Abstract
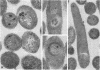
We have presented direct evidence that at least one of the traits associated with killing of paramecia by kappa particles is determined by an extrachromosomal genetic element. Plasmid DNA was isolated from Caedibacter taeniospiralis 47 (commonly known as 47 kappa), which is an obligate cytoplasmic endosymbiont of Paramecium tetraurelia. Fragments of pKAP47 DNA generated by Pst I digestion were inserted into pBR328 and then introduced into Escherichia coli 294 by transformation. Clones carrying recombinant plasmids were screened for toxicity toward sensitive strains of paramecia or for the ability to produce R bodies. None of the clones appeared to be toxic. However, three clones were found to have the ability to produce R bodies, which are proteinaceous ribbons (10-20 microns long, 0.5 microns wide, and 13 nm thick) rolled up inside the cell to form a hollow cylinder about 0.5 microns in diameter and 0.5 microns long. Each of these clones carry plasmids that contain the Pst I B fragment from pKAP47. Subclones of one of the recombinant plasmids, pBQ51, were constructed to determine the approximate location of DNA sequences necessary for R-body synthesis. The left-hand boundary of the required sequences was found to occur within a 600-base-pair region, and the location of the right-hand boundary was determined to occur within a 700-base-pair region. The minimum and maximum sizes of sequences required for R-body synthesis are between 1,300 and 2,600 base pairs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale G. N., Jurand A., Preer J. R. The classes of endosymbiont of Paramecium aurelia. J Cell Sci. 1969 Jul;5(1):65–91. doi: 10.1242/jcs.5.1.65. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Dilts J. A. Covalently closed, circular DNA in kappa endosymbionts of Paramecium. Genet Res. 1976 Apr;27(2):161–170. doi: 10.1017/s0016672300016360. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Hufnagel L. A., Preer L. B. Structure and behavior of R bodies from killer paramecia. J Ultrastruct Res. 1966 Apr;15(1):131–143. doi: 10.1016/s0022-5320(66)80100-4. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Jr, Jurand A. The relation between virus-like particles and R bodies of Paramecium aurelia. Genet Res. 1968 Dec;12(3):331–340. doi: 10.1017/s0016672300011915. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Siegel R. W., Stark P. S. The Relationship between Kappa and Paramecin in Paramecium Aurelia. Proc Natl Acad Sci U S A. 1953 Dec;39(12):1228–1233. doi: 10.1073/pnas.39.12.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer L. B., Jurand A., Preer J. R., Jr, Rudman B. M. The classes of kappa in Paramecium aurelia. J Cell Sci. 1972 Sep;11(2):581–600. doi: 10.1242/jcs.11.2.581. [DOI] [PubMed] [Google Scholar]
- Quackenbush R. L., Dilts J. A., Maser R. L. Physical map of a plasmid from Caedibacter taeniospiralis 51. J Bacteriol. 1982 Nov;152(2):939–942. doi: 10.1128/jb.152.2.939-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]