Abstract
The best approach to control the spread of influenza virus during a pandemic is vaccination. Yet, an appropriate vaccine is not available early in the pandemic since vaccine production is time consuming. For influenza strains with a high pandemic potential like H5N1, stockpiling of vaccines has been considered but is hampered by rapid antigenic drift of the virus. It has, however, been shown that immunization with a given H5N1 strain can prime the immune system for a later booster with a drifted variant. Here, we investigated whether whole inactivated virus (WIV) vaccine can be processed to tablets suitable for sublingual (s.l.) use and whether s.l. vaccine administration can prime the immune system for a later intramuscular (i.m.) boost with a heterologous vaccine. In vitro results demonstrate that freeze-drying and tableting of WIV did not affect the integrity of the viral proteins or the hemagglutinating properties of the viral particles. Immunization experiments revealed that s.l. priming with WIV (prepared from the H5N1 vaccine strain NIBRG-14) 4 weeks prior to i.m. booster immunization with the same virus strongly enhanced hemagglutination-inhibition (HI) titers against NIBRG-14 and the drifted variant NIBRG-23. Moreover, s.l. (and i.m.) immunization with NIBRG-14 also primed for a subsequent heterologous i.m. booster immunization with NIBRG-23 vaccine. In addition to HI serum antibodies, s.l. priming enhanced lung and nose IgA responses, while i.m. priming enhanced lung IgA but not nose IgA levels. Our results identify s.l. vaccination as a user-friendly method to prime for influenza-specific immune responses toward homologous and drifted variants.
KEY WORDS: bird flu, H5N1, mucosal vaccine, sublingual vaccine tablet, whole inactivated virus
INTRODUCTION
Influenza is an infectious disease responsible for morbidity and mortality during yearly epidemics and occasional pandemic outbreaks. Vaccination is an efficient tool in containing the virus by stimulating the immune system and will be of eminent importance in case of a pandemic. However, the immediate availability of influenza vaccine during a pandemic is hindered by the laborious vaccine production process (1). For this reason, it is desirable to stockpile influenza vaccines for future pandemics, at least for influenza strains like H5N1 which have a high pandemic potential (2,3). Yet, limited shelf life of current vaccine formulations and ongoing antigenic drift of the H5N1 virus hamper the stockpiling process (4–6). Therefore, new strategies have to be developed to improve prepandemic preparedness.
H5N1 virus circulates mainly in aquatic birds and in poultry in Southeast Asia and occasionally infects humans who are in close contact with the infected birds. Human to human transmission is rare and very few cases have been reported until today (7,8). Yet, recent studies have shown that a very limited number of mutations may allow for efficient human to human transmission in the future (9–11). Hence, H5N1 poses a constant threat of causing a new pandemic
Much effort has been put in the development of H5N1 vaccines. It appeared that unlike other influenza A vaccines, the H5N1 subtype requires two vaccine doses for effective protection (12,13). The need for two doses of vaccine creates an obstacle to prepandemic preparedness and may contribute to a high risk of vaccine shortage during pandemic outbreaks. Furthermore, H5N1 viruses display distinct antigenic drift; currently nine clades and several subclades are distinguished (14,15). In some cases, influenza vaccines from one clade may provide cross protection against another clade(s). Yet, in other cases, cross-reactivity is very limited (16). Interestingly, a recent clinical study has demonstrated that immunization with one H5N1 virus strain, i.e., A/Vietnam/1203/2004 can prime for boosting with another strain, i.e., A/Indonesia/05/2005 and that the resulting antibodies can neutralize both the virus strains (17). The heterologous boosting of immune responses with vaccines from drifted H5N1 virus has paved the way to pandemic preparedness. Indeed, several countries including the USA have currently stockpiled prepandemic H5N1 vaccines.
The instability of liquid influenza vaccine is an important problem in stockpiling of H5N1 vaccines (18). Influenza vaccines can be stabilized by incorporating them into a glassy matrix of a sugar, e.g., inulin, dextran, or trehalose, for example, by means of freeze-drying techniques (19–22). Dried vaccines can be reconstituted prior to intramuscular (i.m.) injection. Yet, ideally, they would be used in dry form, e.g., as powder or formulated in a unit-dosage form-like tablets. Tablets are easy to distribute and administer during challenging situations like the outbreak of an avian influenza pandemic.
Unfortunately, delivery of influenza vaccines to the gastrointestinal tract as such induces suboptimal immune responses (23). Recently, s.l. delivery of influenza vaccine has raised interest. Preclinical studies in mice have shown promising results: s.l.-administered influenza vaccine is capable of inducing hemagglutination-inhibiting antibodies in serum as well as a local immune response in the upper respiratory tract (24–29). Secreted IgA in the mucosa provides a local immune protection. IgA antibodies in the nose have an added advantage because they can neutralize the virus at the port of entry (21,30).
In this study, we tested the possibility of formulating a whole inactivated virus (WIV) H5N1 vaccine into a stable s.l. tablet and the suitability of such a tablet to prime for an immune response which can be boosted by i.m. immunization with a conventional vaccine formulation. We used inactivated A/Vietnam/1203/2004 vaccine virus (NIBRG-14) belonging to clade 1 for production of the s.l. tablets and NIBRG-14 or A/turkey/Turkey/1/2005 vaccine virus (NIBRG-23), a clade 2.1 virus, for the subsequent i.m. boost.
MATERIALS AND METHODS
Materials
The NIBRG-14 (a reassortant strain of A/PR/8/34 (H1N1) and A/Vietnam/1194/2004 (H5N1)) and NIBRG-23 (a reassortant strain of A/PR/8/34 (H1N1) and A/turkey/Turkey/1/2005 (H5N1)) vaccine strains were obtained from the National Institute for Biological Standards and Controls (NIBSC), Potters Bay, UK and were propagated on embryonated chicken eggs. Inulin 4 kD was procured from Sensus (Roosendaal, The Netherlands). The tablet excipients, i.e., Avicel PH 102 (micro-crystalline cellulose) and Ac-Di-Sol (cross-linked sodium carboxymethylcellulose) were purchased from FMC, Biopolymers (Philadelphia, USA), and mannitol from Bufa (Uitgeest, The Netherlands).
Vaccine
Whole inactivated influenza virus (WIV) was derived from NIBRG-14 and NIBRG-23 viruses by inactivating them with β-propiolactone as described previously (21). Thereafter, WIV was purified by dialyzing overnight against HBS (2 mM Hepes, 125 mM NaCl, 0.9 mM CaCl2, and 0.5 mM MgCl2; pH 7.4). The protein concentration of the inactivated vaccine solution was determined by micro-Lowry assay (31). The hemagglutinin (HA) content of the vaccine was considered to be one third of the total protein content.
Freeze-Drying
The NIBRG-14 vaccine was freeze-dried as described previously (19). In brief, the vaccine was mixed with an aqueous inulin solution with a HA: inulin weight ratio of 1:500 and a final inulin concentration of 5% w/v. Subsequently, 10 ml glass vials were charged with 2 ml of the vaccine dispersion. The samples were frozen by immersing the vials in liquid nitrogen for 10 min. After freezing, they were placed on the shelf of a freeze dryer (Christ Epsilon 2–4 freeze dryer; Salm and Kipp, Breukelen, The Netherlands). The shelf and condenser temperatures were set at −35°C and −85°C, respectively. The freeze-drying process was initiated by reducing the pressure to 0.180 mBar, and the shelf temperature was gradually increased to 4°C over 32 h. Thereafter, the pressure was further reduced to 0.05 mBar while the temperature was gradually increased to 20°C over 11 h. Freeze-drying was continued under these conditions for another 24 h. After freeze-drying, glass vials were closed in a nitrogen atmosphere with a controlled relative humidity of less than 10% and stored at room temperature until further use.
Formulation and Evaluation of s.l. Vaccine Tablets
S.l. tablets weighing 30 mg were formulated by mixing freeze-dried NIBRG-14 vaccine (25% w/w) with Avicel PH 102 (55% w/w), mannitol (10% w/w), and Ac-Di-Sol (10% w/w). The powder mixture was compressed using a single (6 mm × 2 mm capsule-shaped die) tablet press with a compaction force of 10 kN, which was reached within 5 s. The crushing strength of the tablet in radial direction was evaluated using a tablet tester (Model 6D (SG), Pharmatron, Switzerland). The tablet disintegration test recommended by pharmacopeia could not be used, because the size of the tablets was too small for the meshes of the basket. Hence, we adopted a simple method as described by Rawas-Qalaji et al. (32). Briefly, the tablet was immersed into a test tube filled with 2 ml of water, and the time required for breakdown of the tablet into smaller fragments was recorded by visual inspection.
SDS-PAGE
The biochemical integrity of proteins in freeze-dried NIBRG-14 vaccine was analyzed by SDS-PAGE under nonreducing conditions and compared with unprocessed NIBRG-14 vaccine. The freeze-dried samples and the tablets were reconstituted in water. The reconstituted and unprocessed samples and a prestained protein ladder (PageRuler 10–170 kDa, Thermo Scientific, USA) were incubated at 37°C for 10 min. Thereafter, the samples were mixed with sample buffer (Novagen® 4X SDS Sample Buffer, Millipore Corporation, USA). Each sample was then loaded on a precast gel (12% polyacrylamide Mini-PROTEAN TGX Precast Gels, Bio-Rad, USA) and resolved at 100 V for 1.5 h. Subsequently, the polyacrylamide gel was subjected to silver staining as reported earlier (33). The gel was dried and scanned using an HP scanner.
Hemagglutination Assay
The hemagglutination capacity of the unprocessed NIBRG-14 and NIBRG-23 vaccines, reconstituted freeze-dried vaccines, and solubilized tablets was determined as described earlier (34). In brief, a dispersion of the vaccine containing 5 μg of HA in 50 μl phosphate buffer saline (PBS) was prepared and added to the first well of a V-bottom micro-titer plate (Corning Constar, USA). Subsequently, the solution was serially diluted twofold in PBS (pH 7.4). Subsequently, 50 μl of 1% guinea pig red blood cell (RBC) suspension was added to the wells, and hemagglutination was allowed to proceed for 2 h at room temperature. The highest dilution of vaccine capable of agglutinating the RBC was recorded as one hemagglutination unit (HAU). The measurements were performed in triplicate.
Immunization Studies
Animal experiments were evaluated and approved by the Committee for Animal Experimentation (DEC) of the University of Groningen, The Netherlands. Female BALB/c mice (6–8 weeks old) were purchased from Harlan (Zeist, The Netherlands). All procedures in mice were performed under isoflurane/O2 (inhalation) anesthesia.
The mice were immunized on day 0 and day 56 according to the immunization schedule depicted in Table I. To ensure proper s.l. vaccination, the dry vaccine powder was reconstituted in 10 μl PBS and pipetted carefully under the tongue of anesthetized mice. The freeze-dried vaccine powder was used for reconstitution because it was found that the reconstitution of the formulated tablet required more than 10 μl of water, while the sublingual cavity of a mouse can only accommodate maximally 10 μl of liquid. After s.l. immunization, the mice were placed on a flat surface for 30 min under anesthesia to ensure effective immunization. Mice were sacrificed on day 84.
Table I.
Immunization Schedule
| Group | Prime (day 0) | Boost (day 56) | n |
|---|---|---|---|
| 1 | s.l.(NIBRG -14) | i.m. (NIBRG-23) | 8 |
| 2 | i.m. (NIBRG-14) | i.m.(NIBRG-23) | 8 |
| 3 | s.l. (NIBRG-14) | i.m. (NIBRG-14) | 8 |
| 4 | i.m. (NIBRG-14) | i.m. (NIBRG-14) | 8 |
| 5 | s.l. (PBS) | i.m. (NIBRG-23) | 8 |
s.l. sublingual, i.m. intramuscular
Blood samples were taken twice, i.e., on day 28 by orbital vein puncture and on day 84 via cardiac puncture. The samples were centrifuged and the serum was collected. Serum samples were stored at −20°C until further analysis. Nasal wash and bronchoalveolar lavage (BAL) were performed as described earlier (34).
Hemagglutination-Inhibition Assay
The antigen-neutralizing capacity of the collected sera was evaluated by HI assay and was performed according to the procedure used by Audouy et al. (21). In short, serum was inactivated by incubating it with kaolin suspension at 56°C for 20 min. Subsequently, after centrifugation at 1200 rpm, the samples were transferred to the first well of a V-bottom 96-well plate in duplicate and serially diluted twofold in PBS (pH 7.4). Then, 50 μl of either NIBRG-14 or NIBRG-23 vaccine containing four HAU was added to the wells. After 40 min of incubation at room temperature, 50 μl of 1% guinea pig erythrocyte in PBS was added to the wells. After 2 h of incubation at room temperature, the highest serum dilution capable of preventing hemagglutination of RBCs was scored as HI titer. By convention, titers below the detection limit (<8) were assigned a titer of 4 for calculation purposes. An HI titer of ≥40 is considered to be effective in reducing the chance of influenza infection by 50% (35–37). HI titers are presented in 2log scale; a titer of 40 equals a 2log titer of 5.3.
ELISA
ELISA was performed as described previously (34). Briefly, ELISA plates (Greiner Bio-One, Alphen a/d Rijn, The Netherlands) were coated overnight with WIV (NIBRG-14 or NIBRG-23) containing 500 ng of total protein (WIV) and then blocked with a 2.5% aqueous solution of milk powder in PBS at 37°C. The plates were washed and then charged with prediluted samples (serum, nasal, or lung lavages), which were serially diluted till the last well of the plate (12 times), and the plates were incubated for 1.5 h at 37°C. Then, 100 μl of horseradish peroxidase (HRP)-conjugated anti-mouse IgG, anti-mouse IgG1, anti-mouse IgG2a, or anti-mouse IgA (Southern Biotech, Birmingham, USA) diluted 1:5000 in PBST was added followed by incubation at 37°C for 60 min for the detection of IgG, IgG1, IgG2a, and IgA, respectively. Thereafter, the antibodies were detected using 1,2-phenylenediamine dihydrochloride (Sigma Aldrich, USA) as substrate in the phosphate–citrate buffer. The absorbance at 492 nm was measured with a micro-plate reader (Synergy HT, BioTek, USA). IgG antibody titers are given as the reciprocal of the sample dilution calculated to correspond to A492 = 0.2 after background correction, and IgA levels are presented as average of the maximum absorbance of 1:1 diluted nose and lung washes. The measurements were performed in duplicate.
Statistical Analysis
The titers are given as the geometric mean ± standard error of the mean (SEM), unless stated otherwise. The differences in titers between groups were analyzed by Mann–Whitney U test at 95% confidence interval (P < 0.05). Significance is denoted by one symbol (P ≤ 0.05) or two symbols (P ≤ 0.01).
RESULTS
Formulation of WIV as s.l. Tablet and Evaluation of Tablet Properties
In order to assess whether formulation of WIV influenza vaccine as tablet for s.l. administration is feasible, the inactivated NIBRG-14 was freeze-dried using inulin as stabilizer, mixed with other tablet ingredients, and compressed. During this procedure, the vaccine encounters stress related to freeze-drying and tableting. Therefore, to investigate the stability of the vaccine in s.l. tablets, the unprocessed vaccine, the freeze dried vaccine, and the tablets were subjected to different tests such as SDS-PAGE and HA activity assay. Furthermore, the crushing strength and the disintegration time of the tablet were determined.
Stability During Drying and Tableting
First, the proteins in WIV (NIBRG-14) were investigated to establish that they remained intact during freeze-drying and tablet formulation. WIV proteins, i.e., HA, neuraminidase (NA), matrix protein (M1), and nuclear export protein (NS2) of processed WIV were compared with those of unprocessed WIV by SDS-PAGE analysis under nonreducing conditions. Knowing their molecular weights, the WIV proteins could be identified by comparison with the standard protein mixture. The band patterns of HA, NA, M1, and NS2 did not change after processing of WIV by freeze-drying and subsequent compression into tablets (Fig. 1a, compares lanes 2, 3, and 4). The faintness of bands in lane 3 may be due to adsorbent properties of Avicel PH 102 and Ac-Di-Sol (which are insoluble) included in the tablet formulation. This result confirms the biochemical integrity of WIV proteins after freeze-drying and formulation.
Fig. 1.

Stability of NIBRG-14 WIV vaccine. a SDS-PAGE of unprocessed and processed WIV proteins under nonreducing conditions. Lane 1: molecular weight standard, lane 2: unprocessed WIV, lane 3: WIV freeze dried and subsequently processed into a formulated tablet, lane 4: freeze dried WIV. b HA titers of unprocessed WIV (black bar), freeze-dried WIV (dark gray bar) and freeze-dried WIV which was processed into a formulated tablet (light gray bar). The HA assay was performed in triplicate. As no differences in HA titers were found, there are no error bars
Next, the structural stability of inactivated NIBRG-14 was investigated in vitro by determination of the hemagglutination activity. As shown in Fig. 1b, the NIBRG-14 vaccine did not lose any hemagglutinating capacity during freeze-drying and processing of the freeze-dried powder into a tablet.
Tablet-Crushing Strength and Disintegration Time
The tablets should have good mechanical stability in order to withstand the stress related to packaging and transport. The crushing strength of the formulated tablets was found to be 41.80 ± 0.92 N (n = 10), which is high for these small tablets. In addition, the s.l. tablet should disintegrate rapidly to efficiently deliver the enclosed antigen and to prevent swallowing of the vaccine released from the tablet (38). The disintegration time of the formulated s.l tablet was found to be 2 ± 1 s (n = 10).
It can be concluded that inulin sugar glass technology can be successfully applied to prevent deterioration of WIV during stressful process conditions like freeze-drying and compression. The formulated tablets fulfilled the requirements regarding strength and disintegration for s.l. tablets. Hence, a stable influenza vaccine in the form of a sublingual tablet can be made using inulin glass technology.
Effect of s.l. Priming on the Immune Response to i.m. Immunization
In order to evaluate whether s.l. immunization can prime for a subsequent i.m. boost, mice were immunized s.l. with NIBRG-14 (20 μg HA) on day 0 followed by an i.m. booster immunization with NIBRG-14 or the drift variant NIBRG-23 on day 56 (5 μg HA). Control groups received prime and boost via the i.m. route (5 μg HA each) or received PBS on day 0 followed by i.m. immunization with NIBRG-23 (5 μg HA) on day 56. Serum antibodies toward NIBRG-14 and NIBRG-23 were evaluated on day 28 (after prime only) and on day 84 (28 days after the final immunization), respectively. Mucosal antibody responses in lung and nose were determined on day 84 after termination of the animals.
Hemagglutination-Inhibition Titers
On day 28, after a single immunization with NIBRG-14 WIV serum, HI titers against the homologous virus were below the detection limit (2log 3), irrespective of whether the immunization was given via the s.l. or the i.m. route (Fig. 2a, b; left parts, day 28). Similarly, no HI titer against the heterologous drift variant NIBRG-23 was observed 28 days after s.l. or i.m. immunization with NIBRG-14 (Fig. 2a, b; right parts, day 28). However, HI titers toward NIBRG-14 were readily observed upon i.m. boosting of the s.l.- or i.m.-primed mice and exceeded the titer of 40 (2log titer 5.3), regarded as protective in humans (Fig. 2a, left part, day 84). Priming via the s.l. route (dark gray bar) was somewhat less effective than priming via the i.m. route (light gray bar) in this respect. The NIBRG-14 prime/boost immunization regimens resulted in comparable levels of HI antibodies directed against NIBRG-14 (Fig. 2a, left part, day 84) and NIBRG-23 (Fig. 2a, right part, day 84).
Fig. 2.
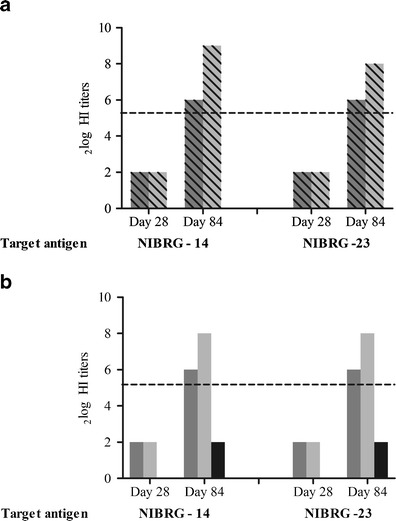
NIBRG-14 and NIBRG-23 specific HI titers of pooled sera after one or two immunizations with H5N1 WIV. a Mice were primed on day 0 with NIBRG-14 WIV given via the s.l. (dark gray bars) or the i.m. (light gray bars) route and boosted with NIBRG-14 WIV via the i.m. route on day 56. HI titers were measured in blood collected on day 28 and 84 against NIBRG-14 (left part) and NIBRG-23 (right part). b Mice were primed on day 0 by s.l. (dark gray bars) or i.m. (light gray bars) immunization with NIBRG-14 WIV and boosted i.m. with NIBRG-23 WIV on day 56. Control mice received PBS on day 0 and NIBRG-23 WIV via the i.m. route on day 56 (black bars). HI titers were measured at day 28 and day 84
Interestingly, s.l. (or i.m.) immunization with NIBRG-14 primed not only for a homologous i.m. boost with NIBRG-14 but also for a heterologous i.m. boost with NIBRG-23 vaccine (Fig. 2b, day 84). Priming with NIBRG-14 followed by boosting with NIBRG-23 resulted in 2log HI titers of 6 (s.l. priming) and 8 (i.m. priming) against NIBRG-14 and NIBRG-23. In contrast, i.m. immunization with NIBRG-23 alone was not capable of inducing HI titers above the detection limit (Fig. 2b, black bars).
Serum IgG Antibody Titers and IgG Subclasses
In contrast to HI antibodies, influenza-specific IgG (reacting with NIBRG-14 as well as NIBRG-23) could already be detected on day 28 after priming of the immune response with NIBRG-14 vaccine administered by the s.l. route (Fig. 3a, b; day 28, dark gray bars). These results clearly indicate that s.l. vaccination with WIV influenza vaccine induced a systemic immune response. Not surprisingly, the response elicited by s.l. priming was of somewhat lower magnitude than the response induced by i.m. priming (Fig. 3a, b; day 28, light gray bars). An i.m. boost with either NIBRG-14 (Fig. 3a) or NIBRG-23 (Fig. 3b) further increased the IgG titers in s.l.-primed mice (Fig. 3a, b compares day 28 with day 84 titers) and had an even greater effect in i.m.-primed animals (Fig. 3a, b compares dark with light gray bars). IgG titers in s.l.-primed and i.m.-boosted animals were significantly larger (P = 0.0008) than in animals which received a single i.m. immunization (Fig. 3b, day 84, compares dark gray bars with black bars). However, the difference was by far not as large as that between the i.m.-primed and i.m.-boosted and the i.m.-immunized group (Fig. 3b, day 84, compares light gray bars with black bars).
Fig. 3.

NIBRG-14 and NIBRG-23 specific IgG titers of sera after one or two immunizations with H5N1 WIV. a Mice were primed on day 0 with NIBRG-14 WIV given via the s.l. (dark gray bars) or the i.m. (light gray bars) route and boosted with NIBRG-14 WIV via the i.m. route on day 56. IgG titers were measured in blood collected on day 28 and 84 against NIBRG-14 (left part) and NIBRG-23 (right part). b Mice were primed and boosted as outlined in the legend to Fig. 2b. IgG in sera of individual mice was determined on day 84
IgG subtypes (IgG1 and IgG2a) were determined on day 84 to elucidate which type of T-helper cell response, i.e., Th1 (IgG2a) or Th2 (IgG1), was predominantly induced by the different vaccination regimens (Fig. 3c, d). In line with the IgG titers, IgG1 and IgG2a titers were lower for s.l.-primed mice than for i.m.-primed mice. For the NIBRG-14/NIBRG-14 scenarios (Fig. 4a), both regimens induced balanced responses with equal amounts of IgG1 and IgG2a. In contrast, for the heterologous NIBRG-14 prime/NIBRG-23 boost scenarios, IgG2a responses were clearly lower than IgG1 responses especially when tested against NIBRG-14 (Fig. 4b). This effect was more pronounced for s.l.-primed mice than for i.m.-primed mice indicating that s.l. priming of IgG2a is somewhat less effective than i.m. priming. Still, IgG1 and IgG2a responses were higher for the mice immunized following a prime/boost regimen than for mice immunized only once i.m. with NIBRG-23 (except for NIBRG-23-specific IgG2a).
Fig. 4.

NIBRG-14 and NIBRG-23 specific IgG1 and IgG2a titers of sera after one or two immunizations with H5N1 WIV. a Mice were primed on day 0 with NIBRG-14 WIV given via the s.l. (dark gray bars) or the i.m. (light gray bars) route and boosted with NIBRG-14 WIV via the i.m. route on day 56. IgG1 and IgG2a titers were measured in blood collected on day 84 against NIBRG-14 (left part) and NIBRG-23 (right part). b Mice were primed and boosted as outlined in the legend to Fig. 2b. IgG1 and IgG2a in sera of individual mice was determined on day 84
Mucosal Immune Response
The mucosal immune responses on day 84 were determined by measuring IgA levels against NIBRG-14 and NIBRG-23 in BAL and nasal washes. IgA reactivity against both viruses was observed in the BAL of all experimental groups except the group which received a single i.m. immunization with NIBRG-23 vaccine (Fig. 5a, b). There was a trend toward higher levels of lung IgA in the i.m. prime/i.m. boost groups as compared to the s.l. prime/i.m. boost groups, but differences were not statistically significant (except for NIBRG-23-specific lung IgA in the groups primed and boosted with NIBRG-14 (Fig. 5a)). In contrast to lung IgA, nose IgA, important for virus neutralization at the port of entry, was significantly better induced by the s.l. prime/i.m. boost regimens than by the i.m. prime/i.m. boost regimens. As expected, no IgA was detected in the nose of mice that had received a single i.m. immunization with NIBRG-23.
Fig. 5.

NIBRG-14 and NIBRG-23 specific IgA responses in BAL and nasal washes a Mice were primed and boosted as outlined in the legend to Fig. 2a. IgA in BAL and nose of individual mice was determined on day 84. b Mice were primed and boosted as outlined in the legend to Fig. 2b. IgA in BAL and nose of individual mice was determined on day 84
These results indicate that s.l. priming is very effective in inducing antibody responses in the upper respiratory tract, the port of entry of the virus. Moreover, the induced IgA responses are cross-reactive across different clades, and vaccine derived from one clade can prime for immunization with another clade.
DISCUSSION
In this study, we found that s.l. administration of NIBRG-14 WIV vaccine can prime for a subsequent i.m. boost. Priming was not only effective for providing a boost with homologous NIBRG-14 but also with heterologous NIBRG-23 WIV vaccine. This prime/boost regimen induced much better immune responses than an i.m. administration of NIBRG-14 or NIBRG-23 alone and resulted in HI titers of more than 40, considered as protective in humans. Moreover, mice immunized by s.l. priming followed by i.m. boosting showed significantly higher nasal IgA antibody levels than mice vaccinated by i.m. priming and boosting. Furthermore, we have shown that WIV vaccine can be formulated as a stable tablet with sufficient mechanical strength and a short disintegration time suitable for sublingual application.
Humans are naïve to H5 antigen. Hence, a single immunization with H5N1 vaccines appeared insufficient to induce protective antibody levels (39) Therefore, a multidose vaccination strategy is required to achieve robust immune responses. Indeed, the necessity of a 2-dose vaccination strategy for H5 vaccines has been proven in several clinical trials (40,41). In 2008, it was shown that an immune response primed by one type of H5N1 vaccine can be boosted with an antigenically distinct H5N1 vaccine for the induction of cross-clade reactive, protective antibodies (12,42,43). This observation opened the way for a pandemic vaccination strategy involving priming with a stockpiled H5N1 vaccine (derived for example from clade 1) followed by boosting with an H5N1 vaccine derived from the pandemic strain (which might be a clade 2 virus). So far, prime/boost strategies have been tested with inactivated virus administered via the i.m. route and with live attenuated virus given by the i.n. route. In this study, we provide evidence that priming is also possible when vaccine is administered via the s.l. route. Moreover, we show that s.l. priming not only enhances antibody titers upon i.m. boosting with a homologous virus strain but also is effective in priming for boosting with a heterologous strain.
In addition to enhancing serum IgG titers, s.l. priming also induced IgA responses in the upper respiratory tract. This is in contrast to i.m. priming which could induce IgA in the lungs but not in the nose. IgA in the upper respiratory tract is very important for neutralization of influenza virus before an infection can get established and IgA was shown to be much more effective in this respect than IgG (44). Thus, next to the advantage of ease of immunization, s.l. antigen delivery has the additional advantage of inducing local immunity at the port of entry of influenza virus.
Sublingual immunization has so far been performed mainly in the context of s.l. immunotherapy (SLIT) in which allergens are administered via the s.l. route to diminish an allergenic immune response. Administration of allergens via the s.l. route has been in use for several years and has been very successful. More recently, the sublingual route has also been used for the induction of immune responses. Generally, s.l. vaccination with influenza vaccine required high doses of antigen and/or use of an adjuvant to elicit a robust immune response (24). In this study, we tested s.l. vaccine administration as a priming strategy. Our results indicate that s.l. priming of immune responses can work even without an adjuvant, at least when performed with WIV which is known to be highly immunogenic due to the presence of single stranded viral RNA (45). The effectiveness of s.l. priming can possibly be further enhanced by addition of suitable adjuvants to the vaccine. Recent studies of influenza vaccines with adjuvants including detoxified cholera toxin and c-di-GMP demonstrate promising results (27,29,46). Although in the animal study reported here, liquid vaccine was given sublingually, we envisage that the WIV vaccine should ideally be converted into a dry and stable product and formulated into a s.l. tablet. Such tablets have two major advantages over the conventional liquid influenza vaccines. First, stockpiling is much easier because no refrigerated conditions are required. Secondly, administration of a s.l. tablet is much more easy than the conventional parenteral administration facilitating a rapid vaccination of the population which is imperative during a pandemic outbreak. In the present study, the NIBRG-14 WIV vaccine was incorporated in an amorphous inulin glass by freeze-drying and then compressed into tablets using appropriate excipients. The antigen stability after tableting was confirmed by SDS-PAGE and the HA assay. In previous studies, we have shown that WIV incorporated in inulin glasses shows an excellent storage stability (22,45). Therefore, although not investigated in the present study, it can be assumed that the WIV vaccine incorporated in the s.l. tablet can be stored at ambient temperatures for prolonged periods of time (years) without loss of antigenicity. The freeze-dried WIV influenza vaccine was formulated with Avicel PH 102, mannitol, and Ac-Di-Sol to fulfill the requirements of the s.l. tablet. The crushing strength (>40 N) and disintegration time (<3 s) of the formulated s.l. tablets were well within the limits (47). The fast disintegration guarantees rapid delivery of antigen for effective immunization (38).
CONCLUSION
Taken together, our results show that s.l. administration of WIV influenza vaccine can successfully prime for a later i.m. booster with a vaccine derived not only from a homologous but also a heterologous influenza strain. This vaccination strategy results in protective levels of serum antibodies as well as IgA in the upper respiratory tract. When incorporated into a polysaccharide matrix, the vaccine can be formulated as a stable tablet, enabling long-term storage and easy vaccine administration. The results indicate that s.l. priming of influenza-specific immune response with s.l. tablet vaccine is an ideal strategy for pandemic preparedness with respect to H5N1, a concept that may also be suitable for other influenza strains.
Footnotes
Anke Huckriede and Wouter L. J. Hinrichs contributed equally to this work.
REFERENCES
- 1.Wyman O. Influenza vaccine strategies for broad global access key findings and project methodology. 2007.
- 2.Jennings LC, Monto AS, Chan PKS, Szucs TD, Nicholson KG, Royal L. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–8. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 3.Longini IM, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DAT, et al. Containing pandemic influenza at the source. Science. 2005;309:1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 4.Luykx DM, Casteleijn MG, Jiskoot W, Westdijk J, Jongen PMJM. Physicochemical studies on the stability of influenza haemagglutinin in vaccine bulk material. Eur J Pharm Sci. 2004;23:65–75. doi: 10.1016/j.ejps.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Coenen F, Tolboom JTBM, Frijlink HW. Stability of influenza sub-unit vaccine: does a couple of days outside the refrigerator matter? Vaccine. 2006;24:525–31. doi: 10.1016/j.vaccine.2005.07.081. [DOI] [PubMed] [Google Scholar]
- 6.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A H5N1. virus in China. Lancet. 2008;371:1427–34. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 8.Ungchusak K, Auewarakul P. Probable person-to-person transmission of avian influenza A H5N1. N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 9.Imai M, Herfst S, Sorrell EM, Schrauwen EJ, Linster M, De Graaf M, et al. Transmission of influenza A/H5N1 viruses in mammals. Virus Res. 2013;178:15–20. doi: 10.1016/j.virusres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell CA, Fonville JM, Brown AEX, Burke DF, Smith DL, James SL, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012;336:1541–7. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–41. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson I, Nicholson KG, Hoschler K, Zambon MC, Hancock K, DeVos J, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N Engl J Med. 2008;359:1631–3. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- 13.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res. 2013;178:78–98. doi: 10.1016/j.virusres.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Updated unified nomenclature system for the highly pathogenic H5N1 avian influenza viruses.2011.
- 15.WHO Continued evolution of highly pathogenic avian influenza A H5N1: updated nomenclature. Influenza Other Respir Viruses. 2012;6:1–5. doi: 10.1111/j.1750-2659.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines 2006; 81:328–30. [PubMed]
- 17.Belshe RB, Frey SE, Graham I, Mulligan MJ, Edupuganti S, Jackson L, et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203:666–73. doi: 10.1093/infdis/jiq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8:E81. doi: 10.1208/pt0804081. [DOI] [PubMed] [Google Scholar]
- 19.Amorij J-P, Meulenaar J, Hinrichs WLJ, Stegmann T, Huckriede A, Coenen F, et al. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine. 2007;25:6447–57. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Geeraedts F, Saluja V, Ter Veer W, Amorij JP, Frijlink HW, Wilschut J, et al. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010;12:215–22. doi: 10.1208/s12248-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audouy SAL, van der Schaaf G, Hinrichs WLJ, Frijlink HW, Wilschut J, Huckriede A. Development of a dried influenza whole inactivated virus vaccine for pulmonary immunization. Vaccine. 2011;29:1–8. doi: 10.1016/j.vaccine.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Murugappan S, Patil HP, Kanojia G, Ter Veer W, Meijerhof T, Frijlink HW, et al. Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. Eur J Pharm Biopharm. 2013;85:716–25. doi: 10.1016/j.ejpb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Boudreault A, Pavilanis V. Oral immunization against influenza virus. Arch Gesamte Virusforsch. 1972;38:177–182. doi: 10.1007/BF01249668. [DOI] [PubMed] [Google Scholar]
- 24.Shim B-S, Choi Y, Cheon IS, Song MK. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013;13:81–5. doi: 10.4110/in.2013.13.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012;8:689–93. doi: 10.4161/hv.19568. [DOI] [PubMed] [Google Scholar]
- 26.Kweon M-NN. Sublingual mucosa: a new vaccination route for systemic and mucosal immunity. Cytokine 2011:10–4. [DOI] [PubMed]
- 27.Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzmán CA, et al. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One 2011;6: doi:10.1371/journal.pone.0026973. [DOI] [PMC free article] [PubMed]
- 28.Shim B-SS, Choi YK, Yun C-HH, Lee E-GG, Jeon YS, Park S-MM, et al. Sublingual Immunization with M2-Based Vaccine Induces Broad Protective Immunity against Influenza. PLoS One 2011;6. doi:10.1371/journal.pone.0027953. [DOI] [PMC free article] [PubMed]
- 29.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–9. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amorij J-P, Hinrichs WL, Frijlink HW, Wilschut JC, Huckriede A. Needle-free influenza vaccination. Lancet Infect Dis. 2010;10:699–711. doi: 10.1016/S1473-3099(10)70157-2. [DOI] [PubMed] [Google Scholar]
- 31.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–50. doi: 10.1016/S0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 32.Rawas-Qalaji MM, Estelle F, Simons R, Simons KJ, Simons FE. Fast-disintegrating sublingual tablets: effect of epinephrine load on tablet characteristics. AAPS PharmSciTech 2006;7: doi:10.1208/pt070241. [DOI] [PMC free article] [PubMed]
- 33.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–9. doi: 10.1002/elps.1150080203. [DOI] [Google Scholar]
- 34.Amorij J-P, Saluja V, Petersen AH, Hinrichs WLJ, Huckriede A, Frijlink HW. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine. 2007;25:8707–17. doi: 10.1016/j.vaccine.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 35.De Jong JC, Palache AM, Beyer WEP, Rimmelzwaan GF, Boon ACM, Osterhaus ADME. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 36.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Lond. 1972;70:767–77. doi: 10.1017/S0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated Influenza vaccines 2013.
- 38.Borde A, Ekman A, Holmgren J, Larsson A. Effect of protein release rates from tablet formulations on the immune response after sublingual immunization. Eur J Pharm Sci. 2012;47:695–700. doi: 10.1016/j.ejps.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res 2013:1–21. [DOI] [PMC free article] [PubMed]
- 40.Belshe RB, Frey SE, Graham I, Mulligan MJ, Edupuganti S, Jackson LA, et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203:666–73. doi: 10.1093/infdis/jiq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Fang H-H, Chen J-T, Zhou J-C, Feng Z-J, Li C-G, et al. Immunogenicity, safety, and cross-reactivity of an inactivated, adjuvanted, prototype pandemic influenza H5N1. vaccine: a phase II, double-blind, randomized trial. Clin Infect Dis. 2009;48:1087–95. doi: 10.1086/597401. [DOI] [PubMed] [Google Scholar]
- 42.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 H5N1. Vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–41. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 43.Vemula S V, Ahi YS, Swaim A-M, Katz JM, Donis R, Sambhara S, et al. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS One 2013;8: doi:10.1371/journal.pone.0062496. [DOI] [PMC free article] [PubMed]
- 44.Renegar KB, Small PA, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 45.Geeraedts F, Goutagny N, Hornung V, Severa M, de Haan A, Pool J, et al. Superior immunogenicity of inactivated whole virus H5N1 Influenza vaccine is primarily controlled by toll-like receptor signalling.PLoS Pathog 2008;8: doi:10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed]
- 46.Cuburu N, Kweon M-N, Song J-H, Hervouet C, Luci C, Sun J-B, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 47.Rudnic EM, Schwartz JB. Remington: the science and practice of pharmacy, oral solid dosage forms.Remington’s pharmaceutical sciences. 21st ed. Lippincott Williams & Wilkins; 2006.


