Abstract
Background
Physicians’ shortage in many countries and demands of high-quality and affordable care make physician-nurse substitution an appealing workforce strategy. The objective of this study is to conduct a systematic review and meta-analysis of randomised controlled trials (RCTs) assessing the impact of physician-nurse substitution in primary care on clinical parameters.
Methods
We systematically searched OVID Medline and Embase, The Cochrane Library and CINAHL, up to August 2012; selected peer-reviewed RCTs comparing physician-led care with nurse-led care on changes in clinical parameters. Study selection and data extraction were performed in duplicate by independent reviewers. We assessed the individual study risk of bias; calculated the study-specific and pooled relative risks (RR) or weighted mean differences (WMD); and performed fixed-effects meta-analyses.
Results
11 RCTs (N = 30,247) were included; most were from Europe, generally small with higher risk of bias. In all studies, nurses provided care for complex conditions including HIV, hypertension, heart failure, cerebrovascular diseases, diabetes, asthma, Parkinson’s disease and incontinence. Meta-analyses showed greater reductions in systolic blood pressure (SBP) in favour of nurse-led care (WMD −4.27 mmHg, 95% CI −6.31 to −2.23) but no statistically significant differences between groups in the reduction of diastolic blood pressure (DBP) (WMD −1.48 mmHg, 95%CI −3.05 to −0.09), total cholesterol (TC) (WMD -0.08 mmol/l, 95%CI -0.22 to 0.07) or glycosylated haemoglobin (WMD 0.12%HbAc1, 95%CI -0.13 to 0.37). Of other 32 clinical parameters identified, less than a fifth favoured nurse-led care while 25 showed no significant differences between groups.
Limitations
disease-specific interventions from a small selection of healthcare systems, insufficient quantity and quality of studies, many different parameters.
Conclusions
trained nurses appeared to be better than physicians at lowering SBP but similar at lowering DBP, TC or HbA1c. There is insufficient evidence that nurse-led care leads to better outcomes of other clinical parameters than physician-led care.
Introduction
A WHO Report showed the global number of health care providers, namely physicians, nurses and midwives, remains lower than required per 1,000 population [1]–[3]. The low number of physicians, changes in working culture and trends in retirement have contributed greatly to this shortage [4]. Furthermore, there are pressing demands for high-quality affordable care due to the escalating growth and ageing of the population, patients’ expectations and the costs incurred managing complex conditions. In response to these changes and healthcare demands, the practice of skill mix has further developed with the aim of maintaining high-quality affordable and accessible care. It refers to a mix of posts, grades, occupations or employees, or to a combination of activities or skills needed for a job [5]. Of the skill mix strategies, substitution of physicians by nurses is a very appealing strategy due to its potential to address workforce shortages, maldistribution of workload, and to reduce cost [2], [6]. Substitution refers to nurses both performing tasks and taking responsibility for care that formerly would have been performed by physicians alone. Two systematic reviews published in 2002 and 2005 found no appreciable differences between nurse-led care and physician-led care on health outcomes but there were only a small number of studies and these also had methodological limitations [7], [8]. We performed a systematic review to compare the effectiveness of nurse-led care and physician-led care on clinical parameters in studies in which nurses substituted physicians.
Methods
We developed a protocol prior to the commencement of the review and followed the PRISMA guidelines [9] for the reporting of systematic reviews (Checklist S1).
Study Inclusion and Exclusion Criteria
We included peer reviewed randomised controlled trials (RCTs) published in English from any country that examined physician-nurse substitution. The studies had to focus on patients of all ages seeking first contact or undergoing care for all conditions including mental health and addiction restricted to primary care; and had to compare care from nurses to care from physicians (family physicians, paediatricians and geriatrician). We further limited the inclusion criteria to studies: in which the intervention or follow-up care had taken place in general practices, community or ambulatory care settings regardless of the recruitment sources; and which reported on clinical parameters that detected changes in the clinical status and/or physiological capability of patients in relation to various forms of disease, e.g. blood pressure for hypertension or cardiovascular disease risk. Based on a published framework [8], we excluded trials where nurses either supplemented the work of physicians (i.e. complemented or extended care) or collaborated with other clinicians and the effect of the intervention between nurse and physician could not be distinguished. We excluded measures of quality of life, satisfaction, mortality, hospital admissions, and progression of disease and process of care.
Study Identification
We comprehensively searched OVID Medline, Embase, The Cochrane Library of Systematic Reviews, CINHAL and the Cochrane Effective Practice and Organisation of Care Group (EPOC) from all available dates until August 2012. The searches - not age-, date- or country-specific - included ‘primary care’, ‘skill mix’, ‘doctor’-‘nurse’ substitution’ (Table S1). We also hand-searched the reference lists of included studies and relevant reviews.
Study Selection
Two authors independently screened titles and abstracts and assessed the full-texts of potentially eligible publications for inclusion, resolving differences through consensus.
Data Extraction
Two authors independently extracted both qualitative data (characteristics of studies, population and interventions) and numeric data (dichotomous and continuous format) using standardised data collection forms designed and developed a-priori, and resolved differences through consensus. Data from more than one control group of interest (e.g. family physicians and paediatricians) were combined and compared as one to the intervention group.
Assessment of Study Quality
Two authors independently assessed the risk of bias of individual trials following established criteria [10], [11] and resolved disagreements by consensus. A composite score was not calculated and we considered bias due to attrition of ≥20% to be of significant concern [12], [13].
Statistical Analyses
We performed meta-analyses when at least three trials reported appropriate data for the same outcome using the generic inverse variance fixed-effects method in Review Manager (Version 5.1) [14]. We calculated the unadjusted relative risks (RR) or the weighted mean differences (WMD) of the absolute endpoint measurements. We report the summary statistics, their 95% confidence intervals (CI) and regard p<0.05 as statistically significant. We quantified heterogeneity using the I2 statistic: values of <25% represent low heterogeneity and ≥50% represent high heterogeneity [15]. There were a maximum of five trials per meta-analysis so we could not inspect publication bias using funnel plots [16]. We decided against further subgroup analyses due to the relatively small number of studies and small number of patients per outcome. For data not combined in meta-analyses, individual trial estimates were calculated and results were compared. If standard deviations (SD) of final measurements were unavailable and could not be calculated from the statistical analyses reported, the baseline SDs were carried forward assuming the intervention would not alter the variability of the outcome [17]. Medians were treated differently from means and are clearly stated. To ensure that all the scales pointed in the same direction, the mean of a set of studies was multiplied by −1 or the mean maximum possible value was subtracted from the scale [17].
Results
Study Identification
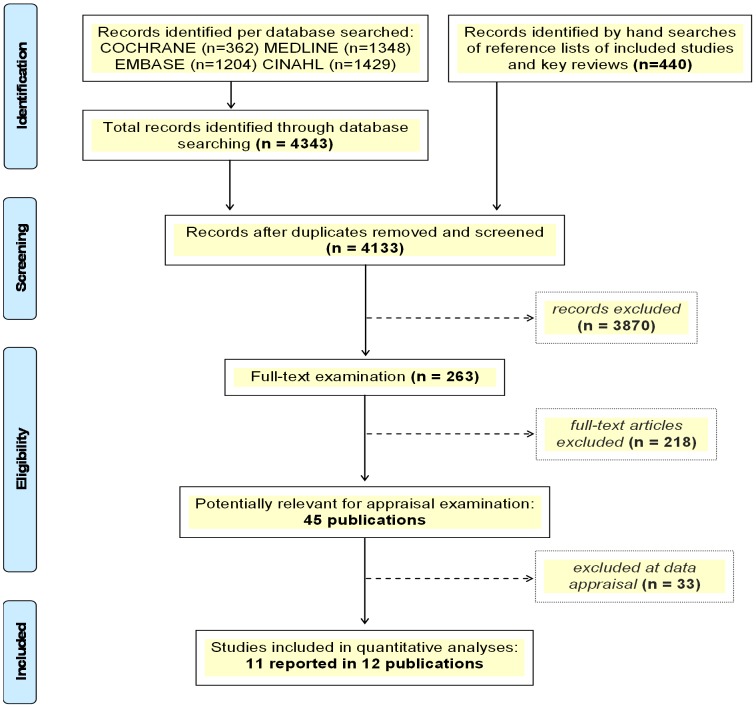
A total of 4,133 original records were identified (Figure 1). Forty-four publications were relevant, of which we excluded 32 for reasons provided in Table S2. In total, 11 RCTs met the inclusion criteria and comprised a total of 30,247 randomised participants, reported in twelve publications [18]–[28]. Table 1 and Table S3 show the characteristics of the populations, interventions and outcomes reported in the included studies.
Figure 1. PRISMA Flow diagram – study selection process.
Table 1. Characteristics of studies included in review.
| Study | Setting | Participants | Nurse group | Physician’s group | Intervention delivered | Outcomes | |||||||||||||||
| First author, y | Location | Design, period* | FUP, m | Facilities, n | Included diagnosis | Nurses, n | Patients, N | Mean age (SD), y | Male, % | Phys., n | Patients, N | Mean age (SD), y | Male, % | By | FCA | GDL | 1stC | UV | OC | C, n | reported |
| Fairall, 2012 [18]. | ZA 2 | cRCT2, 2008–2010. | 18 | Nurse ART clinic, 31. | HIV/AIDS. | 103 | 6415 | 38 (8.9) | 30 | nr | 6479 | 38 (9.63) | 27 | LN | n | y | n | n | y | >1 | CD4 counts for ART continuation and regimens. |
| Fairall, 2012 [18]. | ZA 1 | cRCT1, 2008–2010. | 16–18 | Nurse ART clinic, 31. | HIV/AIDS. | 103 | 6159 | 36 (9.6) | 33 | nr | 4923 | 35 (9.63) | 31 | LN | n | y | n | n | y | >1 | CD4 counts for ART initiation. |
| Houweling, 2011 [20]. | NL 4 | RCT, period nr. | 14 | Practice, 1. | DM2. | 2 | 116 | 67.1 (11) | 53 | 5 | 114 | 69.5 (10.6) | 42 | NP | y | y | n | n | y | >1 | BP, TC, GH, TC/HDL ratio. |
| Kuethe, 2011 [19]. | NL 3 | RCT, 2006–2008. | 24 | Hospital outpatients, 1; Practice, 18. | Asthma. | nr | 36 | 11.2 (2.9) | 64 | nr | 71 (37§, 34‡) | 11.2 (2.5) §; 10.1 (2.6) ‡ | 58 | NP+ | n | y | n | n | y | >1 | PD20, %FEV1, FENO. |
| Voogdt-Pruis 2010 [21]. | NL 2 | RCT, 2006–2007. | 12 | Healthcare centre, 6. | CVD, hypertension, hypercholesterolemia. | 6 | 808 | 64 (9.0) | 58 | 25 | 818 | 64 (9.0) | 62 | NP+ | nr | y | n | n | y | >1 | BP, TC. |
| Andryukhin 2010 [22]. | RU 1 | RCT, 2006–2009. | 6, 18 | Medical centre practice, 1. | Heart Failure with preserved ejection fraction. | 10†† | 50 | 66.5 (3.2) | 27 | 8 | 50 | 68 (4.3) | 34 | NP/LN | n | y | n | n | y | >1 | TC, GH, LDL, Cardiac function/inflammation. |
| Hiss, 2007 [23]. | US 2 | RCT, period nr. | 6 | Community, 2; PHD, 1. | DM2. | nr | 95 | 55.7 (13.1) | 32 | 108¶ | 102 | 57 (11.4) | 35 | NP+ | n | y | n | n | y | >1 | BP, TC, GH. |
| Du Moulin, 2007 [24]. | NL 1 | cRCT, period nr. | 12 | nr. | All forms of incontinence. | 1 NP | 38 | 51 (13.0) | 0 | 28** | 13 | 51 (13.0) | 0 | RN | n | y** | y | n | y | >1 | Incontinence: frequency and volume. |
| Denver, 2003 [26]. | UK 2 | RCT, 2000–2001. | 6 | Nurse clinic hospital based. | DM2, hypertension pre-diagnosis or in receipt of BPLT. | nr | 60 | 58.1 (13.8) | 57 | nr | 60 | 62.4 (9.1) | 70 | NP+ | n | y | n | n | y | >1 | BP, TC, HG, HDL, triglycerides, kidney function. |
| Jarman, 2002 [27]. | UK 1 | RCT, 1996–1999. | 24 | Practice, 438†. | Parkinson’s Disease. | 9 | 1041 | nr | 57 | nr | 818 | nr | 56 | LN | n | nr | n | n | y | >1 | Stand-up and mobility (tests). |
| Mundinger, 2000 [25], [28]. | US 1 | RCT, 1995–1997. | 6–12, 24‡‡ | Community clinic, 4; PC clinic, 1. | Asthma, DM, hypertension, or urgent visits. | 7 | 1181 | 44 | 24 | 11 | 800 | 44.9 | 22 | NP | n | nr | y | y | y | >1 | BP, GH, peak flow. |
Legend.
Studies are listed by year (y) of publication, in decreasing order.
Abbreviations: US = United States; NL = The Netherlands; UK = United Kingdom; ZA = South Africa; RU = Russia; RCT = Randomised Controlled Trial; cRCT = cluster Randomised Controlled Trial; FUP = follow-up; m = months; SD = standard deviation; nr = not reported; Phys.: physicians; PHD = Public health department; PC = Primary Care; ART = Antiretroviral Therapy; DM (2) = Diabetes Mellitus (Type 2); CVD = Cardiovascular Disease; BPLT = Blood Pressure Lowering Treatment; NP = nurse practitioner; NP+ = nurse practitioner with higher degree/course; RN = registered nurse; LN = licensed nurse; y = yes; n = no; FCA = full clinical autonomy; GDL = interventions based on clinical guidelines or protocols; 1stC. = 1st contact; UV = urgent visits; OC = on-going care; n (C, n) = number of consultations; BP = blood pressure; TC = total cholesterol; GH = glycosylated haemoglobin; ART = antiretroviral therapy; CD4 = t-cell surface glycoprotein CD4; HDL = high density lipoprotein; LDL = low density lipoprotein; PD20 = provocative dose of methacholine causing a 20% fall in forced expiratory volume in one second (FEV1); FENO = fraction of exhaled nitric oxide.
* start and end year when studies were conducted.
drawn from nine randomly chosen health authority areas.
paediatricians.
general physicians.
63 were for the control group.
** 9 were physicians and 19 were supervisors; intervention delivered following clinical protocols.
2 were nurse practitioners and 8 were (licensed) nurses.
phase I follow-up: 6–12 months; phase II follow-up: 24 months.
Study and Population Characteristics
Eleven trials - eight RCTs of parallel design and three cluster RCTs - were conducted in the UK (n = 2), The Netherlands (n = 4), USA (n = 2), South Africa (n = 2) and Russia (n = 1) (Table 1). Median follow-up was 18 (range: 6 to 30) months with more than 12 months in six trials [18]–[20], [22], [27] and 12 months or less in the other five. The number of participants ranged from 50 to 12,894 with less than 200 (range: 50 to 197) in five trials [19], [22]–[24], [26] and more than 200 (range: 230 to 12894) in the other six. Mean age reported in ten trials ranged from 11.2 (SD2.9) to 67.1 (SD11.0) years. In ten trials, 35% of the population were male and one trial included women only [24].
Interventions
The number of nurses delivering care was reported in eight trials. It ranged from 1 to 10 in six trials. In two other trials, 31 clinics were randomised with 103 nurses. Six trials reported the number of physicians delivering care, which ranged from 5 to 28 in five trials, and another employed 108. Physicians’ resources, location of practices (e.g. rural or urban) and social settings were scarcely reported. Nurses’ years of experience were not reported in any of the trials but in most of them nurses were already enrolled as staff or took specific courses for delivering care. Nurses’ roles were described under various terminologies and their qualifications and skills varied from practice nurses with or without extra training (e.g. one week training or a specialised degree) to middle nurse managers, registered or licensed nurses.
In all studies nurses were the main figure of care and performed tasks for complex conditions that required specialised skills including cerebrovascular disease, hypertension, heart failure, diabetes mellitus, asthma, incontinence, Parkinson’s disease and HIV (Table 1). Nurses’ interventions were based on more than one consultation in all trials for patients requiring on-going care or both first contact and on-going care [24], [28] and were specifically guideline or protocol based in 82% (n = 9/11) of the trials [18]–[24], [26]. Only one trial addressed urgent visits [28]. In all trials, the physicians performed standard care. Only one trial reported that nurses had full clinical autonomy to manage patients’ disease [20]. In the other ten, there were several tasks for which nurses made independent decisions (e.g. adopting, initiating and prescribing treatment) but still needed minor support or short communication with the physicians, e.g., to discuss patients’ records, to develop action plans, and to sign prescriptions.
Risk of Bias in the Methods of Included Studies
Table 2 summarises the risk of bias in individual studies. The quality varied substantially when assessed against current reporting standards [10], [11]. Among the included studies, 73% reported inclusion and exclusion criteria and funding sources. To measure the success of the intervention, 73% defined a primary outcome. Random sequence generation was adequate in 45.5%, allocation concealment was reported in 45.5% and both were adequate in 36.4%. No trial blinded both patients and providers. Blinded assessment of outcomes was performed in one trial only [22]. Sample size calculation was performed in 91% of the trials but only four held the least target sample required to achieve power (80% to 90%). At baseline, groups were comparable in 64% of the trials and 27% reported to have adjusted for clustering effects. Nearly half (45.5%) of the trials had an attrition rate of ≥20% (range: 11% to 54%) and only 27% used the intention to treat (ITT) techniques principle to deal with missing data.
Table 2. Assessment of risk of bias in studies included in review.
| Study | Inclusion &exclusion criteria | Outcome | Sequencegeneration | Allocationconcealment | Blinding | Samplesize | Attrition, % | Funding | ||
| First author, y | Location | 1ry | 2ry | |||||||
| Fairall, 2012 [18](Cohort 2)§ | ZA 2 | ✓ | ✓ | ✓ | A | A | NP‡‡ | ✓‡ , §§ | ≥20† | G |
| Fairall, 2012 [18](Cohort 1)§ | ZA 1 | ✓|| | ✓ | ✓ | A | A | NP‡‡ | ✓‡ , §§ | ≥20† | G |
| Houweling, 2011 [20] | NL 4 | ✓|| | ✓ | ✓ | I | A | NP | ✓ | <20 | G |
| Kuethe, 2011 [19] | NL 3 | ✓|| | ✓ | A | A | NP | ✓¶ | <20 | NR | |
| Voogdt-Pruis, 2010[21] | NL 2 | ✓ | ✓ | A | U | I†† | ✓ | <20 | P/Ind. | |
| Andryukhin, 2010[22] | RU 1 | ✓ | U | I | ** | ✓¶ | ≥20 | None | ||
| Hiss, 2007 [23] | US 2 | * | U | U | NP | NR | <20 | G | ||
| Du Moulin, 2007 [24] § | NL 1 | ✓|| | ✓ | ✓ | U | U | NP | ✓‡ , *** | ≥20 | NR |
| Denver, 2003 [26] | UK 2 | * | ✓ | ✓ | I | I | NP | ✓¶ | <20† | NR |
| Jarman, 2002 [27] | UK 1 | ✓ | ✓ | ✓ | A | A | NP | ✓ | <20 | P/Ind. |
| Mundinger, 2000[25], [28] | US 1 | * | U | U | NP | ✓¶ | ≥20 | G | ||
Legend.
Studies are listed by year (y) of publication, in decreasing order. A tick indicates that specific criteria were fulfilled. Blinding: whether patients, care providers and outcome assessors were blinded. Attrition: loss of data (≥20% = significant). Intention-to-treat (ITT): whether trial authors performed analyses (e.g. last value carried forward) to take into account all patients who began the intervention regardless of protocol violations, drop-outs or loss of follow-up [11], [12].
Abbreviations: US = United States; NL = The Netherlands; UK = United Kingdom; ZA = South Africa; RU = Russia; I = Inadequate; A = Adequate; U = Unclear; NP = Not Performed; NR = Not Reported; Funding = Government (G), Industry (Ind.) or Private (P) grant.
* report the inclusion criteria only.
used intention-to-treat (ITT) strategies but type not reported.
adjusted for cluster effect or intra-class cluster correlation.
cluster RCT.
trials for which not all factors tested at baseline were comparable (i.e. ≤10% difference between groups in the factors tested).
reached the least target sample required to achieve power in at least one outcome.
** single blinded, nurses and physicians were not aware of patient allocation.
only patients were blinded.
data analysts were partly blinded.
Huber-White cluster effect approach.
*** intra-class cluster correlation approach not reported.
Effectiveness of Interventions on Clinical Parameters
All RCTs [18]–[28] reported quantitative data for most of the clinical endpoints investigated but meta-analyses (Figures 2 and 3) were possible for only three, including blood pressure, systolic (SBP) and/or diastolic (DBP), total cholesterol (TC) and Glycosylated haemoglobin concentration. Thirty-two other measurements were reported in nine RCTs but had mostly one study per outcome and were not combined in meta-analyses. The individual trial estimates of these data are reported in Table 3 and Table 4.
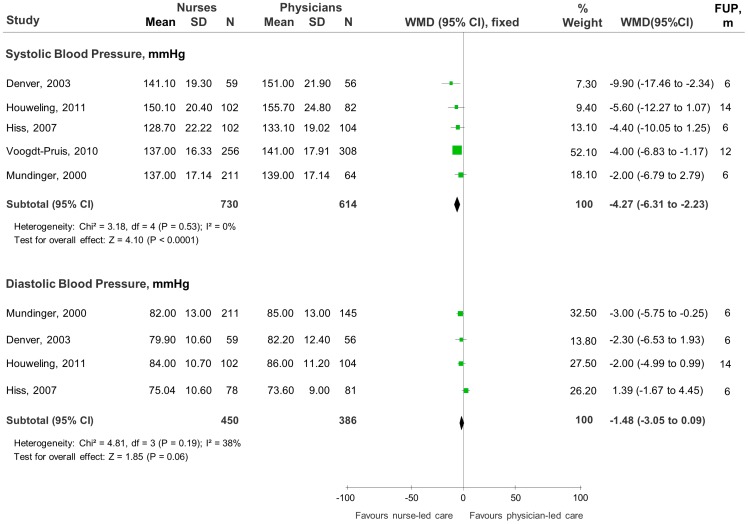
Figure 2. Comparison of blood pressure control between nurse-led care and physician-led care.
Studies are listed in order of decreasing weighted effect size. Abbreviations: mmHg = millimetres of mercury; SD = standard deviation; N = total number of patients in the analysis; WMD = weighted mean differences; CI = confidence interval; df = degrees of freedom; I2 = heterogeneity between trials; FUP = Follow-up; m = months.
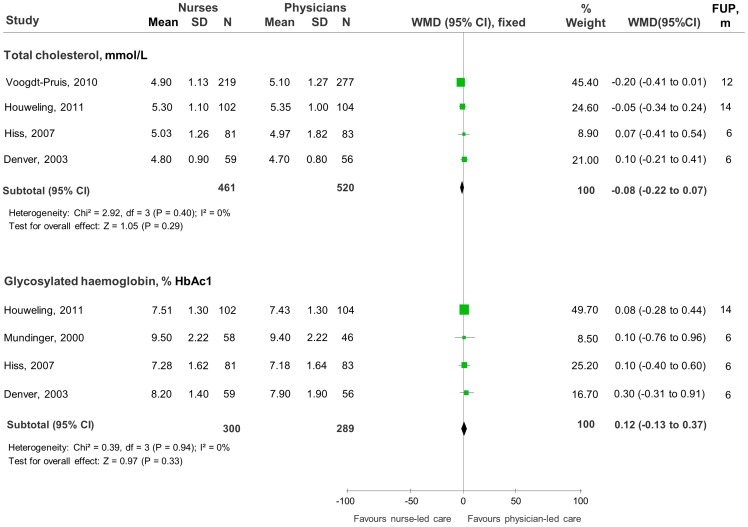
Figure 3. Comparison of total cholesterol and glycosylated haemoglobin control between nurse-led care and physician-led care.
Studies are listed in order of decreasing weighted effect size. Abbreviations: mmol/L = millimoles per litre of blood; % HbAc1 = percent of glycosylated haemoglobin (of total haemoglobin); SD = standard deviation; N = total number of patients in the analysis; WMD = weighted mean differences; CI = confidence interval; df = degrees of freedom; I2 = heterogeneity between trials; FUP = Follow-up; m = months.
Table 3. Individual trial estimates from binary data not combined in meta-analyses.
| Study | Outcome | Nurse group | Physician group | Effect estimate | |||||||
| First author, y | Location | Reported | FUP, m | n | N | n | N | RR (95% CI) | p | ||
| Cholesterol triglycerides and glucose | |||||||||||
| Andryukhin 2010 [22] ¶ | RU 1 | TC regression/stay within 4.5 mmol/l. | 6 | 23 | 40 | 10 | 35 | 2.01 (1.12 to 3.62) | 0.02 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | LDL regression/stay within 2.5 mmol/l. | 6 | 23 | 40 | 9 | 35 | 2.24 (1.2 to 4.17) | 0.010 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | Glucose, decrease/regression/stay within 6 mmol/l. | 6 | 24 | 40 | 22 | 35 | 0.95 (0.67 to 1.37) | 0.800 | ||
| Cardiac function | |||||||||||
| Andryukhin 2010 [22] ¶ | RU 1 | 6MWT exercise capacity decrease/regression. | 6 | 27 | 40 | 7 | 35 | 3.38 (1.68 to 6.77) | 0.001 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | NT-proBNP decrease or regression. | 6 | 13 | 17 | 6 | 16 | 2.04 (1.03 to 4.05) | 0.004 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | LASI decrease or regression. | 6 | 27 | 40 | 19 | 35 | 1.24 (0.86 to 1.8) | 0.250 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | LVEDVI decrease or regression. | 6 | 28 | 40 | 16 | 35 | 1.53 (1.01 to 2.32) | 0.040 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | LVMI decrease or regression. | 6 | 17 | 40 | 8 | 35 | 1.86 (0.92 to 3.77) | 0.090 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | E/A ratio decrease or regression. | 6 | 19 | 39 | 14 | 34 | 1.18 (0.71 to 1.98) | 0.520 | ||
| Andryukhin 2010 [22] ¶ | RU 1 | C-reactive protein levels decrease or regression. | 6 | 24 | 36 | 21 | 32 | 1.02 (0.72 to 1.43) | 0.930 | ||
| Parkinson’s disease | |||||||||||
| Jarman, 2002 [27] | UK 1 | Mobility stand-up test, unable to stand up or had tohold on. | 24 | 329 | 696 | 247 | 558 | 1.07 (0.95 to 1.21) | 0.940 | ||
| Jarman, 2002 [27] | UK 1 | Bone sustaining fractures during study. | 24 | 92 | 696 | 62 | 558 | 1.19 (0.88 to 1.61) | 0.690 | ||
Legend.
Studies are listed in order of increasing length of follow-up, within each category of outcomes.
Abbreviations: UK = United Kingdom; RU = Russia; FUP = follow-up; m = months; n = number of patients with events or number of events; N = total number of patients per group; RR = relative risk; CI = confidence intervals; LDL = Low Density Lipoprotein; TC = Total Cholesterol; NT-proBNP = N-terminal pro-brain natriuretic peptide; LASI = left atrium size index; LVEDVI = left ventricular end-diastolic volume index; LVMI = left ventricular mass index; E/A ratio = ratio of early (E) to late (A) mitral valve flow velocity; 6MWT = six minute walk test to measure of functional exercise capacity; mmol/l = millimoles per litre.
positive decrease/regression corresponded to less than the upper limit of 95% CI.
Table 4. Individual trial estimates from continuous data not combined in meta-analyses.
| Study | Outcome | Nurse group | Physician group | Effect estimate | ||||||
| First author, y | Location | Reported | FUP, m | mean (SD) | N | mean (SD) | N | WMD (95% CI) | p | |
| Cholesterol triglycerides and glucose | ||||||||||
| Denver, 2003 [26] | UK 2 | Mean HDL cholesterol, mmol/l. | 6 | 1.3 (0.3) | 59 | 1.4 (0.5) | 56 | −0.1 (−0.25 to 0.05) | 0.200 | |
| Denver, 2003 [26] | UK 2 | Mean Triglycerides, mmol/l. | 6 | 2.4 (1.7) | 59 | 2.3 (1.4) | 56 | 0.1 (−0.47 to 0.67) | 0.730 | |
| Voogdt-Pruis 2010 [21] | NL 2 | Mean LDL cholesterol, mmol/l. | 12 | 2.9 (1.13) | 218 | 3.1 (1.26) | 270 | −0.2 (−0.41 to 0.01) | 0.06 | |
| Houweling, 2011 [20] | NL 4 | TC/HDL ratio. | 14 | 4.43 (1.1) | 102 | 4.17 (1.2) | 104 | 0.26 (−0.05 to 0.57) | 0.10 | |
| Lung function | ||||||||||
| Mundinger, 2000 [28] | US 1 | Mean peak flow, l/min. | 6 | −297 (108.05) | 107 | −292 (108.05) | 64 | −5 (−38.46 to 28.46) | 0.77 | |
| Kuethe, 2011 [19] | NL 3 | Mean PD20 fall in FEV1. | 12 | −0.12(−0.79 to 1.2)* | −0.04(−0.90 to +0.82)† | nr | 0.96§ | |||
| Kuethe, 2011 [19] | NL 3 | Mean PD20 fall in FEV1. | 24 | 0.75(−0.33 to +1.82)* | 0.10(−0.95 to +1.16)† | nr | 0.55§ | |||
| Kuethe, 2011 [19] | NL 3 | Lung function, % FEV1 of predicted value. | 12 | 3.6 (−0.2 to 7.5)* | 0.5 (−3.3 to 4.3)† | nr | 0.29§ | |||
| Kuethe, 2011 [19] | NL 3 | Lung function, % FEV1 of predicted value. | 24 | 2.5 (−1.1 to 6.2)* | 0.9 (−2.7 to 4.5)† | nr | 0.57§ | |||
| Kuethe, 2011 [19] | NL 3 | Mean p.p.b FENO (3 breath manoeuvres). | 12 | −2.5(−6.6 to 3.4)* | 1.6(−2.8 to 7.8)† | nr | 0.44§ | |||
| Kuethe, 2011 [19] | NL 3 | Mean p.p.b FENO (3 breath manoeuvers). | 24 | −5.1(−9.4 to 0.9)* | −2.9(−8.2 to 3.5)† | nr | 0.36§ | |||
| Kidney function | ||||||||||
| Denver, 2003 [26] | UK 2 | >30 mg/day of urinary albumin excretion = complications. | 6 | 39.2 (16.0 to 200.0)‡ | 30.5 (14.5 to 147.2)‡ | nr | nr | |||
| Denver, 2003 [26] | UK 2 | Mean urine sodium excretion, mmol/day. | 6 | 178.7 (103.1) | 59 | 177.3 (87.7) | 56 | 1.4 (−33.52 to 36.32) | 0.940 | |
| Denver, 2003 [26] | UK 2 | Mean serum creatinine, µmol/day. | 6 | 117.6 (40.2) | 59 | 114.7 (37.2) | 56 | 2.9 (−11.25 to 17.05) | 0.690 | |
| Incontinence | ||||||||||
| Du Moulin, 2007 [24] | NL 1 | Frequency of incontinence episodes. | 6 | 5.3 (7.4) | 35 | 9.6 (7.7) | 10 | −4.3 (−9.67 to 1.07) | 0.120 | |
| Du Moulin, 2007 [24] | NL 1 | Frequency of incontinence episodes. | 12 | 4.8 (7.5) | 35 | 8.6 (5.6) | 10 | −3.8 (−8.07 to 0.47) | 0.080 | |
| Du Moulin, 2007 [24] | NL 1 | Volume of incontinence episodes, number of pads. | 6 | 5 (3.8) | 35 | 6.4 (3.9) | 10 | −1.4 (−4.13 to 1.33) | 0.310 | |
| Du Moulin, 2007 [24] | NL 1 | Volume of incontinence episodes, number of pads. | 12 | 4.2 (4.2) | 35 | 5.8 (4.1) | 10 | −1.6 (−4.5 to 1.3) | 0.280 | |
| HIV/AIDS | ||||||||||
| Fairall, 2012 [18] | ZA 1 | CD4 count for ART initiation. | 12–18 | 161 (175) | 2345 | 141 (161) | 1544 | 20 (9.29 to 30.71) | 0.000 | |
| Fairall, 2012 [18] | ZA 2 | CD4 count for ART continuation and regimens. | 12–18 | 438 (219) | 1733 | 418 (201) | 1691 | 20 (5.93 to 34.07) | 0.005 | |
Legend.
Studies are listed in order of increasing length of follow-up, within each category of outcomes.
Abbreviations: US = United States; NL = The Netherlands; UK = United Kingdom; ZA = South Africa; FUP = follow-up; m = months; N = total number of patients per group; SD = standard deviation; WMD = weighted mean difference; CI = confidence intervals; nr = not reported; ART = Antiretroviral Therapy; FENO = Fraction of Exhaled Nitric Oxide; PD20 = provocative dose of methacholine causing a 20% fall in forced expiratory volume in one second (FEV1); LDL = Low Density Lipoprotein; TC = Total Cholesterol; HDL = High Density Lipoprotein; mmol/l = millimoles per litre; µmol/day = micromoles per day; CD4 = t-cell surface glycoprotein CD4; l/min = litre per minute.
* reported mean (90% CIs) for nurse versus general physician: PD20-FEV1, % predictive value-FEV1, p.p.b FENO.
reported mean (90% CIs) for nurse versus paediatrician: PD20-FEV1, % predictive value-FEV1, p.p.b FENO.
reported the median (interquartile ranges) for nurse and physician groups respectively.
reported between nurse/general physician versus nurse/paediatrician.
Blood Pressure
Five trials provided sufficient quantitative continuous data for meta-analysis (Figure 2). Compared to physician-led care, the pooled WMD revealed a significant SBP-reducing effect of nurse-led care interventions (SBP, mmHg: WMD -4.27, 95%CI -6.31 to -2.23; p<0.0001). The pooled WMD also favoured a DBP-reducing effect of nurse-led care interventions but the confidence intervals crossed the line of no effect (DBP, mmHg: WMD -1.48, 95%CI -3.05 to -0.09; p = 0.06). There was no significant heterogeneity between trials (SBP: I2 = 0%, p = 0.53; DBP: I2 = 38%, p = 0.19).
Cholesterol and Triglycerides
Meta-analysis of four trials demonstrated no significant differences between nurse-led care and physician-led care in reducing the mean levels of total cholesterol (TC) at follow up, with no significant heterogeneity between trials (TC, mmol/l: WMD -0.08, 95%CI -0.22 to 0.07, p = 0.29; I2 = 0%) (Figure 3). Individual trial estimates showed significantly more patients with nurse-led care had a positive decrease or regression in TC and low density lipoprotein (LDL) levels than did patients in the group of physicians [22]. Other trial estimates showed no significant differences between groups in the reduction of LDL, high density lipoprotein (HDL), TC/HDL ratio or triglycerides [20], [21], [26].
Glycosylated Haemoglobin Concentration
Meta-analysis of four trials demonstrated no significant differences between nurse-led care and physician-led care in reducing glycosylated haemoglobin concentrations (HbA1c) at follow up, with no significant heterogeneity between trials (HbA1c, %: WMD 0.12, 95%CI -0.13 to 0.37, p = 0.33; I2 = 0%) (Figure 3). Similarly, trial estimates showed no significant differences in the number of patients with a positive decrease or regression in blood glucose levels [22].
Lung and Kidney Function
Individual trial estimates showed no significant differences between groups in various parameters of lung function including measurements of peak flow at six months [28], and PD20, lung function (%FEV1) or FENO either at 12 or 24 months [19] (Table 4). Similarly, there were no significant differences between groups in the parameters of kidney function including the levels of urine sodium excretion and serum creatinine at six months [26]. The reported median (IQR) levels of urinary albumin excretion tested to detect renal complications were higher in the nurse-led care group [UAER, mmol/day: nurse-led care, median 39.2 (IQR 16.0 to 200.0) vs. physician-led care, median 30.5 (IQR 14.5 to 147.2)].
Cardiac Function
Compared to physician-led care, there were significantly more patients with nurse-led care who had a decrease or regression in the levels of functional exercise capacity, N-terminal pro-brain natriuretic peptide or in the left ventricular end-diastolic volume index [22] (Table 3). There were no significant differences between groups in the levels of C-reactive protein, left atrial size index, and left ventricular mass index or in the ratio of early to late mitral valve flow velocity.
Incontinence
Individual trial estimates showed no significant differences between groups in the frequency (number and volume) or volume (number of pads) of incontinent episodes at either 6 or 12 months follow-up [24] (Table 4).
Parkinson’s Disease
Individual trial estimates showed no significant differences between groups in the fractures sustained during study or in the results from the mobility stand-up test at 24 months follow-up [27] (Table 3).
HIV/AIDS
In one trial, CD4 cell-counts were used as indication for ART initiation (Cohort 1) and ART continuation and management of regimens (Cohort 2) at 12–18 months follow-up [18] (Table 4). Patients receiving nurse-led care had significantly lower CD4 cell-counts compared to patients who received physician-led care.
Discussion
We systematically evaluated the published evidence for the effects of physician-nurse substitution on clinical parameters in 11 RCTs involving more than 30,000 patients with various conditions. The first important and surprising finding of our review is that the number of studies in this area is increasing slowly and studies continue to be of poor quality despite evidence reports published ten years ago [7], [8]. There is also a surprising low volume of literature reporting the outcomes of interest for this review. Most of the studies tend to report more process of care than clinical parameters. There were only three outcomes for which we could quantify the intervention effects using meta-analyses and these comprised a maximum of five studies each. The studies were also generally small. Only 3 of the 11 RCTs had more than 200 patients per arm. Of the studies pooled in meta-analyses, only one had more than 200 patients in each group. Furthermore, no study fulfilled the assessed set of methodological quality criteria. Nearly half of the 11 RCTs suffered from attrition of significant concern (≥20% attrition) and selection (i.e. lack of, or unclear, allocation concealment) biases and only a few were sufficiently powered to detect a true effect. Although we could not investigate the possibility of publication bias, we cannot rule it out since our review was limited to the published literature. Lastly, more than half of the evidence reviewed has been conducted in Europe, mainly the Netherlands. Our review shows the best available evidence however.
The evidence represents interventions for which nurses trained to provide care in various settings and for a wide range of complex conditions. In most cases, (82%), this required specialised skills and the use of guidelines. This suggests that the level of skills may be critical for the success of disease management when physician-nurse substitution takes place. The level of substitution did not seem consistent across studies however. Trials employed nurses of various qualifications and the tasks performed varied regardless of the level of training. Moreover, despite possessing some level of advanced skills, nurses required support or communication with the physicians for various tasks. Thus it was difficult to explore and identify patterns of potential influences of this criterion on the outcomes. On one hand, all studies included in the meta-analyses employed nurse practitioners (with or without further degrees), and the direction of effect remained after systematic exclusion of each trial. On the other hand, studies for which data could not be pooled involved licensed nurses, registered nurses, or nurse practitioners (with or without further degrees). Perhaps the development of nurse-led clinics may be a more appealing strategy to allow nurses to establish full clinical autonomy. The reporting of other clinicians’ characteristics (e.g. nurse-physician-patient ratios, and years of experience) also remains insufficient despite previous findings [7], [8].
There are gaps in the current evidence which merit consideration in further studies. Nurses’ roles and the level of experience required to qualify for substitution need a better definition of boundaries and task allocation in clinical practice. In addition, future research should generate more methodologically sound studies. Consistent and complete reporting of the aforementioned characteristics could improve the understanding and identification of the optimal benefits of this strategy. In spite of these limitations and heterogeneity, our meta-analyses demonstrate a statistically significant systolic blood pressure-reducing effect of nurse-led care (delivered by nurse practitioners) compared to physician-led care but no significant differences in reducing diastolic blood pressure, total cholesterol and glycosylated haemoglobin. Results from the other 32 individual trial estimates reported in nine of the trials suggest that nurse-led care (by nurses with various titles) may be similarly (26 estimates) or more (6 estimates) effective than physicians at managing the variety of clinical parameters evaluated in our review.
Strengths and Limitations of the Review
To our knowledge this is the first systematic review with a focus on clinical parameters in patients undergoing care with the implementation of physician-nurse substitution in primary care. It benefits from a thorough assessment and critical appraisal of RCTs which are at lower risk of bias [29], [30] than observational studies and allow the identification of causal relationships. It also presents the corresponding individual trial estimates and 95% confidence intervals for outcome data for which meta-analyses were not possible. A limitation of our review however, is the small number of studies that met the inclusion criteria, hence threatening the robustness of findings. This may be explained by the fact that the number of studies in this area is increasing slowly, we did not search for grey literature and were unable to identify many studies that reported the outcomes of interest. In addition, almost every study investigated only one condition, resulting in divergent reporting of outcomes that were unique to each study. It was difficult thus to study every area in depth and biases may have arisen from the over-representation of nurse-led care in specialised areas. Although many different clinical parameters were reported among the 11 RCTs, the small number of studies with sufficient and appropriate data limited meta-analyses to three outcomes. This also limited the exploration of effects in pre-specified subgroup analyses. The most probable small study bias thus accounted for the observed effects is publication bias (i.e. when the results of small negative studies are less likely to be published than small studies with positive results). A further potential limitation is the inclusion of publications in English only. We did however use comprehensive searches and screened the reference lists of included studies and relevant reviews (some in foreign languages). We did not contact authors to further obtain or clarify missing information.
Conclusion
Trained nurses appeared to be better than physicians at lowering systolic blood pressure but similar at lowering diastolic blood pressure, total cholesterol or glycosylated haemoglobin. While only a few individual trial estimates of 32 clinical parameters (e.g. kidney and lung function) favoured nurse-led care, there is insufficient evidence to conclude that nurse-led care leads to better outcomes of clinical parameters than physician-led care. The main limiting factor is the insufficient quantity of studies using good quality methods and reporting the same outcome. The current evidence also shows disease specific interventions from a small selection of healthcare systems. In order to provide more general conclusions, far more good quality trials in larger numbers of patients need to be carried out. Furthermore, additional studies should map clinicians’ characteristics, including the wider range of nurse care and tasks provided in many countries and the various levels of training and clinical autonomy.
Supporting Information
Search strategy in Ovid Medline*. *Similar search strategies were performed and run in EMBASE, The Cochrane Library of Systematic Reviews and CINAHL. All included specific search filters for RCTs.
(DOCX)
Studies excluded from the review based on appraisal of full-text articles.
(DOCX)
Participants, interventions and outcomes, in the included studies. Studies are listed by year (y) of publication, in decreasing order. Abbreviations: US = United States; NL = The Netherlands; UK = United Kingdom; ZA = South Africa; RU = Russia; RCT = Randomised Controlled Trial; cRCT = Cluster Randomised Controlled Trial; NR = Not Reported; ART = Antiretroviral Therapy; HbA1c = Haemoglobin; BP = Blood Pressure; TC = Total Cholesterol; GH = Glycosylated Haemoglobin; CD4 = t-cell surface glycoprotein CD4; HDL = High Density Lipoprotein levels; LDL = Low Density Lipoprotein; PD20 = provocative dose of methacholine causing a 20% fall in forced expiratory volume in one second (FEV1); FENO = Fraction of Exhaled Nitric Oxide. * start and end year when studies were conducted.
(DOCX)
PRISMA Checklist.
(DOC)
Acknowledgments
We are grateful to Dr Ulrike Held for her statistical advice and MD Fran Mikulicic for his support in the initial stage of the search strategies.
Funding Statement
This study was funded by the Health Services Research Fund (Bangerter foundation) from the Swiss Academy of Medical Sciences (SAMW) and by the Swiss Association of Family Physicians (Hausärzte Schweiz). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2006) The world health report: working together for health. Geneva, Switzerland: World Health Organization.
- 2.WHO (2008) Task shifting: rational redistribution of tasks among health workforce teams: global recommendations and guidelines. Geneva, Switzerland: World Health Organization.
- 3.WHO (2012) World Health Statistics 2012: Indicator compendium. Geneva, Switzerland: World Health Organization.
- 4. Salsberg E, Grover A (2006) Physician workforce shortages: implications and issues for academic health centers and policymakers. Acad Med 81: 782–7. [DOI] [PubMed] [Google Scholar]
- 5. Sibbald B, Shen J, McBride A (2004) Changing the skill-mix of the health care workforce. J Health Serv Res Policy 9(1): S28–38. [DOI] [PubMed] [Google Scholar]
- 6. Chopra M, Munro S, Lavis JN, Vist G, Bennett S (2008) Effects of policy options for human resources for health: an analysis of systematic reviews. Lancet 371: 668–74. [DOI] [PubMed] [Google Scholar]
- 7. Horrocks S, Anderson E, Salisbury C (2002) Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ 324: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurant M, Reeves D, Hermens R, Braspenning J, Grol R, et al.. (2005) Substitution of doctors by nurses in primary care. Cochrane Database Syst Rev. CD001271. doi: 10.1002/14651858.CD001271.pub2. [DOI] [PubMed]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100 doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 323: 42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fergusson D, Aaron SD, Guyatt G, Hebert P (2002) Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 325: 652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcellus L (2004) Are we missing anything? Pursuing research on attrition. Can J Nurs Res 36: 82–98. [PubMed] [Google Scholar]
- 14.Review Manager (RevMan) (2008) [Computer program]. Version 5.2.4 ed. for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration.
- 15. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 16. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: d4002 doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT GS, Green S, eds. (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available: http://www.cochrane-handbook.org.
- 18. Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, et al. (2012) Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 380: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuethe M, Vaessen-Verberne A, Mulder P, Bindels P, van Aalderen W (2011) Paediatric asthma outpatient care by asthma nurse, paediatrician or general practitioner: Randomised controlled trial with two-year follow-up. Prim Care Respir J 20: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houweling ST, Kleefstra N, van Hateren KJJ, Groenier KH, Meyboom-de Jong B, et al. (2011) Can diabetes management be safely transferred to practice nurses in a primary care setting? A randomised controlled trial. J Clin Nurs 20: 1264–72. [DOI] [PubMed] [Google Scholar]
- 21. Voogdt-Pruis HR, Beusmans GHMI, Gorgels APM, Kester ADM, Van Ree JW (2010) Effectiveness of nurse-delivered cardiovascular risk management in primary care: A randomised trial. Br J Gen Pract 60: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andryukhin A, Frolova E, Vaes B, Degryse J (2010) The impact of a nurse-led care programme on events and physical and psychosocial parameters in patients with heart failure with preserved ejection fraction: a randomized clinical trial in primary care in Russia. Eur J Gen Pract 16: 205–14. [DOI] [PubMed] [Google Scholar]
- 23. Hiss RG, Armbruster BA, Gillard ML, McClure LA (2007) Nurse care manager collaboration with community-based physicians providing diabetes care: a randomized controlled trial. Diabetes Educ 33: 493–502. [DOI] [PubMed] [Google Scholar]
- 24. Du Moulin MFMT, Hamers JPH, Paulus A, Berendsen CL, Halfens R (2007) Effects of introducing a specialized nurse in the care of community-dwelling women suffering from urinary incontinence: a randomized controlled trial. J Wound Ostomy Continence Nurs 34: 631–40. [DOI] [PubMed] [Google Scholar]
- 25. Lenz ER, Mundinger MO, Kane RL, Hopkins SC, Lin SX (2004) Primary care outcomes in patients treated by nurse practitioners or physicians: two-year follow-up. Med Care Res Rev 61: 332–51. [DOI] [PubMed] [Google Scholar]
- 26. Denver EA, Barnard M, Woolfson RG, Earle KA (2003) Management of uncontrolled hypertension in a nurse-led clinic compared with conventional care for patients with type 2 diabetes. Diabetes Care 26: 2256–60. [DOI] [PubMed] [Google Scholar]
- 27. Jarman B, Hurwitz B, Cook A, Bajekal M, Lee A (2002) Effects of community based nurses specialising in Parkinson’s disease on health outcome and costs: randomised controlled trial. BMJ 324: 1072–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mundinger MO, Kane RL, Lenz ER, Totten AM, Tsai WY, et al. (2000) Primary care outcomes in patients treated by nurse practitioners or physicians: a randomized trial. JAMA 283: 59–68. [DOI] [PubMed] [Google Scholar]
- 29. Schulz KF, Altman DG, Moher D (2011) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 9: 672–7. [DOI] [PubMed] [Google Scholar]
- 30. Sibbald B, Roland M (1998) Understanding controlled trials. Why are randomised controlled trials important? BMJ 316: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy in Ovid Medline*. *Similar search strategies were performed and run in EMBASE, The Cochrane Library of Systematic Reviews and CINAHL. All included specific search filters for RCTs.
(DOCX)
Studies excluded from the review based on appraisal of full-text articles.
(DOCX)
Participants, interventions and outcomes, in the included studies. Studies are listed by year (y) of publication, in decreasing order. Abbreviations: US = United States; NL = The Netherlands; UK = United Kingdom; ZA = South Africa; RU = Russia; RCT = Randomised Controlled Trial; cRCT = Cluster Randomised Controlled Trial; NR = Not Reported; ART = Antiretroviral Therapy; HbA1c = Haemoglobin; BP = Blood Pressure; TC = Total Cholesterol; GH = Glycosylated Haemoglobin; CD4 = t-cell surface glycoprotein CD4; HDL = High Density Lipoprotein levels; LDL = Low Density Lipoprotein; PD20 = provocative dose of methacholine causing a 20% fall in forced expiratory volume in one second (FEV1); FENO = Fraction of Exhaled Nitric Oxide. * start and end year when studies were conducted.
(DOCX)
PRISMA Checklist.
(DOC)