Abstract
Background and Objectives
Factors underlying differential responsiveness to opioid analgesic medications used in chronic pain management are poorly understood. We tested whether individual differences in endogenous opioid inhibition of chronic low back pain were associated with magnitude of acute reductions in back pain ratings following morphine administration.
Methods
In randomized, counterbalanced order over three sessions, 50 chronic low back pain patients received intravenous naloxone (8mg), morphine (0.08 mg/kg), or placebo. Back pain intensity was rated pre-drug and again after peak drug activity was achieved using the McGill Pain Questionnaire-Short Form (Sensory and Affective subscales, VAS intensity measure). Opioid blockade effect measures to index degree of endogenous opioid inhibition of back pain intensity were derived as the difference between pre-to post-drug changes in pain intensity across placebo and naloxone conditions, with similar morphine responsiveness measures derived across placebo and morphine conditions.
Results
Morphine significantly reduced back pain compared to placebo (MPQ-Sensory, VAS; P < .01). There were no overall effects of opioid blockade on back pain intensity. However, individual differences in opioid blockade effects were significantly associated with degree of acute morphine-related reductions in back pain on all measures, even after controlling for effects of age, sex, and chronic pain duration (P < .03). Individuals exhibiting greater endogenous opioid inhibition of chronic back pain intensity reported less acute relief of back pain with morphine.
Conclusions
Morphine appears to provide better acute relief of chronic back pain in individuals with lower natural opioidergic inhibition of chronic pain intensity. Possible implications for personalized medicine are discussed.
Personalized medicine principles have not yet been successfully applied to management of chronic pain.1 Incomplete knowledge regarding factors that predict responses to opioid analgesic medications is a significant barrier.1 Ideally, such predictors would reflect known mechanisms rather than simply reflecting empirical associations. Given their shared target receptors,2 individual differences in endogenous opioid function provide a plausible mechanistic predictor of opioid analgesic responses. Animal studies on this topic have been inconsistent, with some suggesting possible synergism and others consistent with opioid analgesic tolerance related to elevated endogenous opioid activity.3-5
We are aware of only 1 human study to date that has directly explored the impact of endogenous opioid function on responses to opioid analgesic medications. We recently reported work addressing this issue using the effects of placebo-controlled opioid blockade (with naloxone) on responses to laboratory evoked pain stimuli as a functional index of endogenous opioid activity.6 This study revealed that greater endogenous opioid inhibition of laboratory evoked pain responses was associated with smaller reductions in those evoked pain responses following morphine administration.6
Other data acquired during the course of this project6 allowed us to address a different, yet related, topic. Our prior study generalized only to the acute pain context, examining endogenous opioid inhibition of brief evoked pain responses as a predictor of morphine analgesic effects on these evoked pain responses. In contrast, the current study examined degree of endogenous opioid modulation of chronic pain intensity, an issue not addressed in our prior work, as a predictor of efficacy of morphine for relieving chronic pain. Whether there would be correspondence across the acute and chronic pain contexts was unclear, given conflicting findings regarding the impact of chronic pain on endogenous opioid function.7-9 If findings for chronic pain were consistent with findings for brief evoked pain outcomes6, we expected that greater endogenous opioid inhibition of chronic pain intensity would be associated with smaller acute analgesic effects of morphine on chronic pain intensity.
METHODS
Design
This study used a 3-session, double-blind crossover design with administration of an opioid antagonist (naloxone), an opioid analgesic (morphine), and saline placebo. Order of drug administration was randomized and counterbalanced. Identical data collection procedures and equipment were employed in a closely coordinated fashion at 2 sites (Vanderbilt University Medical Center and Rush University Medical Center).
Subjects
Subjects included 50 individuals with chronic low back pain who were recruited through advertisements on the Vanderbilt e-mail recruitment system, the Rush Pain Clinic, advertisements in local print media, and posted flyers. General criteria for participation included age 18 to 55 years; no self-reported history of cardiovascular disease, hypertension, liver or kidney disorders, posttraumatic stress disorder, bipolar disorder, psychotic disorder, diabetes, seizure disorder, or alcohol or drug dependence; no use of anti-hypertensive medications; and no daily use of opioid analgesics (with absence of recent use confirmed via urine opiate screen before each laboratory study session). Additional inclusion criteria were chronic daily low back pain of at least 3 months duration, and an average past month severity of at least 3 on a 0 to 10 verbal numeric pain intensity scale. Individuals self-reporting chronic pain related to malignancy, autoimmune disorders, or fibromyalgia were excluded. Potential subjects who were pregnant (determined by urine pregnancy screens) were excluded to avoid unknown effects of naloxone on the fetus. Seven subjects reported occasional use of as-needed opioid analgesics (but none in the preceding 3 days or more) and 2 reported use of antidepressant medications. Brief clinical examinations to determine presence or absence of a radicular back pain pattern (as a surrogate marker for possible neuropathic pain mechanisms) revealed that 54% of subjects displayed evidence of radicular back pain. Presence versus absence of radicular symptoms was not significantly associated with opioid blockade effects or with morphine analgesic effects (all P values > .44). Subjects were compensated $375 for their time upon completion of the 3 study sessions.
Study Drugs
Blockade of opioid receptors was achieved by administration of naloxone, an opioid antagonist with a brief half-life (1.1 hours),10 As in past work,11 an 8 mg dose in 20 mL normal saline was infused intravenously over a 10-minute period through an intravenous cannula placed in the non-dominant arm. At this dosage, naloxone provides effective blockade of all 3 major opioid receptor subtypes.12 The opioid analgesic medication examined in this study was morphine sulfate, the prototypic mu opioid receptor agonist. As in similar laboratory pain studies with morphine,13 the current study employed a dosage of 0.08 mg/kg (in 20mL normal saline), which was infused in the same manner as naloxone. This dosage (approximately 6mg for a 165-pound individual) was selected because it was judged to be sufficient to produce analgesia, but low enough to avoid ceiling effects that might obscure key individual differences in morphine responding. Peak naloxone and morphine activity are both achieved within approximately 15 minutes.14 Normal saline (20 mL) was infused in an identical manner during the placebo condition.
Chronic Pain Measures
Chronic pain intensity was assessed pre- and post-drug during each laboratory session using the McGill Pain Questionnaire-Short Form (MPQ).15 Instructions were modified to refer specifically to the low back pain being experienced “at this moment.” The MPQ is a well-validated measure that allows separate assessment of the sensory and affective qualities of pain.15 The MPQ also includes a 100 mm visual analog scale (VAS) of overall pain intensity. At baseline screening (before the first laboratory session), a 100mm VAS intensity measure was also obtained to assess the “overall intensity” of chronic pain experienced in the past month.
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. All procedures were approved by the Institutional Review Boards at the respective institutions. After providing informed consent, subjects completed a packet of questionnaires, including information regarding demographics and chronic pain. Individuals then participated in three identical experimental sessions (placebo, naloxone, morphine) that were scheduled approximately 1 week apart, at the same time of day to control for variance due to circadian rhythms.
Subjects remained seated upright in a comfortable chair throughout all laboratory procedures. During each session, subjects initially completed a 10-minute seated rest period, after which an indwelling venous cannula was inserted into the dominant arm by a trained research nurse under physician supervision. After a 30-min resting adaptation period, subjects completed the MPQ to describe their current low back pain intensity. Subjects then received (via the cannula) either saline placebo, naloxone, or morphine per the randomization protocol. The investigational pharmacy at each institution prepared and provided the study drugs in blinded fashion to the study nurses. After a 15-minute rest period to allow peak drug activity to be achieved, subjects again described their current level of low back pain using the MPQ. Laboratory evoked pain tasks were conducted following these back pain ratings (procedures and results detailed fully in Bruehl et al6). All subjects remained in the lab under observation for 2 hours after peak drug activity had been achieved to allow drug effects to remit, after which they were released to a responsible adult. The most significant adverse effect noted with any of the study drugs was brief nausea and/or vomiting with morphine in some subjects.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 21 (SPSS Inc., Chicago, Illinois). In preparation for conducting analyses, pre- to post-drug changes in back pain intensity within each drug condition were derived. Next, opioid blockade effects (as an index of endogenous opioid inhibition of chronic pain) were derived separately for the three low back pain intensity measures (MPQ-Sensory, MPQ-Affective, VAS Intensity). These blockade effects were derived by subtracting pre- to post-drug changes when endogenous opioids were intact (placebo condition) from comparable changes when endogenous opioids were blocked (naloxone condition), such that positive blockade effect values indicated that endogenous opioids inhibited chronic back pain intensity. For example, if VAS intensity decreased from 50 to 40 pre- to post-drug in the placebo condition (change of -10), but increased from 50 to 55 after naloxone administration (change of +5), the blockade effect value would be 15, indicating that naloxone produced hyperalgesia relative to the placebo condition. Similar morphine effect measures were also derived for each chronic back pain measure (placebo versus morphine condition) such that larger positive scores indicated greater acute reductions in chronic pain with morphine relative to placebo. For comparability to our prior work,6 all blockade effect and morphine effect data were standardized (using z scores) for presentation in figures.
Analyses of pre- to post-drug changes in low back pain within each drug condition, as well as comparisons of derived change scores between drug conditions, were conducted using paired sample t-tests. To evaluate the primary hypothesis (of associations between degree of endogenous opioid inhibition of chronic pain and the efficacy of morphine for reducing chronic pain), three hierarchical linear regressions were conducted (separately for MPQ-Sensory, MPQ-Affective, and VAS intensity) with relevant morphine effects as the dependent variable. Although age, sex and chronic pain duration were not significantly associated individually with any blockade (naloxone) effect or morphine effect variables (all P values >.11), these three variables were entered in the first step of the regression to help rule out any potential confounding influences. Blockade effects were then entered as the independent variable in the second step of the regression. All analyses used the maximum number of available cases and a two-tailed probability value of P < .05 as the criterion for significance.
RESULTS
Subject Characteristics
Subject characteristics are summarized in Table 1. The sample was predominately female and non-Hispanic Caucasian, with an extended duration of chronic pain. Neither opioid blockade effect nor morphine response measures differed significantly between genders (all P values > .14).
Table 1.
Subject characteristics (n=50).
| Characteristic | |
|---|---|
| Gender (% female) | 68.0 |
| Race: | |
| Caucasian | 60.0 |
| African-American | 34.0 |
| Ethnicity: | |
| Non-Hispanic | 96.0 |
| Age (years) | 36.9 ± 10.70 |
| Past Month VAS Chronic Pain Intensity (0—100) | 56.2 ± 23.80 |
| Median Pain Duration (IQR) | 90.6 (110.39) |
Note: Summary statistics are presented as percentages or means (± SD) except where otherwise indicated.
IQR = Interquartile range.
Changes in Chronic Pain Across Drug Conditions
Raw chronic pain rating values pre- and post-drug are displayed across drug conditions in Table 2. Chronic pain intensity ratings on the MPQ-Sensory subscale decreased significantly from pre- to post-drug in all 3 drug conditions (Placebo: t = 2.45, P < .02; Naloxone: t = 3.18, P < .004; Morphine: t = 4.52, P < .001). Similar reductions on the VAS intensity measure pre-to post-drug were observed in both the naloxone (t = 3.24, P <.003) and morphine conditions (t = 6.33, P < .001), with a trend approaching significance for the placebo condition (t = 2.00, P < .06). These similar findings across drug conditions suggested some degree of pain reduction due to extended rest or possibly placebo effects.
Table 2.
Mean (± SD) chronic back pain ratings pre- and post-drug by drug condition.
| Drug Condition
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Naloxone | Morphine | |||||||
|
| |||||||||
| Chronic Pain Measure | Pre-Drug | Post-Drug | Change | Pre-Drug | Post-Drug | Change | Pre-Drug | Post-Drug | Change |
| MPQ-Sensory | 5.8±6.07 | 4.8±6.29 | -0.98±2.83a | 6.5±7.12 | 5.3±6.19 | -1.21±2.69b | 6.9±6.88 | 3.9±5.99 | -2.97±4.66a,b |
| MPQ-Affective | 1.08±2.04 | 0.7±1.73 | -0.26±1.34 | 1.2±2.07 | 0.8±1.80 | -0.38±1.76 | 1.0±2.05 | 0.6±1.47 | -0.37±1.36 |
| VAS Intensity | 33.2±26.82 | 30.4±27.53 | -2.76±9.76a | 34.3±28.86 | 29.4±28.14 | -4.87±10.64b | 38.6±28.94 | 24.2±28.85 | -14.4±16.07a,b |
Note: Negative values for change measures indicate reduced pain post-drug relative to pre-drug. Change values with the same superscript letter denote significant differences in the change scores across drug conditions at P < .05.
The more important issue was whether changes in pain following either active drug were significantly greater than in the saline placebo condition. Comparisons of drug-related changes in low back pain between placebo and active drug conditions (also in Table 2) revealed significant overall analgesic effects for morphine relative to placebo on both the MPQ-Sensory [t = 2.72, P < .01] and VAS intensity measures [t = 4.40, P < .001]. Magnitude of morphine analgesia was relatively large, with a mean 43.3% reduction in MPQ-Sensory ratings and 37.3% reduction in VAS intensity ratings. All comparisons between placebo and naloxone conditions failed to reach statistical significance (P values > .32).
Is Endogenous Opioid Inhibition of Chronic Pain Related to Morphine Responses?
We next used hierarchical regressions to address whether degree of endogenous opioid inhibition of chronic pain (opioid blockade effects based on naloxone responses) was associated with efficacy of morphine for reducing chronic pain intensity, after controlling for potential confounds (age, sex, and chronic pain duration). For MPQ-Sensory outcomes, opioid blockade (naloxone) effects were found to be a significant predictor of morphine effects, beyond any influence of age, sex, and chronic pain duration (incremental R2 change = 0.43, beta = -0.68, F Change = 40.32, P < .001). The direction of effects revealed that greater endogenous opioid inhibition of low back pain was associated with smaller reductions in back pain intensity with morphine. The finding for incremental R2 change indicates that 43% of the variance in effects of morphine on acute reductions in back pain intensity could be accounted for by the degree to which chronic pain intensity was inhibited by endogenous opioid activity. Findings exhibited a similar pattern for the other two outcome measures. For MPQ-Affective outcomes, opioid blockade effects were a significant predictor of morphine effects beyond any influence of the confounds examined (incremental R2 change = 0.11, beta = -0.35, F Change = 5.73, P < .03). For VAS intensity, opioid blockade effects were also a significant predictor of morphine effects after statistical control for the confounds examined (incremental R2 change = 0.18, beta = -0.43, F Change = 10.19, P < .004).
Scatterplots of the standardized data underlying these associations for the MPQ-Sensory and VAS Intensity measures are presented in Figures 1A and 1B. Inspection of both figures reveals that both correlations derived in large part from links between greater endogenous opioid function (larger positive opioid blockade effects based on naloxone responses) and lower morphine analgesia (smaller positive morphine effects). However, a portion of these associations (particularly for the VAS intensity measure) also appeared to be influenced by several individuals exhibiting prominent paradoxical analgesia with naloxone (larger negative opioid blockade effects) who reported a relatively high degree of back pain relief with morphine (larger positive morphine effects), as well as several individuals exhibiting high levels of endogenous opioid analgesia who experienced apparent hyperalgesic effects with morphine. To further highlight this issue, boxplots to characterize the distributions of standardized opioid blockade (naloxone) effects and morphine effects for both the MPQ-Sensory and VAS intensity measures are presented in Figures 2A and 2B. Comparable data for MPQ-Affective ratings were not informative given the restricted range for this variable related to very low values in all drug conditions.
Figure 1.
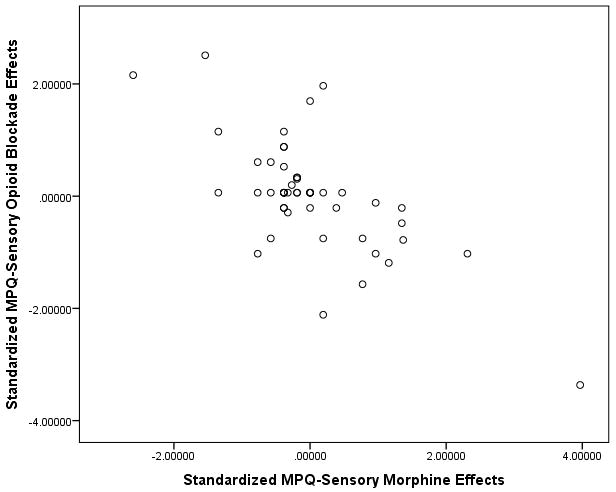
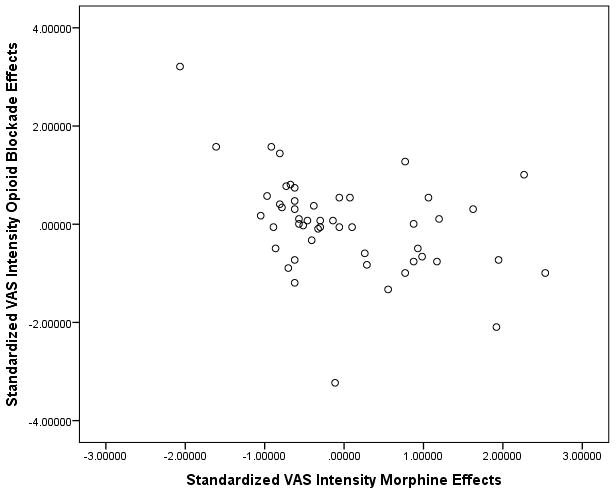
A and B. Scatterplots of associations between standardized opioid blockade effects on chronic low back pain and respective standardized morphine effects for MPQ-Sensory (1A) and VAS Intensity (1B) measures. Positive blockade effects indicate endogenous opioid inhibition of chronic pain intensity and positive morphine effects indicate morphine analgesic effects on chronic pain intensity.
Figure 2.
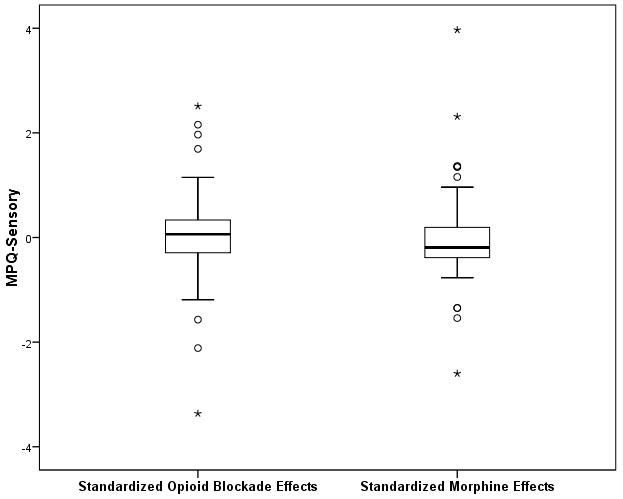
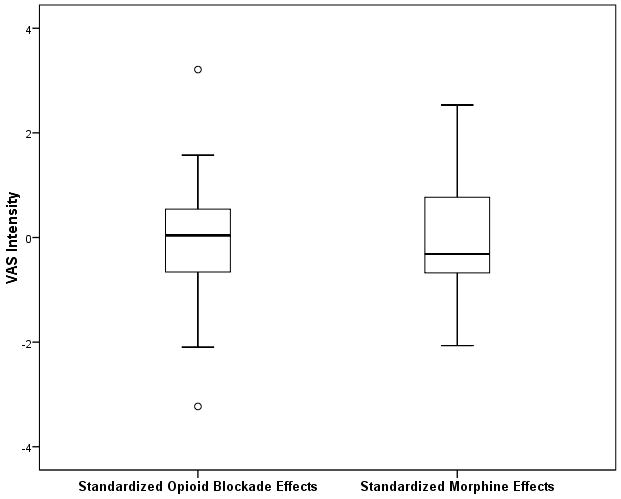
A and B. Boxplots portraying the distribution of standardized opioid blockade effects and standardized morphine effects for MPQ-Sensory (2A) and VAS Intensity (2B) measures. Positive blockade effects indicate endogenous opioid inhibition of chronic pain intensity and positive morphine effects indicate morphine analgesic effects on chronic pain intensity.
Endogenous Opioid Associations with Overall Past Month Back Pain Intensity
Patients reporting higher VAS ratings of past month overall back pain intensity exhibited smaller opioid blockade effects (i.e., less endogenous opioid analgesia) on MPQ-Sensory back pain ratings in the laboratory sessions (r = -0.29, P < .05), consistent with some degree of endogenous opioid inhibition of past month chronic pain intensity. Patients with higher past month back pain intensity also exhibited larger morphine-related reductions in MPQ-Sensory ratings of back pain in the laboratory (r = 0.31, P < .05). We then used the sequential regression approach of Baron and Kenny16 to determine whether differences in endogenous opioid function mediated this latter association. Past month pain intensity was found to account for 9.7% of the variance in laboratory morphine responsiveness when examined alone (p<.03), but when endogenous opioid function (MPQ-Sensory opioid blockade effects) was forced into the regression equation in the first step (removing variance attributable to endogenous opioid differences), the association between past month chronic pain intensity and morphine responsiveness was reduced to nonsignificance (P = 0.29), accounting for only 1.2% of the variance in morphine responsiveness. This 88% reduction in the variance in morphine responses accounted for by past month pain intensity when endogenous opioid differences were controlled is consistent with partial endogenous opioid mediation of this association.16 A Sobel test17 indicated that this endogenous opioid mediation effect was significant (z=-2.01, P < .05). Overall, these results suggest that higher recent back pain intensity as experienced during daily life, even within the moderate intensity range, is a marker for enhanced analgesic responsiveness to morphine in part via endogenous opioid mechanisms.
Comparable analyses between overall past month back pain intensity and both blockade effects and morphine effects for MPQ-Affective and VAS intensity ratings in the lab did not reveal significant associations (P values > 0.22).
DISCUSSION
The influence of individual differences in endogenous opioid inhibition of chronic pain intensity as it relates to effects of opioid analgesic medications on back pain has not previously been explored. This mechanistic information may be important to facilitate development of personalized medicine protocols for use of opioid analgesics.1 The primary aim of this study was to examine under controlled laboratory conditions the degree to which endogenous opioid inhibition of chronic low back pain intensity was associated with magnitude of acute reductions in back pain following morphine administration. Results for all three chronic pain outcomes indicated that individuals displaying greater endogenous opioid inhibition of low back pain exhibited smaller acute reductions in their back pain upon morphine administration. These findings might fit a model in which elevated tonic endogenous opioid activity in a subset of chronic pain patients results in some degree of tolerance which diminishes the efficacy of opioid analgesics. The current results extend to the chronic pain context our previous findings that in both pain-free individuals and those with chronic low back pain, greater endogenous opioid inhibition of evoked laboratory pain responses was associated with reduced analgesic effects of morphine on evoked pain responses6. Previous brain imaging evidence suggests that evoked pain responses and spontaneous chronic pain may in some cases be processed by different brain circuitry18. Nonetheless, the consistency of observed inverse associations between endogenous opioid function and morphine responsiveness in both the evoked pain and chronic pain contexts highlights the potential mechanistic importance of these links.
Another study finding may have more immediate clinical relevance. Results indicated that individuals with greater past month low back pain intensity obtained better acute relief of their back pain with morphine, despite the generally moderate intensity of the back pain in study subjects. Moreover, at least for one study measure (MPQ-Sensory ratings), this association appeared to be accounted for (mediated) by links between higher back pain intensity and lower endogenous opioid function. This finding extends to the chronic pain setting comparable endogenous opioid mediation we observed for associations between greater evoked laboratory pain responsiveness and enhanced efficacy of morphine for reducing evoked pain in our previous work.6
To the extent that clinically feasible biomarkers for endogenous opioid function, such as baseline acute pain sensitivity or chronic pain intensity, can be identified, results of this study imply that it may be possible to predict at least the initial responses to opioid analgesics in chronic pain patients. This information would potentially be useful in selecting starting doses of analgesics. Whether such effects extend to predicting long-term responses to daily opioid analgesics remains to be determined, although results of one study suggest they might.19
Although all three indexes of endogenous opioid function were associated with morphine analgesic responses, mean changes in back pain intensity with naloxone were not significantly different from mean placebo condition changes, suggesting no endogenous opioid inhibition of chronic pain in the sample as a whole in the laboratory. Nonetheless, the significant association observed between greater endogenous opioid inhibition of chronic pain in the lab and lower reports of overall past month chronic pain intensity suggest that endogenous opioid mechanisms did inhibit daily chronic back pain in a subset of subjects. Inspection of Figures 1A and 1B reveal that key associations observed in the study were driven in large part by patients who displayed a higher degree of endogenous opioid inhibition of chronic back pain (positive blockade effects) obtaining less analgesia with morphine. However, the association also appeared to be influenced by several subjects exhibiting paradoxical analgesia with naloxone (negative blockade effects) who also displayed a relatively higher level of analgesia with morphine, and conversely, a small subset exhibiting extreme endogenous opioid analgesia (large positive blockade effects) who displayed paradoxical hyperalgesia with morphine. Although clearly only present in a minority of subjects in this study, paradoxical analgesic effects of opioid antagonists at high doses have been reported in human studies previously using objective laboratory nociceptive reflex measures not subject to reporting bias,20,21 with other studies reporting similar analgesic effects on chronic pain intensity with low dose opioid antagonists.22,23 Although possible anti-inflammatory effects via antagonist actions at non-opioid receptors (Toll-Like Receptor 4) might in theory contribute,23,24 mechanisms for this paradoxical opioid antagonist analgesia remain unclear. Nonetheless, current results suggest such mechanisms might merit further exploration as a determinant of opioid analgesic responsiveness.
Some potential study limitations are noted. Results can be generalized only to the context of a single dose of morphine in individuals not previously taking daily opioids. Application to the more relevant clinical situation of predicting long-term responses to daily opioids awaits future work that integrates baseline laboratory endogenous opioid assessment with a randomized controlled trial of daily opioid analgesics. Subjects in the current study may have been qualitatively different from the typical chronic pain clinic population in their moderate pain intensity and relatively high level of functioning, their relatively young age, their willingness to volunteer for this type of study, and the fact that that none were taking daily opioid analgesic medications. This latter inclusion criterion was necessary for the safety of the subjects, as administration of naloxone to individuals taking daily opioid analgesics would trigger acute withdrawal symptoms. Another potential limitation is that it is not known whether endogenous opioid function might impact differently on responses to opioid analgesic medications other than morphine, a possibility not inconceivable given that different opioid analgesic agents are known to activate distinct signaling pathways.25
In summary, results of this study indicate that individuals exhibiting greater endogenous opioid inhibition of chronic low back pain intensity experience less back pain relief with morphine, at least acutely. Individuals with higher past month intensity of their low back pain experience greater pain relief with morphine, in part through these endogenous opioid mechanisms. Whether these mechanistic findings have clinical utility in personalized-medicine protocols remains to be determined.
Acknowledgments
The authors would like to express their appreciation to Dr Yu Lin for his contributions to this project, and to the research nurses of the Vanderbilt General Clinical Research Center and the department of Anesthesiology at Rush University for their assistance in data collection.
Funding:
This research was supported by NIH Grant R01- DA031726 and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Contents of this work are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to report.
References
- 1.Bruehl S, Apkarian AV, Ballantyne JC, et al. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. J Pain. 2013;14:103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 3.Benedek G, Szikszay M. Sensitization or tolerance to morphine effects after repeated stresses. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:369–380. doi: 10.1016/0278-5846(85)90189-7. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar RJ, Kelly DD, Steiner SS, Glusman M. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav. 1978;8:661–666. doi: 10.1016/0091-3057(78)90263-0. [DOI] [PubMed] [Google Scholar]
- 5.Fazli-Tabaei S, Yahyavi SH, Alagheband P, Samie HR, Safari S, Rastegar F, Zarrindast MR. Cross-tolerance between antinociception induced by swim-stress and morphine in formalin test. Behav Pharmacol. 2005;16:613–619. doi: 10.1097/00008877-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Burns JW, Gupta R, et al. Endogenous opioid function mediates the association between laboratory-evoked pain sensitivity and morphine analgesic responses. Pain. 2013;154:1856–1864. doi: 10.1016/j.pain.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl S, McCubbin JA, Harden RN. Theoretical review: altered pain regulatory systems in chronic pain. Neurosci Biobehav Rev. 1999;23:877–90. doi: 10.1016/s0149-7634(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 8.Corder G, Doolen S, Donahue RR, et al. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martikainen IK, Peciña M, Love TM, et al. Alterations in endogenous opioid functional measures in chronic back pain. J Neurosci. 2013;33:14729–14737. doi: 10.1523/JNEUROSCI.1400-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin WR. Drugs five years later: naloxone. Ann Intern Med. 1976;85:765–768. doi: 10.7326/0003-4819-85-6-765. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S, Burns JW, Chung OY, Chont M. Interacting effects of trait anger and acute anger arousal on pain: the role of endogenous opioids. Psychosom Med. 2011;73:612–619. doi: 10.1097/PSY.0b013e318227cb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–159. [PubMed] [Google Scholar]
- 13.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Berkowitz BA, Ngai SH, Hempstead J, Spector S. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975;195:499–504. [PubMed] [Google Scholar]
- 15.Melzack R. The short form of the McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psych. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 17.Sobel ME. Effect analysis and causation in linear structural equation models. Psychometrika. 1990;55:495–515. [Google Scholar]
- 18.Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15:843.e1–14. doi: 10.1016/j.ejpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja S. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006;104:1243–1248. doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 20.France CR, al’absi M, Ring C, France JL, et al. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol Psychol. 2005;70:168–174. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.France CR, al’Absi M, Ring C, France JL, Harju A, Wittmers LE. Nociceptive flexion reflex and pain rating responses during endogenous opiate blockade with naltrexone in healthy young adults. Biol Psychol. 2007;75:95–100. doi: 10.1016/j.biopsycho.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10:663–672. doi: 10.1111/j.1526-4637.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529–538. doi: 10.1002/art.37734. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


