Abstract
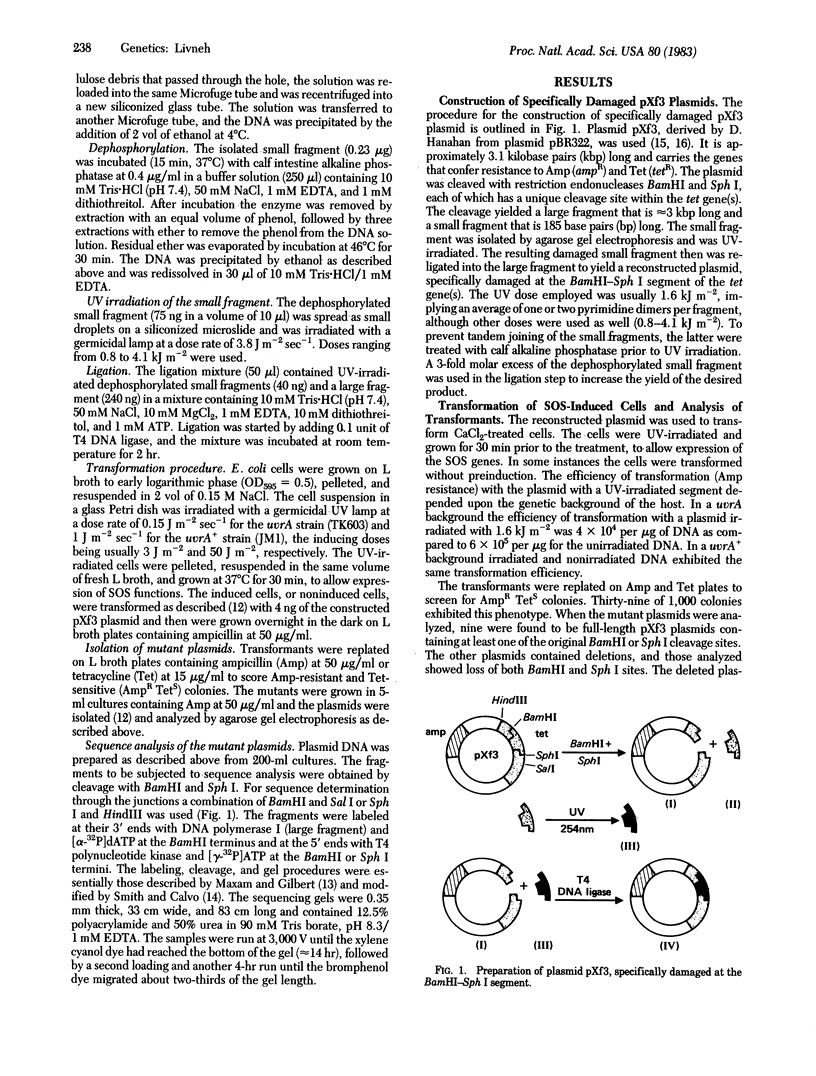
A directed mutagenesis method has been developed for the analysis of mutagen specificity. The method is based on the construction of a plasmid damaged by the mutagen at a specific segment within a given marker gene, followed by screening for mutant plasmids and nucleotide sequence analysis of the damaged segment. By using this method plasmid pXf3 has been specifically damaged by UV radiation at the BamHI-Sph I segment in the tetracycline resistance (tet) gene(s) and used to transform SOS-induced Escherichia coli. Fourteen ampicillin-resistant, tetracycline-sensitive mutants of pXf3 were isolated and subjected to sequence analysis. The data revealed the induction of transitions, a transversion, a frameshift mutation, and deletions. The single base changes all were located within runs of pyrimidines, and the deletions mapped between direct repeats of polypyrimidine tracts. In addition, mutant plasmids were found with no mutation within the damaged segment. In these cases it is likely that "untargeted mutagenesis" in other portions of the tet gene(s) is responsible for the mutant phenotype. The method can be applied to any mutagen that reacts with DNA, and it also can be used for genetic analysis as a means to mutate specific segments of DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brandenburger A., Godson G. N., Radman M., Glickman B. W., van Sluis C. A., Doubleday O. P. Radiation-induced base substitution mutagenesis in single-stranded DNA phage M13. Nature. 1981 Nov 12;294(5837):180–182. doi: 10.1038/294180a0. [DOI] [PubMed] [Google Scholar]
- Brunk C. F. Distribution of dimers in ultraviolet-irradiated DNA. Nat New Biol. 1973 Jan 17;241(107):74–76. doi: 10.1038/newbio241074a0. [DOI] [PubMed] [Google Scholar]
- Chu C. T., Parris D. S., Dixon R. A., Farber F. E., Schaffer P. A. Hydroxylamine mutagenesis of HSV DNA and DNA fragments: introduction of mutations into selected regions of the viral genome. Virology. 1979 Oct 15;98(1):168–181. doi: 10.1016/0042-6822(79)90535-x. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Devoret R. Inducible error-prone repair and induction of prophage lambda in Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1981;26:251–263. doi: 10.1016/s0079-6603(08)60410-9. [DOI] [PubMed] [Google Scholar]
- Echols H. SOS functions, cancer and inducible evolution. Cell. 1981 Jul;25(1):1–2. doi: 10.1016/0092-8674(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Gordon L. K., Lindan C. P., Grafstrom R. H., Shaper N. L., Grossman L. Cleavage of pyrimidine dimers in specific DNA sequences by a pyrimidine dimer DNA-glycosylase of M. luteus. Nature. 1980 Jun 26;285(5767):634–641. doi: 10.1038/285634a0. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Lackey D., Krauss S. W., Linn S. Isolation of an altered form of DNA polymerase I from Escherichia coli cells induced for recA/lexA functions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):330–334. doi: 10.1073/pnas.79.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Absence of relationship between UV-induced reversion frequency and nucleotide sequence at the CYC1 locus of yeast. Mol Gen Genet. 1979;177(1):31–38. doi: 10.1007/BF00267250. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- Overbye K. M., Margolin P. Role of the supX gene in ultraviolet light-induced mutagenesis in Salmonella typhimurium. J Bacteriol. 1981 Apr;146(1):170–178. doi: 10.1128/jb.146.1.170-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Sherman F. Mutagenic specificity: reversion of iso-1-cytochrome c mutants of yeast. J Mol Biol. 1973 Sep 5;79(1):65–82. doi: 10.1016/0022-2836(73)90270-2. [DOI] [PubMed] [Google Scholar]
- Schmid S. E., Daune M. P., Fuchs R. P. Repair and mutagenesis of plasmid DNA modified by ultraviolet irradiation or N-acetoxy-N-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4133–4137. doi: 10.1073/pnas.79.13.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafranovskaya N. N., Trifonov E. N., Lazurkin Y. S., Frank-Kamenetskii M. D. Clustering of thymine dimers in ultraviolet irradiated DNA and the long-range transfer of electronic excitation along the molecule. Nat New Biol. 1973 Jan 10;241(106):58–60. doi: 10.1038/newbio241058a0. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




