Abstract
This study focuses on the co-engineering of salbutamol sulphate (SS), a common bronchodilator, and mannitol (MA), a mucolytic, as a potential combination therapy for mucus hypersecretion. This combination was chosen to have a synergic effect on the airways: the SS will act on the β2-receptor for relaxation of smooth muscle and enhancement of ciliary beat frequency, whilst mannitol will improve the fluidity of mucus, consequently enhancing its clearance from the lung. A series of co-spray-dried samples, containing therapeutically relevant doses of SS and MA, were prepared. The physico-chemical characteristics of the formulations were evaluated in terms of size distribution, morphology, thermal and moisture response and aerosol performance. Additionally, the formulations were evaluated for their effects on cell viability and transport across air interface Calu-3 bronchial epithelial cells, contractibility effects on bronchial smooth muscle cells and cilia beat activity using ciliated nasal epithelial cells in vitro. The formulations demonstrated size distributions and aerosol performance suitable for inhalation therapy. Transport studies revealed that the MA component of the formulation enhanced penetration of SS across the complex mucus layer and the lung epithelia cells. Furthermore, the formulation in the ratios of SS 10−6 and MA 10−3 M gave a significant increase in cilia beat frequency whilst simultaneously preventing smooth muscle contraction associated with mannitol administration. These studies have established that co-spray dried combination formulations of MA and SS can be successfully prepared with limited toxicity, good aerosol performance and the ability to increase ciliary beat frequency for improving the mucociliary clearance in patients suffering from hyper-secretory diseases, whilst simultaneously acting on the underlying smooth muscle.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-014-9560-4) contains supplementary material, which is available to authorized users.
KEY WORDS: cilia responce, epithelia transport, lung delivery, mannitol, salbutamol, smooth muscle responce
INTRODUCTION
Chronic lung diseases including chronic obstructive pulmonary disease (COPD), bronchiectasis and genetic disease such as cystic fibrosis (CF), where the pulmonary system is implicated, are often associated with bronchoconstriction, airflow limitation, inflammation, thick hyper-viscous mucus production and subsequent disruption in mucociliary clearance (1,2). This will periodically result in cycles of infection and inflammation, leading to severe decline in lung function, high morbidity and mortality rates (3,4). There is currently no cure for these diseases and current treatments focus on slowing progression and delaying the onset of irreversible damage to the respiratory system and other organs, ultimately improving survival but not healing (5). Some of these pharmacological strategies include antibiotics and antifungals as a prophylactic or control of airway infections, steroids and non-steroidal anti-inflammatory to limit inflammatory responses, intermittent bronchodilators to optimise bronchodilations and mucolytics to promote mucus clearance (6,7). However, there are still urgent unmet needs in the treatment of these patients, including alternative methods of administration of currently available drugs, new antibiotics to treat multi-resistance bacterial infections and ultimately a cure.
Previous studies (8,9) have investigated the effects of inhaled mannitol, an airway ‘hydrator’ for enhancing mucociliary clearance in CF and bronchiectasis patients, and have shown favourable outcomes with respect to bronchial mucus clearance. In these studies, the patients were pre-treated with short acting β-agonist to prevent or reverse the airway narrowing associated with this hyperosmolar challenge. Recent evidence have also shown that β-agonists are also able to enhance mucociliary clearance in patients with asthma (10) and chronic bronchitis (11), through increased ciliary beat frequency shown in vitro (12). However, it is important to consider both the viscosity of the mucus and frequency of mucociliary clearance, as merely increasing the ciliary beat frequency may not be effective if the mucus is too thick, as seen in patients with chronic lung diseases. This may be the reason for conflicting results seen in patients with CF (13), since previous studies have shown that CF patients have normal cilia structure and function (14).
Hence, this study focuses on the co-engineering of salbutamol sulphate (SS), a common bronchodilator, and mannitol, a mucolytic, as a potential combination therapy for chronic lung diseases with mucus hyper-secretion. This combination was chosen to have a synergic effect on the airways: the SS will act on the β2 receptor for relaxation of the smooth muscle, whilst mannitol will improve mucus fluidity via osmotic pressure, consequently enhancing its clearance from the lung. Co-spray drying of both drugs has the potential to not only improve efficacy but also enhance compliance. A series of co-spray-dried samples, containing therapeutically relevant doses of salbutamol and mannitol were prepared and tested in terms of physico-chemical characteristics and in vitro aerosol performance. Additionally, the formulations were evaluated for their effects on epithelia cell viability, transport across an established air interface Calu-3 cell-line coupled with a twin-stage impinger, ciliary beat frequency and smooth muscle contraction.
MATERIALS AND METHODS
Materials
Salbutamol sulphate, referred to as SS hereafter, was purchased from S & D Chemicals (Sydney, Australia). Mannitol (Pearlitol® 160C), referred to as MA hereafter, was obtained from Rocquette Frères (Lestrem, France). Non-essential amino acids solution, CelLytic™ M Cell Lysis, protease inhibitor cocktail, and fluorescein sodium were purchased from Sigma-Aldrich (Sydney, Australia). Other cell culture reagents including trypsin–EDTA solution (2.5 g/L trypsin, 0.5 g/L EDTA), Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline, l-glutamine solution (200 mM), fetal bovine serum and Hank’s balanced salt solution (HBSS) were obtained from Invitrogen (Sydney, Australia). Transwell cell culture inserts (0.33 cm2 polyester, 0.4 μm pore size) were purchased from Corning Costar (Lowell, MA, USA), and all other sterile culture plastic wares were from Sarstedt (Adelaide, Australia). All solvents used were of analytical grade and were supplied by Biolab (Victoria, Australia).
Preparation of Micro-particulates
Single and combination powders were produced by spray drying aqueous solutions of MA and/or SS) using a Buchi B-290 spray dryer (Flawil, Switzerland) under the conditions outlined in supplementary materials Table 1. The relative concentrations of each drug in the feed solution were adjusted so that the final dry weight percentages (% w/w) were 100% MA, 99.95%MA/0.05%SS and 99.9%MA/0.1% SS, respectively. Briefly, the formulations were prepared by dissolving MA and SS in water to the desired concentrations, and the feed solution was spray-dried at the following conditions: feed concentration of 20 mg/mL, feed rate of 4 mL/min, aspiration rate of 47.6 m3 h−1, atomising pressure of 600 kPa and inlet temperature of 150°C.
Physical Characterisation
Particle Size Analysis
Particles size distributions of each micro-particulate system were measured using laser diffraction (Mastersizer 3000, Malvern Instruments, Malvern, UK) to ensure that the particles generated were within the respirable range. The particles were dispersed in chloroform with 1% (v/v) of Tween and sonicated for 1 min to prevent particle agglomeration. An aliquot of the suspension was transferred to the small dispersion cell of the Malvern particle sizer until an obscuration of 15–30% was achieved. Particle size distributions were measured using refractive index of 1.52 for mannitol and 1.44 for chloroform. Each sample was analysed in triplicate.
Scanning Electron Microscopy
The morphologies of the micro-particles were visualised using a field emission scanning electron microscopy (SEM; Zeiss Ultra Plus, Carl Zeiss NTS GmbH, Oberkochen, Germany) at 5 kV. The samples were mounted onto carbon sticky tapes, which were attached to aluminium stubs and gold-coated at 30 nm thickness (Sputter coater S150B, Edwards High Vacuum, UK) under vacuum prior to imaging.
Thermal Analysis of the Micro-particulate Systems
The thermal responses of each micro-particulate system were analysed using differential scanning calorimetery (DSC-DSC823e, Mettler-Tolledo, Switzerland). Approximately 10 mg of powder were weighed and crimp-sealed in DSC sample pans with the lid pierced to ensure constant pressure. Thermal properties were analysed using a 10°C/min temperature ramp over 40–400°C. Data were normalised for initial mass. Temperatures of each exothermic and endothermic peak and onset were determined using STARe V11.0× software (Mettler-Tolledo).
Additionally, the mass related thermal response of each micro-particulate system was assessed using thermal gravimetric analysis (TGA; Mettler-Toledo ltd, Switzerland). Approximately 25 mg of dry powder was weighed into open aluminium crucible pans. The samples were subsequently evaluated for weight loss on heating with temperatures ranging from 25 to 400°C with a scanning rate of 10°C/min, under a nitrogen purge. The weight loss on heating was characterised as a percentage of the initial weight.
Dynamic Vapour Sorption
The moisture sorption characteristics and stability of the spray-dried micro-particles with respect to humidity were assessed using dynamic vapour sorption (DVS). Samples (ca. 10 mg) were added to sample pans and dried at 0% relative humidity (RH) before exposure to a full sorption cycle of 0–90% RH, at a 10% increment and a set temperature of 25°C. Equilibrium moisture content at each step was determined when a change in mass to time ratio (dm/dt) ≤ 0.0005% min−1. An automated water sorption analyser (DVS-1, Surface Measurement Systems Ltd., UK) was used to determine the moisture sorption profiles of samples.
Mannitol and Salbutamol Analysis
Quantification of SS was performed using high-performance liquid chromatography (HPLC) with a system (Shimadzu Prominence UFLC system, Shimadzu Corporation, Japan) that consisted of: a SPD-20A UV–Vis detector, LC-20AT liquid chromatography, RID 10A refractive index detector, SIL-20A HT autosampler and an Optimal ODS-H column (3 μm, 150 × 4.6 mm, Capital HPLC Limited, Broxburn, West Lothian, Scotland). The mobile phase was a mixture of methanol and 0.1 M sodium dihydrogen phosphate at a 30:70 (v/v) ratio, with pH adjusted to 3.35 with phosphoric acid. The flow rate was set to 0.8 mL/min, and 100 μL of each sample was injected into the column. The UV detector was set to 276 nm, and linearity was obtained between 0.01 and 10 μg/mL (R2 = 0.99) with a retention time of 3.5 min.
For the quantification of mannitol, a refractive index detector (RID 10A refractive index detector, Shimadzu, Kyoto, Japan) was used. Samples were injected into the HPLC system with a Resolve C-18 column (5 μm, 150 × 3.9 mm, Waters, MA, USA) using deionised water as the mobile phase. The HPLC was set to a flow rate of 1 mL/min, injection volume of 100 μL and retention time of 1.5 min. Linearity was obtained with concentrations ranging from 0.05 to 20 mg/mL (R2 = 0.99).
In Vitro Aerosol Performance Studies
The aerosolisation efficiency of the dry powder formulations was evaluated using a multi-stage liquid impinger (MSLI) (Apparatus A, European Pharmacopoeia, Chapter 2.9.18; Copley Scientific, Nottingham, UK) and an Aerolizer® dry powder inhaler (Norvartis Surrey, UK). The flow rate through the MSLI was controlled by a Novi rotary vein pump and solenoid valve timer (Westech Scientific Instruments, Bedfordshire, UK). Prior to the experiment, the flow rate was tested and set to 60 L/min using a calibrated flow meter (TSI, Shoreview, MN, USA). Twenty milliliters of Milli-Q water was accurately added to each collection stage of the MSLI.
A total dose of 200 mg was chosen for delivery, which were pre-weighed as 40 mg powder aliquots into five size 3 hard gelatine capsules (Capsugel, Sydney, Australia). These capsules were carefully placed into the dosage chamber of an Aerolizer® and administered consecutively at a flow rate of 60 mL/min for 4 s into the MSLI. The Aerolizer® was fitted into a mouthpiece adapter, which was connected to the MSLI using a US Pharmacopoeia throat. After actuation, the device, capsule, throat, all stages and filter were washed separately using water and analysed using HPLC. Each sample was tested in triplicate.
In Vitro Bio-Characterisation
Cell Culture
Human bronchial smooth muscle cells in primary culture from a healthy donor were purchased from PromoCell GmBH (Heidelberg, Germany), thawed at passage 2 and used within the first seven passages. Cells were cultured in smooth muscle cell growth medium supplemented with 5% (v/v) fetal bovine serum, 0.5 ng/mL epidermal growth factor, 2 ng/mL basic fibroblast growth factor, 5 μg/mL insulin, 50 μg/mL amphotericin B and 50 ng/mL gentamycin, based on the manufacturer’s recommendations (PromoCell).
Calu-3 bronchial epithelial cells were from the American Type Culture Collection (Manassas, USA). The cells were cultured between passages 35–45 in pre-warmed Dulbecco’s Modified Eagle’s Medium—F12 supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) non-essential amino acid solution and 1% (v/v) l-glutamine solution. Cells were incubated at 37°C in 5% CO2 and 95% humidity until confluency was reached. Medium was exchanged every 2–3 days and the cells passaged weekly according ATCC recommended guidelines.
Cell Toxicity Assay
To better understand the range of concentrations suitable for use on pulmonary cells, in vitro pulmonary cytotoxicity tests were conducted. The cytotoxicity profiles of SS were assessed using CellTiter 96® AQueous One Solution Cell proliferation Assay (Promega, USA) on Calu-3 as previously described (15,16). Briefly, the cells were seeded onto sterile 96-well microtitre plates at a density of 5 × 104 cells/well and left overnight to allow for cell attachment. Cells were treated with increasing concentrations of SS and mannitol in complete DMEM medium with a final volume of 200 μL. The drugs were dissolved in water and subsequently sterile-filtered before adding to complete medium in a final water concentration of <1%. Untreated controls and vehicle controls were included in each experiment. Plates were subsequently incubated at 37°C in 5% CO2 and 95% humidity for 72 h. The cells were analysed for viability following the addition of 20 μL of AQueous One Solution to each well and incubation for 3 h at 37°C in 5% CO2 and 95% humidity. Absorbance of the wells was measured at 490 nm using a fluorescence plate reader (SpectraMax M2; Molecular devices, USA). The cell viability (%) was calculated based on this formula: [(average absorbance of treated cells/average absorbance of control cells) × 100]. The concentration that produced a 50% decrease in cell viability after 72 h (IC50) was calculated following the 72-h treatment period.
Transepithelial Transport Studies
Culture conditions of the air interface Calu-3 cells is described in detailed elsewhere (15,16). Experiments on the cells were performed between days 11 and 14, from passages 35 to 45 to allow for cell differentiation. A modified glass twin stage impinger (TSI; Copley Scientific, UK) was used for the deposition of dry powder formulations onto the Calu-3 epithelial cells. The apparatus was set up and performed as previously described (15,17). The formulations delivered from the Aerolizer® were tested at a flow rate of 60 L/min for 4 s to allow particles ≤6.4 μm to deposit onto the epithelial cells, mimicking deposition of particles in the bronchiolar region of the lungs. Samples were collected every 30 min up to 4 h, together with drugs recovered from the cell surface and intracellularly at the end of the transport experiment; the sum of these samples is the estimated amount of SS deposited onto the Calu-3 cells. Approximately 1 mg of dry powder formulation was weighed into size 3 hard gelatine capsules, which was actuated into the TSI assembly using the same protocol as for the MSLI.
Assessment of the Calu-3 monolayer’s integrity was performed using transepithelial electrical resistance (TEER) measurements, as previously described (15,17), using an epithelial voltohmmeter (EVOM, World Precision Instruments, USA). TEER measurements were performed on untreated-control cells and after the deposition experiments were complete.
Ciliary Activity
The effects of SS, MA and combination of both drugs on ciliary activity was analysed using primary nasal epithelial cells from healthy volunteers (n = 5). The nasal epithelial cells were obtained through nasal brushings (31). The cells were suspended in approximately 5 mL of Medium 199 (Sigma, Australia) to maintain cell viability and aliquots of cell suspensions were treated with the drugs for 15–30 min. The range of concentrations of SS and MA tested ranged from 10−8 to 10−3 M. Based on the results of ciliary beat frequency (CBF) stimulation from individual drug and previous study by Devalia et al. (18), indicated that the optimal concentrations to achieve significant increase in CBF were between 10−6 and 10−4 M on cultured bronchial epithelial cells. Consequently, in this study, the concentrations of SS used were 0.5 × 10−6 M and 1 × 10−6 M together with 10−3 M of MA based on the combination therapy formulated. These concentrations were also selected taking into consideration the solubility of MA and toxicity of both drugs on the epithelial cells. Analysis of the CBF was performed under a light microscope (Olympus IX70, Japan) connected to a photo-multiplier (Zeiss, West Germany). The CBF was measured based on the electronic signals from the photometer (Tektronix), which was delivered through an oscilloscope and subsequently recorded by Mac-Lab recording system. For each sample, three measurements of CBF were taken from different regions of interest that contains strips of ciliated epithelial cells to obtain precise frequency values.
Single Cell Smooth Muscle Contraction Assay
Smooth muscle cells of passages 4–7 were used. Cells were seeded onto a 12-well cell culture plate at a density of 9.6 × 104 cells/well and left overnight for attachment. Prior to the experiment, the cells were partially detached from the plastic culture dish by replacing medium with a non-enzymatic cell dissociation buffer (Sigma, Australia) and incubated at 37°C for 15 min. The contraction of the smooth muscle cells in response to histamine (10−3 M), which acts as a positive control, MA (10−3 M), SS (10−6 M) and combination of SS and MA were visualised using a Nikon Elipse TI time-lapse microscope with NIS-Elements AR 3.2 software (Nikon, Tokyo, Japan). Images were captured prior to analysis and at 5–10-s interval for 5 min after drug treatment. A number of cell images were randomly selected and manually outlined. The enclosed cross-sectional area was quantified using image analysis software (Image J 1.47v). Subsequently, the cross-sectional areas of each cell were normalised by dividing the cross-sectional area at each time point by the cross-sectional area of the same cell at time 0 (prior to drug exposure). A decrease in relative cross-sectional area was interpreted as an indication of cell contraction. All studies were performed in triplicate.
Intracellular Cyclic Adenosine Monophosphate Assay
Intracellular cyclic adenosine monophosphate (cAMP) was assayed to determine and confirm its role in regulating smooth muscle contraction. Approximately 9.6 × 104 cells/well of smooth muscles cells were seeded onto a six-well culture plate and cultured for 2 days. Prior to drug exposure, the cells were pre-incubated with 500 mM of isobutyl-1-methylxanthine (IBMX, Sigma) for 30 min at 37°C. Subsequently, the cells were treated with MA (10−3 M), SS (10−6 M) and combination of SS and MA for 10 min. The cells were then lysed using 0.1 M hydrochloric acid for 20 min, and cell suspension was centrifuge at 1,000 × g for 10 min. cAMP in the supernatant was measured using an EIA assay kit (Cayman Chemical, USA), according to manufacturer’s recommendation.
Statistical Analysis
All results are expressed as mean ± standard deviation of at least three separate determinants. To determine significance between groups and control, unpaired two-tailed t tests or one-way ANOVA were performed (quoted at the level of p < 0.05).
RESULTS AND DISCUSSION
The aim of this study was to develop a series of combined SS and MA formulations as a potential therapy for mucus hyper-secretive lung diseases. The study was broadly divided into two main sections: (1) physico-chemical characterisation of the formulations and (2) in vitro bio-characterisation of the interaction between the formulations and Calu-3 lung epithelium cell line, human bronchial smooth muscle cells, and primary nasal epithelial cells with functional cilia, respectively.
Physical Characterisation of Formulations
Particle Size Distributions of the Micro-particulate Systems
The particle size distributions of the spray-dried MA and co-spray-dried SS with MA are shown in supplementary materials Fig. 1. The particles had similar size distribution with small deviations, which could be attributed to the systems being subjected to similar spray drying conditions and concentrations. A median volume diameter of 2.50 ± 0.01 μm, 3.63 ± 0.31 μm and 3.24 ± 0.16 μm μm (n = 3 ±SD) were found for spray-dried 100% (w/w) MA, 0.1% SS/99.9% MA and 0.05%/99.95% MA (w/w), respectively. These sizes fall within the range for respiratory delivery (19) when assuming a density <1.4 g/cm3 as that for mannitol.
Scanning Electron Microscopy
Scanning electron micrographs of single spray-dried MA and co-spray-dried SS-MA are shown in Fig. 1. Generally, the particle diameters were in good agreement with particle size data. Overall, the microparticles displayed a spherical morphology with crystalline ‘finger-like’ projections on the surface, typical of spray-dried MA (20). The combined formulation of SS and MA did not show any particular difference in morphology and size, most likely due to the low concentrations of SS in the formulations.
Fig. 1.

Scanning electron micrographs of a Spray-dried mannitol. b Co-spray dried 0.05 SS/99.95 MA% (w/w). c Co-spray-dried 0.10 SS/99.90 MA% (w/w), respectively
Thermal Analysis of the Micro-particulate Systems
The thermal properties of the SS and MA formulations were investigated to analyse the influence of heat flow on the micro-particulate system, using DSC and TGA. The response of each sample to the temperature ramp from the DSC is displayed in Fig. 2. The raw and single spray-dried MA displayed a characteristic endothermic melting peak at approximately 168°C and decomposition at 303°C. Similarly, the raw SS demonstrated a sequence of endothermic peaks at 205 and 285°C, attributed to a multiple decomposition pathway. These thermal responses are in good agreement with previous studies (20–23). As for the combination formulations (SS-MA), they exhibit the characteristic endothermic peak of crystalline MA at ∼168°C and another smaller peak at 285°C that could be attributed to the decomposition of SS. These results are expected, as the formulations are comprised of mostly MA.
Fig. 2.
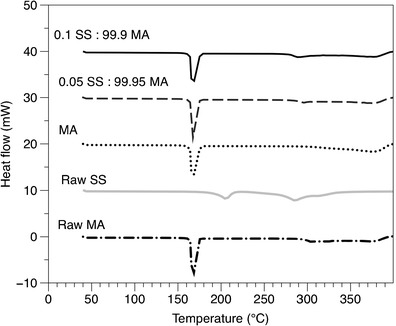
DSC thermograms of raw SS, MA and the spray-dried combined formulations (SS-MA)
These results were further confirmed by TGA (Fig. 3), which evaluated the influence of heat on weight lost. Spray-dried MA displayed a rapid mass loss at approximately 350°C, which could be attributed to the decomposition of the sample. However, the rapid mass loss for the combination formulations was shifted to the left, with the majority lost at approximately 270°C due to the decomposition of SS influencing the decomposition of the MA bulk phase at a lower temperature.
Fig. 3.
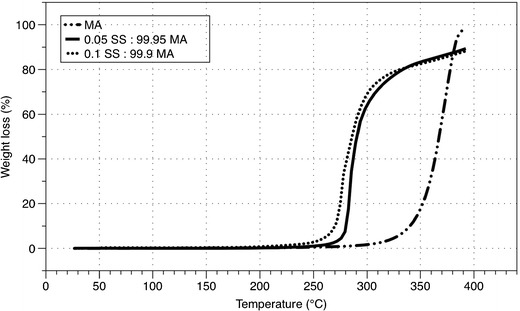
TGA thermogram of the salbutamol sulphate (SS) and mannitol (MA) spray-dried formulations (SS-MA)
Dynamic Vapour Sorption
To further understand the relative stability of the formulations’ solid state, the effect of humidity on moisture sorption was analysed by DVS. The moisture sorption profiles (first sorption ad desorption cycles) for spray-dried mannitol and co-spray-dried formulations are shown in Fig. 4. All the formulations adsorbed approximately 0.3% (w/w) water, between 0 and 90% RH in agreement with previously published results (20,24,25).
Fig. 4.
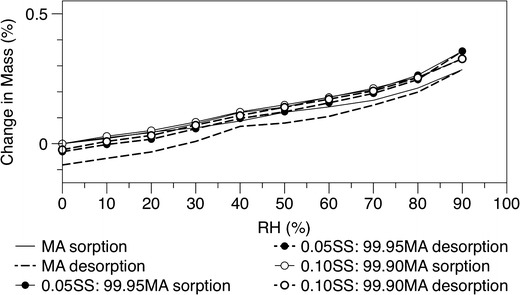
DVS isotherm of the initial single spray-dried MA and co-spray dried SS-MA at the two different concentrations
In Vitro Aerosol Performance Studies
The in vitro aerosolisation efficiency of each formulation were tested using the MSLI and is shown in Fig. 5. Mass deposition of both MA and SS is represented as the percentage of the total drug deposited in the device/capsule and on each stage of the MSLI over the total mass recovery. The fine particle fraction (FPF) was calculated as the cumulative percentage of drug deposited from stage 3 to the filter of the MSLI, representing particles with aerodynamic diameter of <6.8 μm. Although the formulations mostly comprised of MA, the addition of small percentage of SS significantly influenced the deposition of either component on each stage of the MSLI. Additionally, the percentage of the individual component on each stage was not significantly different, suggesting a homogenous composite particle rather than a binary system that contains individual components that will likely result in each component having a different deposition pattern.
Fig. 5.
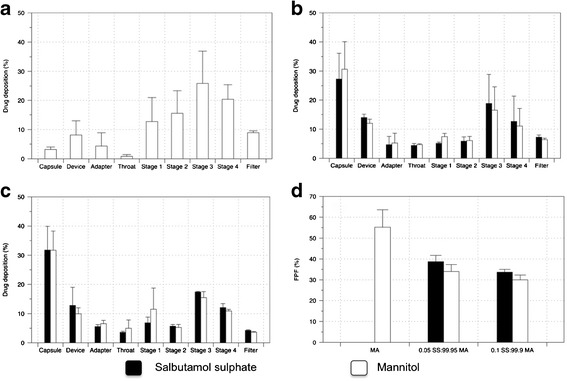
In vitro drug deposition the different formulations using the MSLI and the Aerolizer® DPI device at a 60 L/min flow rate: a spray-dried MA alone, b 0.05/99.95% (w/w) SS/MA, c 0.10/99.90% (w/w) SS/MA and d FPF of the formulations. Data represents the mean ± SD of three independent experiments
Further analysis demonstrated that the spray-dried MA alone had a significantly higher FPF compared to both the co-spray-dried SS-MA formulations. The total emitted dose (the total drug recovered from all stages excluding device and capsules) of MA recovered from the co-spray dried formulations were found to be equivalent at approximately 122 ± 34 mg, whereas the emitted dose of SS was found to be 45.1 ± 7.2 μg and 106 ± 17 μg for 0.05% SS/99.95% MA and 0.1%/99.9% MA (w/w), respectively. However, once emitted from the device the FPF of the emitted dose of all the formulations and each component were not significantly different from each other. This indicates that the co-spray-dried formulations are more cohesive, promoting the adhesion of these particles to the capsules and device. Interestingly, previous studies have demonstrated that SS generally has low aerosolisation efficiency with approximately FPF of 10%, but this was seen to be marginally improved in the presence of mannitol as a carrier (26,27). In this study, however, the co-spray-dried MA-SS as one particle will change the surface chemistry (and consequently the surface energy) and subsequent particle interactions and cohesion forces of the formulations, resulting in an improved aerosol efficiency.
In Vitro Bio-characterisation of Formulations and Cell Interactions
Cell Toxicity Assay
The first step in the development of the co-spray-dried SS-MA formulations as a potential inhaled therapy for patients with mucus hyper-secretion in chronic lung disease was to investigate its toxicity on bronchial cells to better understand the range of concentrations suitable for pulmonary drug delivery. Delivery of these formulations creates a high local concentration upon deposition onto the epithelial cells in the bronchiolar airway regions. Both drugs were shown to be safe and non-toxic, up to a concentration of approximately 1 mM (supplementary materials Fig. 2). Hence, in vitro biological assays were performed within these concentrations to ensure the drugs used were not toxic to the cells.
Transepithelial Transport Studies
The air interface Calu-3 cell model coupled with the TSI helps to simulate the impaction of respirable particles onto the mucosa surfaces of the lung epithelial cells, which by extension will have similar proportion of the particles that will reach the patients lungs for its pharmacological actions. This model has been well established for evaluation of the drug transport and toxicity (28). The transport profiles of SS from the combination formulations across the epithelia cells are shown in Fig. 6, where drug amounts are expressed as the mean cumulative percentage (±SD) of the total recovery. Equivalent amounts of SS were deposited onto the Calu-3 epithelial layer with an average of 0.077 ± 0.018 μg. A lower amount of SS was deposited onto the cells in comparison to the fine particle fraction measured. This could be due to the fact that the drug could either deposit on the cells surface or escape though the openings around the transwell insert placed at the second lower jet of the TSI. Over the 4 h of the experiment, approximately 76% of the drug was transported across the epithelial cells. No significant difference was found between the SS transport profiles of both the combination formulations investigated.
Fig. 6.
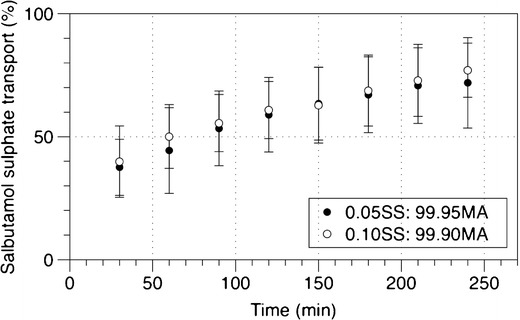
The transport of SS across air interface Calu-3 cells from co-spray-dried formulations. Data represent the mean ± SD of three independent experiments
However, these results were slightly, but significantly enhanced compared to the study conducted by Haghi et al. (21) whereby the transport of salbutamol was around 50% over the same 4-h period. Interestingly, however, the TEER measurements performed after the experiment to measure the integrity of the cell monolayer demonstrated a significant increase (660 ± 85 Ω cm2) compared to control (473 ± 73 Ω cm2) (supplementary materials Fig. 3). Although the enhancement of tight junctions was concurrent with results showed in the study by Ong et al. (17), the increase in drug transport in this study was contrary to the reduced transport of the antibiotic ciprofloxacin following MA deposition found in the previous study by Ong et al. The observed effect could be attributed to the deposition of MA, which raises the osmolarity of the airway surface liquid inducing transepithelial water flux from the cells and subsequent increase in surface liquid volume (29). Hence, given that the process is mainly driven by diffusion, the increase in water content in the mucus could have further facilitated the dissolution of SS into the epithelial surface lining and hence transport across the epithelia cells. This may be beneficial to patients with mucus hyper-secretion to enhance penetration of SS into the tenacious mucus and hence elucidate its pharmacological actions on its target sites, specifically the ciliated epithelial cells and smooth muscles beneath. This study has highlighted the complex interactions between the drug and formulation components to the mucus and/or the presence of different active transport mechanisms in the epithelium that could govern the drug absorption process across the lung epithelium.
Ciliary Activity
The effect of SS, MA and the combination of both drugs on ciliary activity was evaluated using primary nasal epithelial cells with functioning cilia. These cells were utilised because of the sampling collection easiness of nasal brushing, unlike the more invasive and complex bronchoscopy or lung explants. Furthermore, previous studies (30–32) have used nasal epithelial cells as a substitute or surrogate to bronchial epithelial cells and have shown to have identical morphologies with similar expression of receptors and responses to cytokine stimulation. Analyses of the nasal CBF in response to the different concentrations of drugs are shown in Fig. 7. Salbutamol sulphate was shown to significantly increase CBF compared to baseline (control: 6.7 ± 1.3 Hz) from a concentration of 10−7 M onwards, but no statistical differences were found between the concentrations. Interestingly, similar enhancement of CBF was also observed when the cells were exposed to MA within the range of concentrations studied. This is in good agreement with previous studies by Devalia et al. and Yaghi et al. (18,33), which also found a significant enhancement of CBF following SS and MA exposure to bronchial epithelial cells. The increase in the CBF by both drugs may be mediated via the β2 adrenergic pathway, leading to the release in cAMP and increase in intracellular calcium in response to hyperosmolar stimuli, respectively (34). Although CBF was augmented when the drugs were given in combination, no significant difference was found between the treatment groups (i.e. combination and individual drugs). As both MA and SS increase CBF when administered separately, it is expected that an augmented response can be achieved when administered in combination. Consequently, there is evidence to suggest that the ciliary activity had reached its maximal response. It should be highlighted however that the method of obtaining ciliated epithelium in this study involved placing small pieces of sampled nasal tissues in a large volume of nutrient medium. This would greatly dilute the mucus components and other factors that could have an influence on mucociliary function, and more so in patients with cystic fibrosis and COPD. Further studies to establish an in vitro air-interface nasal epithelial cell model with functional cilia and mucus production are currently in progress. Nevertheless, nasal CBF has been shown to be a key-determining factor in mucociliary clearance (32,35,36).
Fig. 7.
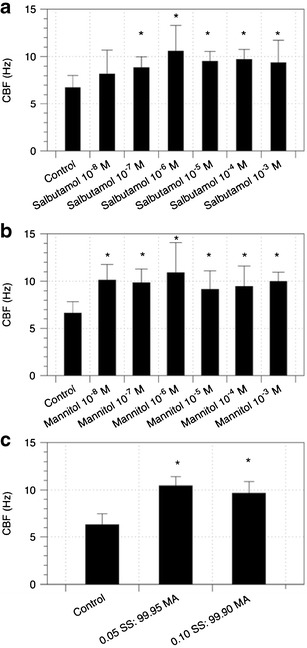
Effects on ciliary beat frequency (CBF) of primary nasal epithelial cells after exposure to different concentrations of a SS, b MA and c combination formulations (SS 0.5 × 10-6 M, SS 1 × 10-6 M in combination with MA 10−3 M) compared to baseline (control). Data represent the mean ± SD of at least five separate determinations. *p < 0.05, statistically different to control
Single Cell Smooth Muscle Contraction Assay
It has been well established that MA could cause bronchoconstriction in individuals with airway hyper-responsiveness and hence have been used as a tool for diagnosis of asthma through measurement of this airway hyper-responsiveness (37,38). To determine the effectiveness of the combination formulation to prevent this, single-cell contraction assays was used to study the pharmacological responses of the individual and combined treatments on human bronchial smooth muscle cells. Figure 8 shows representative photomicrographs of human bronchial smooth muscle cells before and after exposure to SS, MA, both drugs and histamine that acts as a positive control. Quantification of the cross-sectional area at the different time points is presented in Fig. 9. The cells had to be partially detached from the plate for contraction to occur, where contraction will cause further detachment and shrinkage in cell surface area. However, the contracted cells do not undergo significant reversible relaxation. Subsequently, the effect of the drugs to inhibit cell contraction rather than induce cell relaxation was determined (39). Although relatively simple and convenient, this method has been well established to study functional changes of muscle cells (39–43).
Fig. 8.
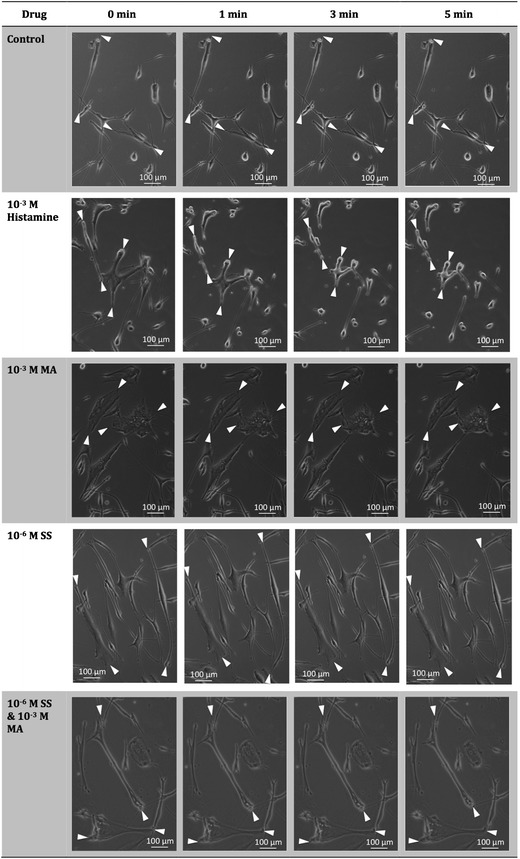
Representative photomicrographs of normal human bronchial smooth muscle cells before (0 min) and after (1, 3 and 5 min) exposure to histamine, MA, SS or both MA and SS. The decrease in relative cross-sectional area was interpreted as contraction of smooth muscle cells
Fig. 9.
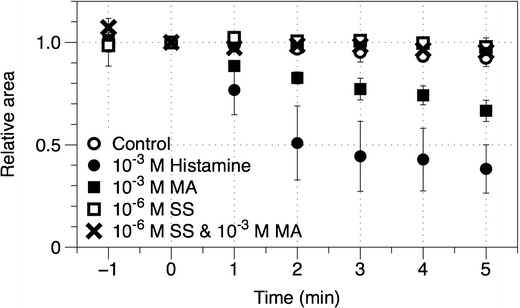
Time course profiles of the effects of various drugs and drug combination on bronchial smooth muscle contraction. Drugs were added at time 0. Each data point represents mean ± SD of at least three separate determinations
Experimental control with no drugs showed a negligible change in surface areas from baseline. Histamine on the other hand, caused the cells to contract rapidly to approximately 20–30% of their original size after only 1 min of exposure, indicating its ability to contract in response to triggers. Drug concentrations used in this study were established based on the toxicity studies, ciliary activity and the combination therapy formulated. The exposure of the smooth muscle cells to MA showed a significant contraction but to a lower extent compared to histamine. This could be attributed to the cells being obtained from healthy individual, whereby hyper-responsive patients have been shown to have more contractile airway smooth muscle cells in response to stimuli (40,43). Salbutamol sulphate, on the other hand, did not induce any significant smooth muscle contraction, which is expected as it is used for relieving bronchoconstriction. Interestingly, exposure to both SS and MA in combination also did not show a significant change in contraction, validating the protective effects of SS towards MA challenge.
Intracellular Cyclic Adenosine Monophosphate Assay
To further confirm the previous findings, intracellular cAMP was quantified and compared to basal levels (unstimulated), whereby the formation of this second messenger could indirectly attenuate cell contraction and also increase CBF (44). The quantified cAMP levels within the bronchial smooth muscle cells are shown in Fig. 10. Exposure to MA showed no significant difference to baseline values, whereas exposure to SS and the combination drugs demonstrated a similar stimulation of cAMP by adenylyl cyclase. Hence, it is clear in this study that the increase in cAMP levels is mainly responsible for the inhibition of smooth muscle contraction in response to MA. It is also possible that the increase in CBF of ciliated nasal epithelial cells after SS exposure could be modulated through the increase in this cAMP level (45). It is envisaged that these findings coupled with the enhanced SS penetration across the complex mucus layer and epithelia cells towards the smooth muscles could improve efficacy and hence may enhance health-care outcomes in patients with mucus hyper-secretion.
Fig. 10.
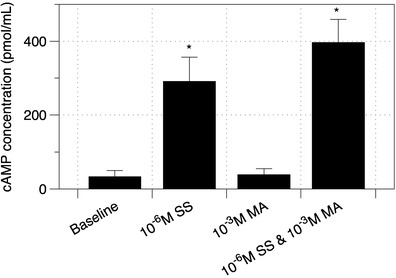
Assay of intracellular cAMP after 10 min exposure to SS, MA and combination of the drugs on bronchial smooth muscle cells. Data represent the mean ± SD of at least five separate determinations. *p < 0.05, statistically different to baseline
CONCLUSIONS
Inhalable dry powder combination formulations of mannitol and salbutamol sulphate were successfully produced and characterised in vitro. This formulation approach has proven to enhance penetration of salbutamol sulphate across the Calu-3 epithelial cells, showed increased ciliated beat frequency in vitro and, more importantly, displayed the ability to simultaneously prevent smooth muscle contraction associated with mannitol administration. Future work should focus on the correlation of these in vitro finding to in vivo and also the development of an air-interface nasal epithelium with functional cilia and mucus production for pre-clinical evaluation of inhaled formulations. Therefore, this formulation has the potential to provide a promising dry powder therapy for the treatment of hyper-mucus production for pulmonary diseases whilst simultaneously prevent broncho-constriction and improve patient’s compliance.
Electronic supplementary material
Spray drying conditions for single mannitol (MA) and co-spray-dried mannitol/salbutamol sulphate (SS). (DOCX 51 kb)
Particle size distribution of single spray-dried mannitol (MA) and co-spray-dried salbutamol sulphate with mannitol (SS/MA) measured using laser diffraction. Data represents the mean ± SD of at least three replicate experiments. (JPEG 214 kb)
The effect of SS and MA on Calu-3 cells viability after 72-h drug exposure. The data represented the mean ± SD of at least three separate determinations. (JPEG 357 kb)
Transepithelial electrical resistance (TEER) of Calu-3 cells after transport experiments. Data represents the mean ± SD of at least three separate determinations. (JPEG 167 kb)
Acknowledgements
A/Professor Traini is the recipient of an Australian Research Council Future Fellowship (project number FT12010063). A/Professor Young is the recipient of an Australian Research Council Future Fellowship (project number FT110100996). This project was supported by ARC-Linkage grant no. LP100100451. The authors would also like to acknowledge Lynn Moir for her expert advice on the smooth muscle contraction studies.
REFERENCES
- 1.Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 2.Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007;19(1):83. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 3.Barker AF, Couch L, Fiel SB, Gotfried MH, Ilowite J, Meyer KC, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162(2):481–485. doi: 10.1164/ajrccm.162.2.9910086. [DOI] [PubMed] [Google Scholar]
- 4.Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54(5):658–670. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Tsifansky MD, Shin S, Lin Q, Yeo Y. Mannitol-guided delivery of ciprofloxacin in artificial cystic fibrosis mucus model. Biotechnol Bioeng. 2011;108(6):1441–1449. doi: 10.1002/bit.23046. [DOI] [PubMed] [Google Scholar]
- 6.Anderson P. Emerging therapies in cystic fibrosis. Ther Adv Respir Dis. 2010;4(3):177–185. doi: 10.1177/1753465810371107. [DOI] [PubMed] [Google Scholar]
- 7.Wood AJJ, Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335(3):179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 8.Brannan JD, Anderson SD, Perry CP, Freed-Martens R, Lassig AR, Charlton B. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4.5%) saline. Respir Res. 2005;6(1):144. doi: 10.1186/1465-9921-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson S, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J. 2001;14(3):678–685. doi: 10.1034/j.1399-3003.1999.14c30.x. [DOI] [PubMed] [Google Scholar]
- 10.Hasani A, Toms N, O’Connor J, Dilworth J, Agnew J. Effect of salmeterol xinafoate on lung mucociliary clearance in patients with asthma. Respir Med. 2003;97(6):667–671. doi: 10.1053/rmed.2003.1498. [DOI] [PubMed] [Google Scholar]
- 11.Fazio F, Lafortuna C. Effect of inhaled salbutamol on mucociliary clearance in patients with chronic bronchitis. Chest. 1981;80(6 Suppl):827–830. doi: 10.1378/chest.80.6.827. [DOI] [PubMed] [Google Scholar]
- 12.Bennett WD. Effect of β-adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol. 2002;110(6, Supplement):S291–S297. doi: 10.1067/mai.2002.129704. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen J, Hansen A, Falk M, Nielsen I, Groth S. Reduced effect of inhaled beta 2-adrenergic agonists on lung mucociliary clearance in patients with cystic fibrosis. CHEST J. 1993;103(3):805–811. doi: 10.1378/chest.103.3.805. [DOI] [PubMed] [Google Scholar]
- 14.Robinson M, Bye PTB. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33(4):293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 15.Ong HX, Traini D, Bebawy M, Young PM. Epithelial profiling of antibiotic controlled release respiratory formulations. Pharm Res. 2011;28(9):2327–2338. doi: 10.1007/s11095-011-0462-1. [DOI] [PubMed] [Google Scholar]
- 16.Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev Ind Pharm. 2010;36(10):1207–1214. doi: 10.3109/03639041003695113. [DOI] [PubMed] [Google Scholar]
- 17.Ong HX, Traini D, Salama R, Anderson SD, Daviskas E, Young PM. The effects of mannitol on the transport of ciprofloxacin across respiratory epithelia. Mol Pharm. 2013;10(8):2915–2924. doi: 10.1021/mp400030n. [DOI] [PubMed] [Google Scholar]
- 18.Devalia J, Sapsford R, Rusznak C, Toumbis M, Davies R. The effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitro. Pulm Pharmacol. 1992;5(4):257–263. doi: 10.1016/0952-0600(92)90068-R. [DOI] [PubMed] [Google Scholar]
- 19.Labiris N, Dolovich M. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Phamacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adi H, Young PM, Chan H-K, Agus H, Traini D. Co-spray-dried mannitol–ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. Eur J Pharm Sci. 2010;40(3):239–247. doi: 10.1016/j.ejps.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Haghi M, Traini D, Bebawy M, Young PM. Deposition, diffusion and transport mechanism of dry powder microparticulate salbutamol, at the respiratory epithelia. Mol Pharm. 2012;9(6):1717–1726. doi: 10.1021/mp200620m. [DOI] [PubMed] [Google Scholar]
- 22.Palacio M, Cuffini S, Badini R, Karlsson A, Palacios S. Solid-state characterization of two polymorphic forms of R-albuterol sulfate. J Pharm Biomed Anal. 2007;43(4):1531–1534. doi: 10.1016/j.jpba.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Shariare M, de Matas M, York P. Effect of crystallisation conditions and feedstock morphology on the aerosolization performance of micronised salbutamol sulphate. Int J Pharm. 2011;415(1):62–72. doi: 10.1016/j.ijpharm.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Begat P, Young PM, Edge S, Kaerger JS, Price R. The effect of mechanical processing on surface stability of pharmaceutical powders: visualization by atomic force microscopy. J Pharm Sci. 2003;92(3):611–620. doi: 10.1002/jps.10320. [DOI] [PubMed] [Google Scholar]
- 25.Heng D, Lee SH, Kwek JW, Ng WK, Chan H-K, Tan RB. Assessing the combinatorial influence of climate, formulation and device on powder aerosolization using the Taguchi experimental design. Powder Technol. 2012;226:253–260. doi: 10.1016/j.powtec.2012.04.056. [DOI] [Google Scholar]
- 26.Tee S, Marriott C, Zeng X, Martin G. The use of different sugars as fine and coarse carriers for aerosolised salbutamol sulphate. Int J Pharm. 2000;208(1):111–123. doi: 10.1016/S0378-5173(00)00553-6. [DOI] [PubMed] [Google Scholar]
- 27.Young PM, Edge S, Traini D, Jones MD, Price R, El-Sabawi D, et al. The influence of dose on the performance of dry powder inhalation systems. Int J Pharm. 2005;296(1):26–33. doi: 10.1016/j.ijpharm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Ong HX, Traini D, Young PM. Pharmaceutical applications of the Calu-3 lung epithelia cell line. Expert Opin Drug Deliv. 2013;0(0):1–16. doi: 10.1517/17425247.2013.805743. [DOI] [PubMed] [Google Scholar]
- 29.Hirsh AJ. Altering airway surface liquid volume: inhalation therapy with amiloride and hyperosmotic agents. Adv Drug Deliv Rev. 2002;54(11):1445–1462. doi: 10.1016/S0169-409X(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 30.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39(5):560. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutland J, Cole PJ. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981;36(9):654–658. doi: 10.1136/thx.36.9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen I, Camner P, Jensen PL, Philipson K, Proctor DF. A comparison of nasal and tracheobronchial clearance. Arch Environ Health. 1974;29(5):290–293. doi: 10.1080/00039896.1974.10666589. [DOI] [PubMed] [Google Scholar]
- 33.Yaghi A, Zaman A, Dolovich MB. The direct effect of hyperosmolar agents on ciliary beating of human bronchial epithelial cells. J Aerosol Med Pulm Drug Deliv. 2012;25(2):88–95. doi: 10.1089/jamp.2011.0914. [DOI] [PubMed] [Google Scholar]
- 34.Daviskas E, Anderson SD, Eberl S, Chan HK, Young IH, Seale JP. Effects of terbutaline in combination with mannitol on mucociliary clearance. The Eur Respir J. 2002;20(6):1423–1429. doi: 10.1183/09031936.02.00301502. [DOI] [PubMed] [Google Scholar]
- 35.Duchateau GS, Merkus FW, Zuidema J, Graamans K. Correlation between nasal ciliary beat frequency and mucus transport rate in volunteers. Laryngoscope. 1985;95(7):854–859. doi: 10.1288/00005537-198507000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Boek WM, Graamans K, Natzijl H, van Rijk PP, Huizing EH. Nasal mucociliary transport: new evidence for a key role of ciliary beat frequency. Laryngoscope. 2002;112(3):570–573. doi: 10.1097/00005537-200203000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Anderson SD, Brannan J, Spring J, Spalding N, Rodwell LT, Chan K, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. 1997;156(3):758–765. doi: 10.1164/ajrccm.156.3.9701113. [DOI] [PubMed] [Google Scholar]
- 38.Leuppi JD, Brannan JD, Anderson SD. Bronchial provocation tests: the rationale for using inhaled mannitol as a test for airway hyperresponsiveness. Swiss Med Wkly. 2002;132(13/14):151–158. doi: 10.4414/smw.2002.09850. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto N, Zhao C, Shichi H. The effects of prostaglandins E2 and F2α on porcine ciliary muscle cells in culture. Curr Eye Res. 1995;14(12):1155–1163. doi: 10.3109/02713689508995822. [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Cheng Z, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 41.Pang I-H, Shade DL, Tamm E, DeSantis L. Single-cell contraction assay for human ciliary muscle cells. Effect of carbachol. Invest Ophthalmol Vis Sci. 1993;34(5):1876–1879. [PubMed] [Google Scholar]
- 42.Oldenburg PJ, Wyatt TA, Sisson JH. Ethanol attenuates contraction of primary cultured rat airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;43(5):539–545. doi: 10.1165/rcmb.2009-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govindaraju V, Michoud M-C, Ferraro P, Arkinson J, Safka K, Valderrama-Carvajal H, et al. The effects of interleukin-8 on airway smooth muscle contraction in cystic fibrosis. Respir Res. 2008;9(1):76. doi: 10.1186/1465-9921-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. The J Clin Investig. 1994;93(1):371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salathe M. Effects of β-agonists on airway epithelial cells. J Allergy Clin Immunol. 2002;110(6):S275–S281. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spray drying conditions for single mannitol (MA) and co-spray-dried mannitol/salbutamol sulphate (SS). (DOCX 51 kb)
Particle size distribution of single spray-dried mannitol (MA) and co-spray-dried salbutamol sulphate with mannitol (SS/MA) measured using laser diffraction. Data represents the mean ± SD of at least three replicate experiments. (JPEG 214 kb)
The effect of SS and MA on Calu-3 cells viability after 72-h drug exposure. The data represented the mean ± SD of at least three separate determinations. (JPEG 357 kb)
Transepithelial electrical resistance (TEER) of Calu-3 cells after transport experiments. Data represents the mean ± SD of at least three separate determinations. (JPEG 167 kb)


