermore, an interaction between tractography fiber termination and anatomy was found (P‐value < 0.001), suggesting that fiber occurrence is a better predictor of language‐related gamma‐sites within at least one of the three tested anatomical regions when compared with the others. Figure 4 depicts, by anatomical region, the percentage of active and nonactive supra‐Sylvian ECoG targets receiving at least one tractography fiber from language‐related ECoG sites of the temporal lobe. It can be observed that the greatest predictive value of diffusion‐weighted tractography for language‐related gamma‐activity was within the precentral gyrus (positive predictive value [PPV] = 0.89; negative predictive value [NPV] = 0.76), the lowest predictive value in the postcentral gyrus (PPV = 0.45; NPV = 0.57), and an intermediate predictive value in Broca's area (PPV = 0.78; NPV = 0.52). This suggests that diffusion‐weighted tractography seeded from language‐related gamma‐sites in the left temporal region made a good overall prediction of significant language‐related gamma‐augmentation only within the precentral gyrus.
Figure 4.
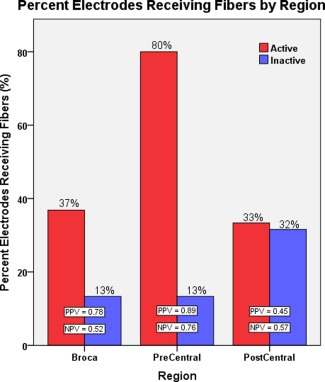
Percent of electrodes receiving tractography fibers by region. The percentage of active or inactive anterior electrodes receiving at least one tractography fiber from language‐related gamma‐sites of the temporal lobe is displayed by region. The overall predictive value scores suggest that diffusion‐weighted tractography from posterior language‐related sites shows the greatest discrimination for anterior language‐related sites within the precentral gyrus. Within Broca's area, only the positive occurrence of a nearby fiber termination exhibited an appreciable predictive value for whether or not a particular ECoG site would show language‐related gamma‐augmentation; the lack of a nearby fiber termination provided no predictive value. As expected, fiber terminations within the postcentral gyrus provided no predictive value for associated language‐related ECoG sites. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Tractography Seeded From Anterior Regions
As a purely qualitative validation step, we performed tractographic analysis in the reverse direction using seeds in the anatomical regions of the precentral gyrus and within Broca's area. Within each patient, we found that more fibers from the precentral gyrus when compared with those from Broca's area arrived at the targets here defined as language‐related gamma‐sites of the lateral temporal lobe. Indeed, no fibers seeded from pars triangularis of the inferior frontal gyrus reached these temporal targets. Figure 5A,B depicts fibers seeded from Broca's area and precentral gyrus to language‐related sites of the lateral temporal lobe, respectively, in Patient 5. Strong U‐fiber tracks could be isolated between the precentral gyrus and both pars opercularis and pars triangularis within Broca's area. Although no fibers seeded from pars triangularis reached any of the language‐related temporal lobe sites, fibers were found to reach the inferior portion of the precentral gyrus; this is depicted in Figure 5C. Overall, diffusion‐weighted tractography in the reverse direction, from frontal to temporal, supported our other findings.
Figure 5.
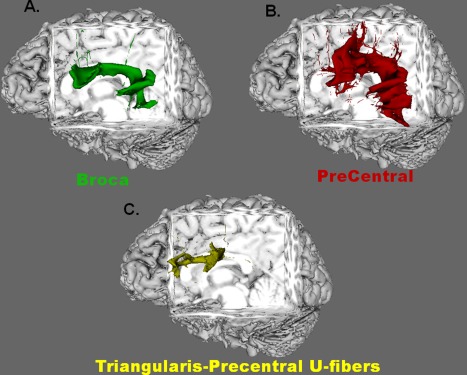
Reversed tracking. Selected results from tractography seeded from the anterior anatomical sites in Patient 5 are shown. A and B: Connectivity maps of fibers seeded from Broca's area and precentral gyrus, respectively, to language‐related gamma‐sites of the lateral temporal lobe. All fibers from Broca's area that reach the posterior language sites originated specifically from pars opercularis of the inferior frontal gyrus; no fibers from pars triangularis reached the temporal language sites. The overall tract seeded from the precentral gyrus is qualitatively larger and more robust than that from Broca's area. Although the entire precentral gyrus was used as the seed region, only those fibers originating from the inferiolateral portion tracked through the arcuate fasciculus to the temporal language sites. Fibers shown in (C) depict U‐fibers that were seeded from pars triangularis and reached the inferior portion of the precentral gyrus. This finding occurred even though no fibers seeded from pars triangularis reached the temporal language sites. Similar U‐fibers were observed between pars opercularis and the precentral gyrus, regardless of which region was used as the seed. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Primary Findings
We failed to find evidence in our analyses that posterior language‐related cortices of the left temporal lobe possess direct white matter connections to Broca's area in the inferior frontal gyrus that are more developed than those to the precentral gyrus. This by no means indicates that Broca's area is completely devoid of arcuate fasciculus terminations. However, the fibers generated from posterior language areas failed to exhibit any significant preference for Broca's area above that for the postcentral gyrus, a region used here as a negative control.
Our approach showed that diffusion‐weighted tractography connectivity patterns may be related to the results of language‐related ECoG analysis. It is shown here that tractographic connectivity with posterior language‐related sites was best at predicting language‐related gamma‐sites of the precentral gyrus. This finding suggests that tractography fibers from posterior language‐related sites had some appreciable ability to discriminate between specific sites within the precentral gyrus that may or may not possess language‐related function. Indeed, this ability for language‐related functional discrimination was greater within the precentral gyrus than within Broca's area in our study.
These results add to the growing literature suggesting that the arcuate fasciculus, arising from the left lateral temporal region showing language‐related gamma‐augmentation, has an anterior termination that is best approximated as the inferior portion of the precentral gyrus. In our small cohort, a well‐developed pathway could be traced from posterior language‐related areas to the precentral gyrus in each subject. Within Broca's area, fibers could only be occasionally traced to the pars opercularis of the inferior frontal gyrus. In none of the patients could fibers be traced to the pars triangularis; a finding repeated elsewhere with various diffusion‐weighted tractography and anatomical dissection methods [Bernal and Altman, 2010; Brauer et al., 2011; Diehl et al., 2010; Martino et al., 2013; Perani et al., 2011].
A New Model for Language Connectivity
The Wernicke–Lichtheim model, proposed over one century ago, was powerful in that it allowed for the prediction of language‐related functional deficits from lesion location in the brain [Graves, 1997; Lichtheim, 1885]. This model was the first to propose a direct anatomical communication between posterior language sites of the temporal lobe, Wernicke's region, and anterior language sites within Broca's area [Graves, 1997]. Indeed, a particular set of symptoms was attributed to lesions of this tract, later identified as the arcuate fasciculus [Geschwind, 1970], in which the ability of speech repetition is compromised [Bernal and Ardila, 2009; Lichtheim, 1885]; this is known today as conduction aphasia. In essence, the model describes that Wernicke's region provides the processes required for comprehension of auditory language and Broca's area, receiving this essential information via the arcuate fasciculus, provides the cortical machinery to code language into its articulatory forms [Geschwind, 1970]. Such a model not only predicts conduction aphasia but also predicts the long‐term occurrence of disturbances in speech output that can occur with Wernicke's aphasia [Geschwind, 1970], described as a lesion to Wernicke's region primarily affecting the processes of comprehension [Lichtheim, 1885]. The idea that the primary purpose of the arcuate fasciculus is to provide a direct link between Wernicke's region and Broca's area in order to mediate a language‐specific function has dominated both scientific and clinical thinking as a persistent dogma about the function of language even to the present time [Brauer et al., 2011; Henning Stieglitz et al., 2012; Kwon and Jang, 2011; Mohades et al., 2012].
Our study showed a primary anterior termination of the arcuate fasciculus, specifically the portions arising from lateral temporal sites showing language‐related gamma‐augmentation, within the inferior precentral gyrus and failed to support the conventional model of language connectivity within the brain [Geschwind, 1970]. The inferior portion of the precentral gyrus possesses both premotor (BA 6) and primary motor (BA 4) cortex [Strotzer, 2009]; primary motor cortex residing largely along the central sulcus with the premotor cortex lying more laterally on the gyrus. Others have yielded similar findings regarding the anterior termination of the arcuate fasciculus with diffusion MRI methods [Anwander et al., 2007; Catani et al., 2005; Perani et al., 2011], structural MRI of lesion locations [Bizzi et al., 2012], and cadaver dissection [Martino et al., 2013]. New models of language are beginning to incorporate the premotor cortex of the inferior portion of the lateral precentral gyrus [Amunts and Zilles, 2012; Friederici, 2012]. One well‐developed model incorporating recent findings from imaging and lesion studies regarding language‐related structural connectivity, presented by Bernal and Ardila [2009], hypothesizes that the arcuate fasciculus mediates a direct communication between Wernicke's region and premotor (BA 6) cortex of the inferior precentral gyrus. Regarding both Broca's area and primary motor cortex for the face and mouth, this portion of premotor cortex is hypothesized to serve as a bidirectional relay station coordinating the language‐related processes among these frontal and temporal language‐related regions [Bernal and Ardila, 2009]. Further studies are warranted to confirm this bidirectional connectivity between the two regions using techniques such as measurement of cortico‐cortical evoked potentials [Matsumoto et al., 2004]; indeed, we plan to validate the arcuate pathway on DTI tractography using such evoked potential data as a follow‐up to this study.
It is hypothesized that the arcuate fasciculus may serve as a supportive, language‐related role that is more crucial during language development in childhood but nonessential for language maintenance in adulthood [Bernal and Altman, 2010; Bernal and Ardila, 2009]. Indeed, classical conduction aphasia is so rare that some have been prompted to suggest that it is not associated with the arcuate fasciculus at all [Anderson et al., 1999]; the literature presents a case of complete recovery from aphasia associated with complete and permanent disruption of the arcuate fasciculus of the dominant hemisphere only 30 months after injury and with only 3 months of therapy in a middle‐aged adult [Kwon and Jang, 2011]. Diffusion MRI of left hemisphere stroke patients (22 months poststroke, on average) showed that reduced fractional anisotropy of the arcuate fasciculus correlates with decreased repetition ability [Breier et al., 2008]. However, in such a study, it is nearly impossible to exclude cortical involvement; thus, it is difficult to prove with such findings that the arcuate fasciculus is itself essential for repetition. This was acknowledged in a separate MRI study involving patients with Broca‐like or Wernicke‐like conduction aphasia, where the location of reduced anisotropy, anterior or posterior, was related to the type of conduction aphasia that can present [Song et al., 2011]. Recovery with time, a common characteristic of conduction aphasia, suggests that the brain is able to compensate for loss of the arcuate fasciculus, either within or between the hemispheres [Berthier et al., 2011]. It is more likely that permanent conduction aphasia is due to a cortical dysfunction than to any lesion in the white matter [Hickok, 2012].
Clinical Significance
Although not the goal of this study, it is difficult to ignore the potential clinical application of this simple methodology. Our results suggest that diffusion‐weighted tractography from posterior language‐related electrode sites may be capable of predicting language‐related sites of the precentral gyrus. This is a promising finding in which a noninvasive modality might have some, albeit limited, value in predicting invasive results. Within Broca's area, only positive prediction of language‐related gamma‐sites was adequate. It is well known that ECoG coverage is often very limited. A method such as this could be useful when ECoG samples the posterior superior temporal region but not the inferior portions of the precentral gyrus, as was the case with Patient 2 in this study. Surface tracking from language‐related sites of the lateral temporal lobe may serve to “fill in the gap” to some extent for presurgical language mapping in such cases. Further validation of this finding is necessary. Applicability to language‐related sites of the right hemisphere should also be investigated.
Methodological Issues
When evaluating cortical terminations of white matter tracts, diffusion‐weighted tractography has several limitations. Indeed, the spatial resolution of images used for diffusion‐weighted tractography is limited. Furthermore, we were limited to the use of clinical scans. For example, in this study, the spatial resolution was on the millimeter scale, which is no better than a gross anatomical dissection study [Martino et al., 2013]; the value of diffusion‐weighted tractography lies in its ability to enable in vivo study. Using 55‐direction scans, we were able to generate a probability distribution function that considered information about diffusion in many directions. We used a nonparametric probabilistic tractography method that should have improved our ability to compensate for situations in which fibers cross [Aganj et al., 2010] or make sharp turns. Further compensation of crossing fibers from the corticospinal tract was provided by inclusion of the postcentral gyrus as a control, or reference, target region. The postcentral gyrus also provided a reference for the effect of proximity to the tractography seeds, as shorter distance may increase the likelihood of apparent tractographic connectivity. Despite that, our tractography fibers will have the general tendency to follow the path that is dominated by the greatest number of axons oriented in a common direction. Therefore, our findings suggest only that it is more likely [Jones, 2008] that more of the axons associated with the arcuate fasciculus are terminating within the precentral gyrus. It may be that there exist other axons associated with the arcuate fasciculus that extend to Broca's area or to other nearby areas of the frontal lobe, such as BA 8 [Frey et al., 2008], that are simply hidden due to partial volume effects associated with relatively low spatial resolution. Indeed, it has been shown that the actual tractography method used can change the depicted shape of cerebral tracts, including the arcuate fasciculus [Dell'Acqua and Catani, 2012]. It is impressive that we were able to find a good predictive value for ECoG results in the precentral gyrus even in the setting of such limited diffusion‐weighted tractography information.
We applied several strict parameters to our tractography analysis including an exclusion mask preventing the traversal of gray matter, probabilistic anisotropy correction, and the restriction of seed and target regions of the gray–white interface to 8 mm from the cortical surface associated with a specific anatomical region or ECoG electrode. Beyond these restrictions, however, we allowed for any path between the seeds and targets, that is, no assumptions about or “dissections” of the dominant fiber path were applied. This approach allowed fibers to be influenced greatly by individual variability between patients. Despite that, nearly all fibers in each patient appeared to follow a path resembling that of the arcuate fasciculus [Catani and Thiebaut de Schotten, 2008]. It may be that our strict tractography parameters have prevented the visualization of other potential tracts that may provide a direct connection between posterior and anterior language sites, for example, the extreme capsule fiber system [Frey et al., 2008]. As our seed and target regions did not include the inferior parietal lobe, only the direct, long segment of the arcuate fasciculus could be evaluated [Catani et al., 2005, 2007]. We did not have consistent coverage of the inferior parietal lobe, sometimes referred to as Geschwind's territory [Catani et al., 2007], in our patients, and thus, we left ECoG‐constrained analysis of the indirect segments to future work. Indeed, we were primarily interested in the direct segment of the arcuate fasciculus at the onset of this study and, in particular, to determine where the dominant physical termination may be. Within anterior regions, other tracts may exist that interact with portions of the arcuate fasciculus. The corticospinal tract is well known to cross the arcuate fasciculus along the central sulcus. The frontal aslant tract, which may possess some function in language [Catani et al., 2012], appears to connect pars opercularis to supplementary motor and presupplementary motor areas, thus potentially interacting physically with the more anterior portions of the arcuate fasciculus. We built in a simple method to account for problems that may arise from such crossing fibers as described above. Further evaluation of these pathways with ECoG‐constrained tractography is warranted for future studies.
We did not use electrical brain stimulation to define posterior language sites serving as seed regions. This is because stimulation is insensitive to language mapping in children [Kojima et al., 2012; Schevon et al., 2007]. Indeed, the task‐relevant superior temporal sites used here as seed sites are often not detected by stimulation [Brown et al., 2008; Kojima et al., 2012, 2013]. Our recent study of 77 patients who underwent epilepsy surgery showed that the mapping of language‐related gamma‐augmentation on ECoG, using the naming task mentioned herein, was capable of predicting postsurgical language impairment better than electrical stimulation [Kojima et al., 2013]. Although it is frequently treated as a clinical gold standard in brain mapping, electrical brain stimulation suffers from many of its own methodological deficits [Su and Ojemann, 2013]. Nonetheless, we can report that clinically oriented stimulation was able to confirm left‐hemispheric essential language sites in all five patients under study. Taken together, studies by our group and studies by Kojima et al. [2012, 2013] and Cervenka et al. [2013] combine as converging evidence to create the grounds for us to claim that language mapping by ECoG using an auditory descriptive naming task is a highly promising methodology for both clinical and scientific application.
All findings present herein were generated from a data set based on patients with focal epilepsy and may be influenced by such a disease process. However, there is no objective evidence that epileptiform discharges, structural lesions, antiepileptic drugs, or ECoG sampling limitations have a differential affect on termination patterns of the arcuate fasciculus. All patients had the seizure onset zone and interictal epileptiform discharges recorded away from the inferior frontal region. It has been reported that antiepileptic drugs reduced the duration of interictal epileptiform high‐frequency oscillations at >80 Hz in only 3% of patients [Zijlmans et al., 2009]; we successfully found language‐related gamma‐sites of the temporal lobe highly concordant with language‐related temporal lobe sites on functional neuroimaging [Brown et al., 2012]. The left superior temporal gyrus, especially in the posterior portion traditionally considered a part of Wernicke's region [Bogen and Bogen, 1976], was well sampled by ECoG electrodes.
As our study included a small cohort of children aged from 6 to 21 years, we cannot rule out an effect of patient age on our findings. A volumetric MRI study reported that the white matter volume in the frontal lobes continues to increase until 12 years [Giedd et al., 1999]. Myelination of language‐related pathways, the arcuate fasciculus in particular, is known to be relatively protracted. Indeed, at 5 years, myelination of the arcuate fasciculus remains detectably incomplete when compared with that of adults [Su et al., 2008]; although having progressed through more than 80% of change from newborn to adult values, based on structural MRI measures. Diffusion MRI imaging is not completely dependent on myelination [Berman et al., 2005], as anisotropic diffusion can be observed in 3‐day‐old newborns in whom major portions of the arcuate fasciculus can be studied despite a general lack of myelination [Perani et al., 2011]. When compared with adults (age: 24.4–32.4 years), tractography has yielded evidence that the portion of the direct segment of the arcuate fasciculus connecting Wernicke's region to pars opercularis (within Broca's area) may be underdeveloped in children (age: 7 years) [Brauer et al., 2011]; this conclusion was based on reduced fractional anisotropy and the apparent utilization of alternative pathways in children. In the current study, the dominant termination of the arcuate fasciculus residing within the inferior precentral gyrus was seen not only in a 6‐year‐old child but also in the remaining four patients older than 12 years. In three of our five patients (including the youngest one aged 6 years), we could demonstrate a component of the arcuate fasciculus connecting lateral temporal language‐related sites to pars opercularis of the inferior frontal gyrus in addition to precentral gyrus.
CONCLUSION
The purpose of this study was to constrain diffusion‐weighted tractography with language‐related functional information from ECoG measures to evaluate the anterior termination of language‐related white matter connectivity. Even though no assumptions about “correct” fiber pathways were applied, a tract resembling the traditional arcuate fasciculus vastly dominated the results. It was shown that the dominant anterior termination of this tract was within the inferior portion of the precentral gyrus and could best predict the result of language‐related gamma‐measures within that region. Our findings contribute to mounting evidence that the traditional view of language organization within the brain requires some modification. This, along with other scientific and clinical data regarding the arcuate fasciculus, suggests that the arcuate fasciculus might be best thought of as a language‐related structure providing support for the development of effective auditory communication. New models of language structure and function may benefit from incorporating the robust termination of the arcuate fasciculus within the inferior precentral gyrus.
ACKNOWLEDGMENTS
The authors are grateful to Katsuaki Kojima, M.D., Harry T. Chugani, M.D., Carol Pawlak, R.EEG./EP.T., Sarah Minarik, R.N., B.S.N., Alanna Marie Carlson, M.A., Elizabeth Bohme, M.A., and the staff of the Division of Electroneurodiagnostics at the Children's Hospital of Michigan, Wayne State University's School of Medicine, for the collaboration and assistance in performing the studies. Cynthia L. Arfken, Ph.D., Associate Professor of Psychiatry and Behavioral Neurosciences, Wayne State University, graciously provided statistical consultation. The authors thank two anonymous reviewers of Human Brain Mapping for their helpful suggestions and comments. The first author thanks the Translational Neuroscience Program and the MD/PhD Program of Wayne State University's School of Medicine for providing an environment in which to perform translational research and grow as an investigator.
REFERENCES
- Aganj I, Lenglet C, Sapiro G, Yacoub E, Ugurbil K, Harel N (2010): Reconstruction of the orientation distribution function in single‐ and multiple‐shell q‐ball imaging within constant solid angle. Magn Reson Med 64:554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, Chugani HT (2009): Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res 87:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Zilles K (2012): Architecture and organizational principles of Broca's region. Trends Cogn Sci 16:418–426. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K (2003): Broca's region: Cytoarchitectonic asymmetry and developmental changes. J Comp Neurol 465:72–89. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, Beversdorf DQ, Cibula J, Rogish M III, Kortencamp S, Hughes JD, Gonzalez Rothi LJ, Heilman KM (1999): Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke‐Geschwind model. Brain Lang 70:1–12. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR (2007): Connectivity‐based parcellation of Broca's area. Cereb Cortex 17:816–825. [DOI] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT (2005): Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia 46:1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhasz C, Shah A, Sood S, Chugani HT (2009a): Role of subdural electrocorticography in prediction of long‐term seizure outcome in epilepsy surgery. Brain 132:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S (2009b): Differential visually‐induced gamma‐oscillations in human cerebral cortex. Neuroimage 45:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auranen T (2002): Nonparametric statistical analysis of time–frequency representations of magnetoencephalographic data Master's MS thesis. Espoo, Finland: Helsinki University of Technology. [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG (2005): Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage 27:862–871. [DOI] [PubMed] [Google Scholar]
- Bernal B, Altman N (2010): The connectivity of the superior longitudinal fasciculus: A tractography DTI study. Magn Reson Imaging 28:217–225. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A (2009): The role of the arcuate fasciculus in conduction aphasia. Brain 132:2309–2316. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Garcia‐Casares N, Walsh SF, Nabrozidis A, Ruiz de Mier RJ, Green C, Davila G, Gutierrez A, Pulvermuller F (2011): Recovery from post‐stroke aphasia: Lessons from brain imaging and implications for rehabilitation and biological treatments. Discov Med 12:275–289. [PubMed] [Google Scholar]
- Bizzi A, Nava S, Ferre F, Castelli G, Aquino D, Ciaraffa F, Broggi G, DiMeco F, Piacentini S (2012): Aphasia induced by gliomas growing in the ventrolateral frontal region: Assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex 48:255–272. [DOI] [PubMed] [Google Scholar]
- Bogen JE, Bogen GM (1976): Wernicke's region—Where is it? Ann N Y Acad Sci 280:834–843. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD (2011): Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex 21:459–466. [DOI] [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC (2008): Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol 29:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E (2008): In vivo animation of auditory‐language‐induced gamma‐oscillations in children with intractable focal epilepsy. Neuroimage 41:1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Matsuzaki N, Juhász C, Shah AK, Atkinson MD, Fuerst D, Mittal S, Sood S, Diwadkar VA, Asano E (2012): Evaluating reverse speech as a control task with language‐related gamma activity on electrocorticography. Neuroimage 60:2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell'Acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M (2012): Short frontal lobe connections of the human brain. Cortex 48:273–291. [DOI] [PubMed] [Google Scholar]
- Cervenka MC, Corines J, Boatman‐Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, Crone NE (2013): Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage 69:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ (2011): Cortical gamma responses: Searching high and low. Int J Psychophysiol 79:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS (2008): Localization of neurosurgically implanted electrodes via photograph‐MRI‐radiograph coregistration. J Neurosci Methods 174:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AC, Hinkley DV (1999): Bootstrap Methods and Their Application Studentized Bootstrap Method. Cambridge: Cambridge University Press; pp 161–175. [Google Scholar]
- Dell'Acqua F, Catani M (2012): Structural human brain networks: Hot topics in diffusion tractography. Curr Opin Neurol 25:375–383. [DOI] [PubMed] [Google Scholar]
- Diehl B, Piao Z, Tkach J, Busch RM, LaPresto E, Najm I, Bingaman B, Duncan J, Luders H (2010): Cortical stimulation for language mapping in focal epilepsy: Correlations with tractography of the arcuate fasciculus. Epilepsia 51:639–646. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba‐Zizen MT, Cabanis EA (2007): Paul Broca's historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain 130:1432–1441. [DOI] [PubMed] [Google Scholar]
- Ellmore TM, Beauchamp MS, O'Neill TJ, Dreyer S, Tandon N (2009): Relationships between essential cortical language sites and subcortical pathways. J Neurosurg 111:755–766. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M (2008): Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28:11435–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2012): The cortical language circuit: From auditory perception to sentence comprehension. Trends Cogn Sci 16:262–268. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhasz C, Nishida M, Sood S, Asano E (2010): Cortical gamma‐oscillations modulated by listening and overt repetition of phonemes. Neuroimage 49:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N (1970): The organization of language and the brain. Science 170:940–944. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Graves RE (1997): The legacy of the Wernicke–Lichtheim model. J Hist Neurosci 6:3–20. [DOI] [PubMed] [Google Scholar]
- Henning Stieglitz L, Seidel K, Wiest R, Beck J, Raabe A (2012): Localization of primary language areas by arcuate fascicle fiber tracking. Neurosurgery 70:56–65. [DOI] [PubMed] [Google Scholar]
- Hickok G (2012): The cortical organization of speech processing: Feedback control and predictive coding the context of a dual‐stream model. J Commun Disord 45:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M (2004): BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topogr 16:233–238. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Ossandón T, Hamamé CM, Senova S, Dalal SS, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, Lachaux JP (2009): Task‐related gamma‐band dynamics from an intracerebral perspective: Review and implications for surface EEG and MEG. Hum Brain Mapp 30:1758–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2008): Studying connections in the living human brain with diffusion MRI. Cortex 44:936–952. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H (2000): Handedness and hemispheric language dominance in healthy humans. Brain 123:2512–2518. [DOI] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, Atkinson M, Mittal S, Fuerst D, Sood S, Asano E (2012): Multimodality language mapping in patients with left‐hemispheric language dominance on Wada test. Clin Neurophysiol 123:1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki N, Shah A, Atkinson M, Basha M, Mittal S, Sood S, Asano E (2013): Clinical significance and developmental changes of auditory‐language‐related gamma activity. Clin Neurophysiol 124:857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HG, Jang SH (2011): Excellent recovery of aphasia in a patient with complete injury of the arcuate fasciculus in the dominant hemisphere. Neurorehabilitation 29:401–404. [DOI] [PubMed] [Google Scholar]
- Lichtheim L (1885): On aphasia. Brain 7:433–484. [Google Scholar]
- Logothetis NK (2003): The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23:3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H (2013): Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: A fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO (2004): Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain 127:2316–2330. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Makeig S, Hebb AO, Rao RP, denNijs M, Ojemann JG (2007): Cortical electrode localization from X‐rays and simple mapping for electrocorticographic research: The “Location on Cortex” (LOC) package for MATLAB. J Neurosci Methods 162:303–308. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Shenoy P, den Nijs M, Sorensen LB, Rao RN, Ojemann JG (2008): Beyond the gamma band: The role of high‐frequency features in movement classification. IEEE Trans Biomed Eng 55:1634–1637. [DOI] [PubMed] [Google Scholar]
- Moddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T (2009): Atypical language lateralization in epilepsy patients. Epilepsia 50:1505–1516. [DOI] [PubMed] [Google Scholar]
- Mohades SG, Struys E, Van Schuerbeek P, Mondt K, Van De Craen P, Luypaert R (2012): DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Res 1435:72–80. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT (2007): Multimodality data integration in epilepsy. Int J Biomed Imaging 2007:13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhasz C, Fukuda M, Nishida M, Akiyama T, Sood S, Asano E (2010a): Cortical gamma‐oscillations modulated by auditory‐motor tasks‐intracranial recording in patients with epilepsy. Hum Brain Mapp 31:1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhasz C, Nishida M, Sood S, Asano E (2010b): Cortical gamma‐oscillations modulated by visuomotor tasks: Intracranial recording in patients with epilepsy. Epilepsy Behav 18:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P (1977): Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13:135–145. [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD (2011): Neural language networks at birth. Proc Natl Acad Sci USA 108:16056–16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH (1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Emmorey K, Hickok G, Pylkkanen L (2012): Towards a new neurobiology of language. J Neurosci 32:14125–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hari R (1994): Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60:537–550. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Carlson C, Zaroff CM, Weiner HJ, Doyle WK, Miles D, Lajoie J, Kuzniecky R, Pacia S, Vazquez B, Luciano D, Najjar S, Devinsky O (2007): Pediatric language mapping: Sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia 48:539–545. [DOI] [PubMed] [Google Scholar]
- Simes RJ (1986): An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754. [Google Scholar]
- Song X, Dornbos D III, Lai Z, Zhang Y, Li T, Chen H, Yang Z (2011): Diffusion tensor imaging and diffusion tensor imaging‐fibre tractograph depict the mechanisms of Broca‐like and Wernicke‐like conduction aphasia. Neurol Res 33:529–535. [DOI] [PubMed] [Google Scholar]
- Strotzer M (2009): One century of brain mapping using Brodmann areas. Klin Neuroradiol 19:179–186. [DOI] [PubMed] [Google Scholar]
- Su DK, Ojemann JG. Electrocorticographic sensorimotor mapping. Clin Neurophysiol 2013;124:1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Kuan CC, Kaga K, Sano M, Mima K (2008): Myelination progression in language‐correlated regions in brain of normal children determined by quantitative MRI assessment. Int J Pediatr Otorhinolaryngol 72:1751–1763. [DOI] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U (1997): A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage 5:S514. [Google Scholar]
- Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, Shah A, Mittal S, Fuerst D, Sood S, Asano E (2011): Gamma‐oscillations modulated by picture naming and word reading: Intracranial recording in epileptic patients. Clin Neurophysiol 122:1929–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J (2009): High‐frequency oscillations mirror disease activity in patients with epilepsy. Neurology 72:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]


