Abstract
Purpose
To compare the frequency and the spectrum of karyotype abnormality in the first trimester miscarriages in women aged under and over 35 years, who conceived naturally (NC) and who conceived through in vitro fertilization (IVF).
Methods
Comparative analysis of cytogenetic data obtained by karyotyping of miscarriages in patients who conceived naturally, and who conceived through IVF. Patients were subcategorized by their age: <35 years (NC, n = 173; IVF, n = 108) and ≥35 years (NC, n = 107; IVF, n = 111).
Results
A total of 499 miscarriage karyotypes was analyzed. The spectrum and the relative proportions of different cytogenetic categories in karyotypically abnormal miscarriages differed neither between the NC and IVF patients aged <35 years, nor between the NC and IVF patients aged ≥35 years. In the patients aged <35 years, the incidence of abnormal miscarriage karyotype was lower in the IVF group (37.04 % vs 62.43 %). In the patients aged ≥35 years, the incidence of miscarriages with cytogenetic pathology did not differ between the NC and the IVF group (75.70 % vs 58.56 %). The lowest frequency of karyotypically abnormal miscarriages (29.82 %) was detected in the young IVF-treated patients at <7 weeks of gestation.
Conclusions
IVF does not increase the risk of a pregnancy loss because of abnormal embryonic karyotype, nor does it increase the preponderance for any specific type of cytogenetic abnormality in both patients aged under and over 35 years. In young IVF-treated women early pregnancy loss is generally caused by non-cytogenetic factors. Identification of a cytogenetically normal spontaneous abortion is clinically significant and reinforces the importance of developing an appropriate diagnosis and treatment strategies for IVF patients in order to reduce the risk of euploid pregnancy loss.
Keywords: Pregnancy loss, Miscarriage karyotype, Chromosomal abnormalities, Maternal age, Natural conception, Assisted reproduction technology, In vitro fertilization
Introduction
Up to 15 % of all recognized pregnancies end in spontaneous abortions, making miscarriage a significant problem in obstetrics and gynecology [1]. It affects both naturally conceived pregnancies and pregnancies conceived through assisted reproduction technology (ART). Numerous reasons for pregnancy loss are discussed. These include infections and immunological pathologies, endocrinological, epigenetic and genetic changes [2–5]. Cytogenetic abnormalities are a significant factor in pregnancy wastage. Numerical and structural chromosomal aberrations are detected in up to 70 % of miscarriages [5–8]. Maternal age has a significant impact on the embryonic aneuploidy rate, which is especially pronounced in women older than 35 years [9–12]. Whether the way of conception—natural or ART—affects the rate of abnormal embryonic karyotype is still discussed [13–16].
In the present study, we address the question whether a conception through standard in vitro fertilization (IVF) affects the rate and the spectrum of miscarriage karyotype abnormality in women aged < and ≥35 years. A detailed comparison of miscarriage karyotype between IVF-treated women and those who conceived naturally at a different age could provide a valuable information for further improvement of treatment strategies and pregnancy monitoring, which has particular importance for IVF patients.
Materials and methods
Studied groups
Study was performed on two groups of patients with pregnancy loss at 4–12 weeks of gestation (w.g.): those who conceived naturally (NC group, n = 280) and those who conceived through standard IVF (IVF group, n = 219). Patients who conceived through intrauterine and intracervical insemination or through intracytoplasmic sperm injection with partner or donor semen were not enrolled in the study. The patients’ age in the studied groups ranged from 22 to 45 years.
The diagnosis of missed abortion was based on standard ultrasound between 4 and 12 w.g. Both NC and IVF patients had the initial ultrasound at 4–5 w.g. to visualize the gestational sac and exclude ectopic implantation and/or at 5–6 w.g. to visualize the cardiac activity of the embryo. The first trimester ultrasound was performed at 11–14 w.g.. In the period between the initial and the first trimester ultrasound, an additional US-examination was performed in the event of the patient’s complaints of abdominal pain and/or vaginal bleeding. In our practice, all the patients diagnosed with missed abortion were offered dilation and curettage (D&C) with karyotyping of the chorionic villi obtained. D&Cs were performed within 48 h after the diagnosis of missed abortion.
To evaluate the impact of age on the rate and the spectrum of miscarriage karyotype abnormality, patients in both groups were subcategorized by their age: <35 years (NC group, n = 173; IVF group, n = 108) and ≥35 years (NC group, n = 107; IVF group, n = 111). The date of the last menstrual period and US measurements were used for the calculation of the gestational age. All the patients’ data were collected from medical case records.
Specimen preparation and karyotyping
Products of conception (POCs) were obtained by curettage of uterine cavity in different clinics of St. Petersburg in the period between February 2007 and May 2013. The POCs were placed in 0.9 % NaCl and sent for karyotyping to the Laboratory for Prenatal Diagnosis of Human Inborn and Inherited Diseases of D.O. Ott Research Institute of Obstetrics and Gynecology. Chorionic villi were selected and released from the maternal decidua and blood clots under the Leica M125 stereomicroscope.
Metaphase chromosomes were prepared from the chorionic villi samples by the direct technique [17] with modifications , which allows rapid analysis and eliminates the risk of contamination with maternal cells. The chorionic villi were divided into small pieces and placed into vials (5–10 mg per vial) in 5 ml of hypotonic solution (0.9 % sodium citrate) supplemented with colchicines at the final concentration 2.5 μg/ml. After 60–80 min of incubation at room temperature, 2.5 ml of hypotonic solution was replaced with 2.5 ml of freshly prepared fixative (ethanol : glacial acetic acid, 3:1). After 25–35 min of incubation at room temperature, all the solution in vials was replaced with cold fixative (ethanol : glacial acetic acid, 3:1). The chorionic villi were fixed at 4 °C for at least 90 min. Then approximately 2.5 ml of room-temperature distilled water was added into the vials. 2–5 min later, the chorionic villi were extracted from the vials and dried on filter paper. They were put on prewarmed slides into drops of freshly prepared 60 % acetic acid and macerated for 30–90 s. Saturation of acetic acid with cells was controlled under the Leica M125 stereomicroscope. Then the chorionic villi were discarded from the slides with forceps. Drops of 60 % acetic acid saturated with cells from chorionic villi were spread on the slides, fixed with 2–3 drops of freshly prepared fixative (ethanol : glacial acetic acid, 3:1) and air-dried for at least 12 h.
The rate and the spectrum of miscarriage karyotype abnormality were determined by chorionic villi karyotyping, performed on QFH/AcD-stained metaphases. From 5 to 11 metaphases per person were analyzed at a 400-band level.
Statistical analysis
Statistical analysis was performed using STATISTICA 8 and GraphPad InStat Demo. Categorical variables (the number of karyotypically abnormal miscarriages in the studied groups, the number of miscarriages with a certain cytogenetic abnormality) were compared using the chi-square test; the null hypothesis was rejected if p < 0.001. Inter-group comparison of the patients’ age was carried out using the Mann–Whitney test.
IRB approval and ethical issue
The study was approved by the Institutional Review Board of D.O. Ott Research Institute of Obstetrics and Gynecology. All the personal data, including medical history data, was processed with the written informed consent of the patients. The study was performed in accordance with the Declaration of Helsinki.
Results
The available data included the analysis of 499 miscarriages among patients aged < and ≥35 years, those who conceived through IVF and those with a spontaneous conception. We checked for age-matching subgroups of patients aged <35 and ≥35 years from the NC and the IVF group. The Mann–Whitney test did not show any statistically significant difference, either between the subgroups of patients aged <35 years (p = 0.09), or between the subgroups of patients aged ≥35 years (p = 0.46).
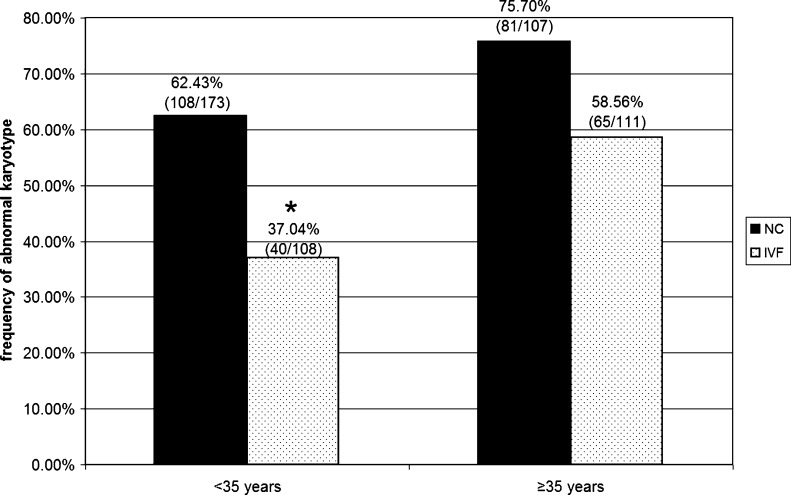
When comparing the incidence of miscarriage karyotype abnormality between the patients aged <35 years, we revealed that 108 of 173 pregnancy losses (62.43 %) in the NC group had an abnormality compared with 40 of 108 pregnancy losses (37.04 %) in the IVF group (Fig. 1). The incidence of abnormal karyotype was significantly lower in the IVF group (p < 0.001). In contrast, among the patients aged ≥35 years, an abnormal embryonic karyotype was registered in 81 of 107 pregnancy losses (75.70 %) in the NC group and in 65 of 111 pregnancy losses (58.56 %) in the IVF group (Fig. 1). The chi-square test did not show statistically significant difference between these rates. Thus, a lower incidence of abnormal miscarriage karyotype was detected in IVF patients aged <35 years in contrast to their counterparts who conceived naturally and patients aged ≥35 years.
Fig. 1.
The incidence of abnormal karyotype in miscarriages of patients aged < and ≥35 years who conceived naturally (NC) and who conceived through in vitro fertilization (IVF). *The incidence of cytogenetically abnormal miscarriages is significantly lower in IVF patients aged <35 years than in their NC counterparts (p < 0.001)
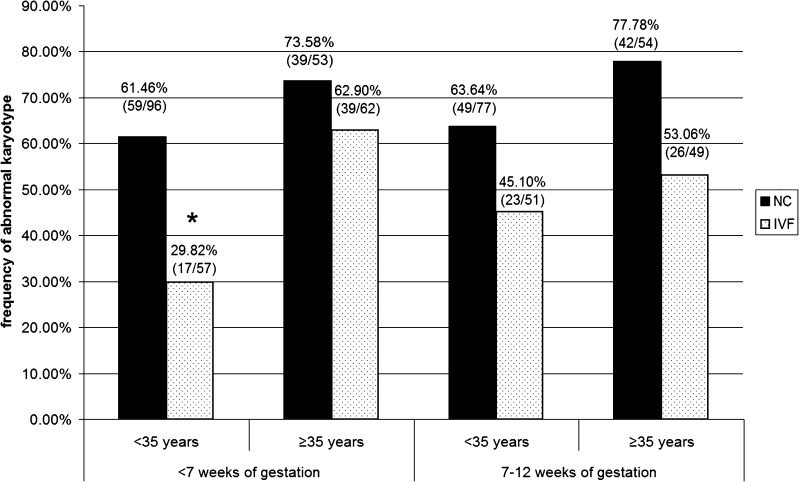
To evaluate the impact of gestational age on the rate of embryonic karyotype abnormality, we calculated frequencies of miscarriages with abnormal karyotype at <7 and 7–12 w.g. (Fig. 2). At <7 w.g. in the <35 years subgroups, 59 of 96 miscarriages (61.46 %) in the NC group and 17 of 57 miscarriages (29.82 %) in the IVF group had cytogenetic abnormalities, while in the ≥35 years subgroups, 39 of 53 miscarriages (73.58 %) in the NC group and 39 of 62 miscarriages (62.9 %) in the IVF group were cytogenetically abnormal. At 7–12 w.g. in the <35 years subgroups, 49 of 77 miscarriages (63.64 %) in the NC group and 23 of 51 miscarriages (45.1 %) in the IVF group had karyotype abnormalities; in the ≥35 years subgroups cytogenetic abnormalities were detected in 42 of 54 miscarriages (77.78 %) in the NC group and in 26 of 49 miscarriages (53.06 %) in the IVF group (Fig. 2). Difference in the frequency of abnormal miscarriage karyotype between the NC and the IVF groups achieved statistical significance only for the patients aged <35 years at <7 w.g. (p < 0.001). Thus, in young IVF-treated women the incidence of abnormal miscarriage karyotype at <7 w.g. was lower than in their NC counterparts.
Fig. 2.
The incidence of abnormal karyotype in miscarriages at <7 and 7–12 weeks of gestation in the age subgroups of patients who conceived naturally (NC), and who conceived through in vitro fertilization (IVF). *The incidence of cytogenetically abnormal miscarriages at <7 w.g. is significantly lower in IVF-treated women aged <35 years than in their NC counterparts (p < 0.001)
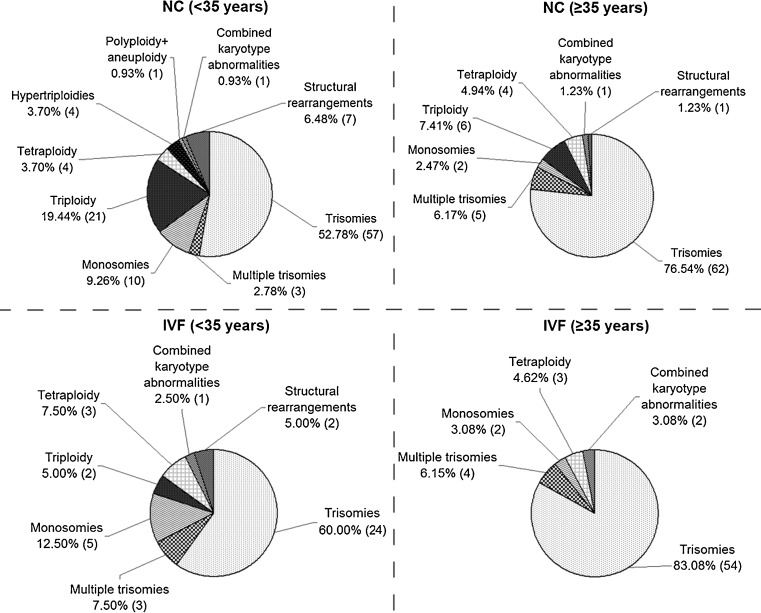
Then we analyzed the spectrum and the frequency of miscarriage karyotype abnormalities in the age subgroups (Fig. 3). The cytogenetic analysis results are summarized in the Table 1. The most prevalent abnormalities were trisomies: 57 out of 108 (52.78 %) in the NC and 24 out of 40 (60 %) in the IVF patients aged <35 years; 62 out of 81 (76.54 %) in the NC and 54 out of 65 (83.08 %) in the IVF patients aged ≥35 years. Monosomies were detected in 10 out of 108 miscarriages (9.26 %) in the NC and in 5 out of 40 miscarriages (12.5 %) in the IVF patients aged <35 years; in 2 out of 81 miscarriages (2.47 %) in the NC and in 2 out of 65 miscarriages (3.08 %) in the IVF patients aged ≥35 years. Other karyotype abnormalities, including multiple trisomies, tetraploidy, structural chromosomal rearrangements and combined pathology (structural + numerical abnormalities) were registered in the NC and the IVF age subgroups with low frequencies. When comparing frequencies of all detected cytogenetic abnormalities between the NC and the IVF age subgroups, a statistically significant difference was not registered. Triploidy was more typical for the NC age subgroups: 21 out of 108 (19.44 %) and 6 out of 81 (7.41 %) compared to 2 out of 40 (5 %) and 0 out of 65 (0 %) in the IVF patients aged <35 years and ≥35 years, respectively. However, difference in the triploidy rate between the NC and the IVF age subgroups did not achieve statistical significance. Thus, the spectrum and the relative proportions of miscarriage karyotype pathology differed neither between the NC and the IVF patients aged <35 years, nor between the NC and the IVF patients aged ≥35 years.
Fig. 3.
The relative proportions of different cytogenetic categories in karyotypically abnormal miscarriages of patients aged < and ≥35 years who conceived naturally (NC) and who conceived through in vitro fertilization (IVF)
Table 1.
Cytogenetic data of miscarriages in patients aged < or ≥35 years who conceived naturally (NC) and who conceived through IVF
| Miscarriage karyotype | <35 years | ≥35 years | |||
|---|---|---|---|---|---|
| Number of cases (frequency, %) | Number of cases (frequency, %) | ||||
| Group | Group | ||||
| NC | IVF | NC | IVF | ||
| Normal | 46,XX | 27 (15.61 %) | 39 (36.11 %) | 16 (14.95 %) | 24 (21.62 %) |
| 46,XY | 38 (21.96 %) | 29 (26.85 %) | 10 (9.35 %) | 22 (19.82 %) | |
| Abnormal | 108 (62.43 %) | 40 (37.04 %) | 81 (75.70 %) | 65 (58.56 %) | |
| Trisomies | Trisomy 1 | – | – | – | – |
| Trisomy 2 | 2 (1.16 %) | – | 1 (0.93 %) | 3 (2.71 %) | |
| Trisomy 3 | 2 (1.16 %) | – | 1 (0.93 %) | – | |
| Trisomy 4 | 3 (1.73 %) | – | 1 (0.93 %) | – | |
| Trisomy 5 | – | 1 (0.93 %) | – | – | |
| Trisomy 6 | 1 (0.58 %) | 1 (0.93 %) | – | 2 (1.80 %) | |
| Trisomy 7 | 3 (1.73 %) | 1 (0.93 %) | 1 (0.93 %) | 1 (0.90 %) | |
| Trisomy 8 | 2 (1.16 %) | – | 2 (1.87 %) | 2 (1.80 %) | |
| Trisomy 9 | 1 (0.58 %) | 1 (0.93 %) | 2 (1.87 %) | – | |
| Trisomy 10 | 1 (0.58 %) | 1 (0.93 %) | 2 (1.87 %) | 1 (0.90 %) | |
| Trisomy 11 | 1 (0.58 %) | 1 (0.93 %) | – | – | |
| Trisomy 12 | – | 1 (0.93 %) | 4 (3.74 %) | 2 (1.80 %) | |
| Trisomy 13 | 4 (2.31 %) | 3 (2.77 %) | 4 (3.74 %) | 2 (1.80 %) | |
| Trisomy 14 | 2 (1.16 %) | – | 1 (0.93 %) | 1 (0.90 %) | |
| Trisomy 15 | 3 (1.73 %) | 2 (1.85 %) | 8 (7.48 %) | 10 (9.01 %) | |
| Trisomy 16 | 21 (12.13 %) | 7 (6.48 %) | 15 (14.02 %) | 12 (10.81 %) | |
| Trisomy 17 | – | – | – | 1 (0.90 %) | |
| Trisomy 18 | 2 (1.16 %) | – | 1 (0.93 %) | 3 (2.71 %) | |
| Trisomy 19 | – | – | – | – | |
| Trisomy 20 | 2 (1.16 %) | – | 1 (0.93 %) | 2 (1.80 %) | |
| Trisomy 21 | 4 (2.31 %) | 1 (0.93 %) | 5 (4.68 %) | 2 (1.80 %) | |
| Trisomy 22 | 2 (1.16 %) | 3 (2.77 %) | 13 (12.15 %) | 9 (8.11 %) | |
| XXY | 1 (0.58 %) | 1 (0.93 %) | – | 1 (0.90 %) | |
| Multiple trisomies | 3 (1.73 %) | 3 (2.77 %) | 5 (4.68 %) | 4 (3.61 %) | |
| Monosomies | Monosomy X | 9 (5.19 %) | 4 (3.70 %) | 2 (1.87 %) | 1 (0.90 %) |
| Monosomy 21 | 1 (0.58 %) | 1 (0.93 %) | – | 1 (0.90 %) | |
| Polyploidies | Triploidy | 21 (12.13 %) | 2 (1.85 %) | 6 (5.62 %) | – |
| Hypertriploidy | 4 (2.31 %) | – | – | – | |
| Tetraploidy | 2 (1.16 %) | 3 (2.77 %) | 4 (3.74 %) | 2 (1.80 %) | |
| Hypertetraploidy | 2 (1.16 %) | – | – | 1 (0.90 %) | |
| Polyploidy + aneuploidy | 1 (0.58 %) | – | – | – | |
| Combined karyotype abnormalities | Monosomies combined with trisomies | – | – | – | 1 (0.90 %) |
| Сhromosomal rearrangements combined with genomic mutations | 1 (0.58 %) | 1 (0.93 %) | 1 (0.93 %) | 1 (0.90 %) | |
| Structural | Chromosomal rearrangements | 5 (2.89 %) | 2 (1.85 %) | 1 (0.93 %) | – |
| Marker chromosomes | 2 (1.16 %) | – | – | – | |
| Total | 173 | 108 | 107 | 111 | |
Discussion
Pregnancy loss is the most common complication of human reproduction, with ~15 % of recognized pregnancies resulting in spontaneous abortion [1]. The majority of pregnancy failures are associated with cytogenetic abnormalities, with over 50 % of early miscarriages exhibiting abnormal karyotypes [18]. Karyotype abnormality in pregnancy losses arises from errors in gametogenesis, fertilization and cleavage divisions. The risk of trisomy resulting from nondisjunctional events rises dramatically for women aged ≥35 years [19]. Thus, it could be expected that ART, including IVF, has an effect on the spectrum and the overall rate of miscarriage karyotype abnormality, especially in women older than 35 years.
In this study we have not found any difference in the spectrum of miscarriage karyotype abnormality between women who conceived through IVF and those who conceived naturally. In both IVF and NC groups the spectrum included nondisjunctional events: monosomies, trisomies, and multiple trisomies; errors in fertilization: triploidy; errors in the first mitotic cleavage division: tetraploidy; structural rearrangements and combined pathology (structural + numerical abnormalities). The relative proportions of different cytogenetic abnormality types did not differ statistically between the NC and the IVF patients. However, a notable observation was a low incidence or absence of triploid miscarriages in the IVF patients, which can be due to the artificial negative selection of the triploid zygotes at the pronuclear stage in IVF clinics. We observed a dramatic increase in the rate of trisomy with maternal age in both IVF and NC groups: from 55.56 % in the NC patients aged <35 years to 82.71 % in those aged ≥35 years, and from 67.5 % in the IVF patients aged <35 years to 89.23 % in those aged ≥35 years. Our data show that the distribution and the relative proportions of types of abnormalities among the aberrant miscarriage karyotypes are similar in IVF and NC patients.
A possible risk of increase in the overall incidence of abnormal embryonic karyotype after IVF is discussed. Bettio and colleagues have reported that 54.5 % and 71.5 % of miscarriages are cytogenetically abnormal after IVF and natural conceptions, respectively, with no statistically significant difference [13]. Martínez and colleagues have shown that the incidence of cytogenetic abnormality in miscarriages amounts to 47.82 % after IVF and to 55.14 % after natural conceptions, also with no statistically significant difference [20]. Our karyotyping results of miscarriages in patients aged ≥35 years from the IVF (58.56 %) and the NC group (75.70 %) are in conformity with this data. In the young IVF-treated women we detected a significantly lower rate of abnormal miscarriage karyotype (37.04 %), compared with those who conceived naturally (62.43 %). A remarkably low incidence of abnormal miscarriage karyotype in young IVF patients at <7 w.g. is likely to contribute essentially to an overall decrease of abnormal embryonic karyotype incidence. This is an unexpected finding because, generally, the earlier the developmental age, the greater the likelihood of an abnormal karyotype in a spontaneous pregnancy loss. Boué and colleagues found that 66 % of losses between 2 and 7 weeks and 23 % of those between 8 and 12 weeks had abnormal karyotypes [21]. A low incidence of karyotypically abnormal pregnancy losses in IVF patients, detected in our study, could be induced by several factors. On the one hand, supporting hormonal therapy of ART patients may delay spontaneous abortion of an embryo with abnormal karyotype to a later gestational age. On the other hand, there is a number of reasons underlying early euploid pregnancy loss. Sporadic miscarriages may be provoked by immunological abnormalities due to qualitative and quantitative changes of decidual and uterine natural killer cells, immunological incompatibility between mother and fetus [4, 22–24]. Numerous endocrinological and endometrial factors are known to trigger pregnancy loss [25, 26]. Pathomorphological changes including chronic inflammation have been shown to be a common cause of abortions with normal karyotype [2]. The impact of thrombophilia, antiphospholipid syndrome, and thyroid autoimmunity on sporadic fetal loss have been also demonstrated [27]. All the mentioned conditions can cause implantation failure and infertility, thus becoming a reason for IVF treatment. In the case of an underdiagnosis or insufficient treatment, the listed pathologies negatively affect the pregnancy progress and increase the rate of miscarriages with normal karyotype in IVF patients. Non-cytogenetic reasons for pregnancy loss are likely to be more pronounced in IVF patients aged <35 years, as in older women most of miscarriages are caused by abnormal embryonic karyotype. Our data is in line with a recent study showing the increase in karyotypically normal miscarriages in young overweight and obese women, who conceived through IVF [28].
Along with the woman’s health, another important condition for normal prenatal development is a correct epigenetic marking of the embryonic genome. After fertilization, a human embryo undergoes global changes of DNA methylation patterns, necessary for the subsequent setting of tissue-specific epigenetic marks [29–32]. Epimutations leading to inappropriate DNA methylation may contribute to the incidence of early euploid pregnancy loss. This concept is supported by recent studies demonstrating altered methylation patterns in a number of genes in miscarriages [33, 34]. An increased risk of epigenetic mistakes associated with ART has not been proved yet [35, 36]; however, the outcome of many studies suggests that it should be taken into account [3, 37, 38].
In conclusion, our data show that IVF does not increase the rate of a pregnancy loss because of abnormal embryonic karyotype, nor does it increase the preponderance for any specific type of cytogenetic abnormality. Our results support the safety of IVF as an effective treatment for infertility. A low incidence of abnormal miscarriage karyotype in young IVF-treated women, especially at the initial stages of pregnancy, suggests that cytogenetic causes for early pregnancy loss play a minor role in this group. Identification of a cytogenetically normal spontaneous abortion may be more important clinically than identification of an aberrant gestation, because of an increased risk of repeat miscarriage [18]. Our data reinforces the importance of developing a rational approach for an appropriate diagnosis and treatment of patients prior to IVF cycle in order to reduce the risk of euploid pregnancy loss.
Acknowledgments
The authors would like to thank Ksenia O. Khudadyan, Igor Yu. Kogan, Inna V. Kareva, Sergey V. Mylnikov, Anton V. Kiselev and Anna A. Egorova for helpful advice during preparation of the manuscript.
This study is supported by Russian Foundation of Basic Research (11-04-01639-а, 13-04-01978-a), Administration of St. Petersburg and a stipend from RF President (1305.2012.4).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule IVF does not increase the risk of a pregnancy loss because of abnormal embryonic karyotype, nor does it increase the preponderance for any specific type of cytogenetic abnormality. The incidence of abnormal miscarriage karyotype is low in young IVF-treated women. Appropriate diagnosis and treatment of patients prior to IVF cycle are important to reduce the risk of euploid pregnancy loss.
References
- 1.Simpson JL, Bombard AT. Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counselling. In: Edmonds K, Bennett MJ, editors. Spontaneous abortion. London: Blackwell; 1987. pp. 51–76. [Google Scholar]
- 2.Redline RW, Zaragoza M, Hassold T. Prevalence of developmental and inflammatory lesions in nonmolar first-trimester spontaneous abortions. Hum Pathol. 1999;30(1):93–100. doi: 10.1016/S0046-8177(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 3.Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18(12):2508–2511. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- 4.Yamada H, Shimada S, Morikawa M, Iwabuchi K, Kishi R, Onoé K, et al. Divergence of natural killer cell receptor and related molecule in the decidua from sporadic miscarriage with normal chromosome karyotype. Mol Hum Reprod. 2005;11(6):451–457. doi: 10.1093/molehr/gah181. [DOI] [PubMed] [Google Scholar]
- 5.Morales C, Sanchez A, Bruguera J, Margarit E, Borrell A, Borobio V, et al. Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am J Med Genet A. 2008;146:66–70. doi: 10.1002/ajmg.a.32058. [DOI] [PubMed] [Google Scholar]
- 6.Guerneri S, Bettio D, Simoni G, Brambati B, Lanzani A, Fraccaro M. Prevalence and distribution of chromosome abnormalities in a sample of first trimester internal abortions. Hum Reprod. 1987;2:735–739. doi: 10.1093/oxfordjournals.humrep.a136623. [DOI] [PubMed] [Google Scholar]
- 7.Nagaishi M, Yamamoto T, Iinuma K, Shimomura K, Berend SA, Knops J. Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J Obstet Gynaecol Res. 2004;30:237–241. doi: 10.1111/j.1447-0756.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf RZ, Naeem R. Cytogenetic abnormalities in products of conception: a relationship revisited. Am J Reprod Immunol. 2004;52:88–96. doi: 10.1111/j.1600-0897.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 9.Snijders RJ, Holzgreve W, Cuckle H, Nicolaides KH. Maternal age-specific risks for trisomies at 9-14 weeks’ gestation. Prenat Diagn. 1994;14(7):543–552. doi: 10.1002/pd.1970140706. [DOI] [PubMed] [Google Scholar]
- 10.Snijders RJ, Sebire NJ, Nicolaides KH. Maternal age and gestational age-specific risk for chromosomal defects. Fetal Diagn Ther. 1995;10(6):356–367. doi: 10.1159/000264259. [DOI] [PubMed] [Google Scholar]
- 11.Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril. 2004;81(5):1265–1269. doi: 10.1016/j.fertnstert.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Grande M, Borrell A, Garcia-Posada R, Borobio V, Muñoz M, Creus M, et al. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27(10):3109–3117. doi: 10.1093/humrep/des251. [DOI] [PubMed] [Google Scholar]
- 13.Bettio D, Venci A, Levi Setti PE. Chromosomal abnormalities in miscarriages after different assisted reproduction procedures. Placenta. 2008;29(Suppl B):S126–S128. doi:10.1016/j.placenta.2008.08.015 [DOI] [PubMed]
- 14.Kim JW, Lee WS, Yoon WK, Lee WS, Cho JH, Kim YS, et al. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153. doi: 10.1186/1471-2350-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bingol B, Abike F, Gedikbasi A, Tapisiz OL, Gunenc Z. Comparison of chromosomal abnormality rates in ICSI for non-male factor and spontaneous conception. J Assist Reprod Genet. 2012;29(1):25–30. doi: 10.1007/s10815-011-9646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner M, Reh A, Grifo J, Perle MA. Characteristics of chromosomal abnormalities diagnosed after spontaneous abortions in an infertile population. J Assist Reprod Genet. 2012;29(8):817–820. doi: 10.1007/s10815-012-9781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranov VS, Lebedev VM, Poleev AV, Mikhaĭlova EP, Rybalko AV, Shved NV. Fast direct method of obtaining metaphase and prometaphase chromosomes from chorion biopsy cells and human embryos during the lst trimester of pregnancy. Biull Eksp Biol Med. 1990;110(8):196–198. [PubMed] [Google Scholar]
- 18.Pflueger SMV. The cytogenetics of spontaneous abortion. In: Gersen SL, Keagle MB, editors. The principles of clinical cytogenetics. New York: Springer; 2013. pp. 275–292. [Google Scholar]
- 19.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez MC, Méndez C, Ferro J, Nicolás M, Serra V, Landeras J. Cytogenetic analysis of early nonviable pregnancies after assisted reproduction treatment. Fertil Steril. 2010;93(1):28992. doi: 10.1016/j.fertnstert.2009.07.989. [DOI] [PubMed] [Google Scholar]
- 21.Boué A, Boué J, Gropp A. Cytogenetics of pregnancy wastage. Adv Hum Genet. 1985;14:1–57. doi: 10.1007/978-1-4615-9400-0_1. [DOI] [PubMed] [Google Scholar]
- 22.Clifford K, Flanagan AM, Regan L. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric study. Hum Reprod. 1999;14(11):2727–2730. doi: 10.1093/humrep/14.11.2727. [DOI] [PubMed] [Google Scholar]
- 23.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, et al. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod. 1999;14(9):2386–2391. doi: 10.1093/humrep/14.9.2386. [DOI] [PubMed] [Google Scholar]
- 24.Tuckerman E, Laird SM, Prakash A, Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod. 2007;22(8):2208–2213. doi: 10.1093/humrep/dem141. [DOI] [PubMed] [Google Scholar]
- 25.Li TC, Spuijbroek MD, Tuckerman E, Anstie B, Loxley M, Laird S. Endocrinological and endometrial factors in recurrent miscarriage. BJOG. 2000;107(12):1471–1479. doi: 10.1111/j.1471-0528.2000.tb11670.x. [DOI] [PubMed] [Google Scholar]
- 26.Tuckerman E, Laird SM, Stewart R, Wells M, Li TC. Markers of endometrial function in women with unexplained recurrent pregnancy loss: a comparison between morphologically normal and retarded endometrium. Hum Reprod. 2004;19(1):196–205. [PubMed] [Google Scholar]
- 27.Bellver J, Soares SR, Alvarez C, Muñoz E, Ramírez A, Rubio C, et al. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. Hum Reprod. 2008;23(2):278–284. doi: 10.1093/humrep/dem383. [DOI] [PubMed] [Google Scholar]
- 28.Kroon B, Harrison K, Martin N, Wong B, Yazdani A. Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertil Steril. 2011;95(5):1827–1829. doi: 10.1016/j.fertnstert.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 29.Fulka H, Mrazek M, Tepla O, Fulka J., Jr DNA methylation pattern in human zygotes and developing embryos. Reproduction. 2004;128(6):703–708. doi: 10.1530/rep.1.00217. [DOI] [PubMed] [Google Scholar]
- 30.Baranov VS, Pendina AA, Kuznetsova TV, Efimova OA, Fedorova ID, Leont’eva OA, et al. Peculiarities of metaphase chromosome methylation pattern in preimplantation human embryos. Tsitologiia. 2005;47(8):723–730. [PubMed] [Google Scholar]
- 31.Pendina AA, Efimova OA, Kaminskaia AN, Kuznetsova TV, Baranov VS. Immunocytochemical analysis of human metaphase chromosome methylation status. Tsitologiia. 2005;47(8):731–737. [PubMed] [Google Scholar]
- 32.Pendina AA, Efimova OA, Fedorova ID, Leont’eva OA, Shilnikova EM, Lezhnina JG, et al. DNA methylation patterns of metaphase chromosomes in human preimplantation embryos. Cytogenet Genome Res. 2011;132(1–2):1–7. doi: 10.1159/000318673. [DOI] [PubMed] [Google Scholar]
- 33.Sazhenova EA, Skryabin NA, Sukhanova NN, Lebedev IN. Multilocus epimutations of imprintome in the pathology of human embryo development. Mol Biol. 2012;46(2):183–191. doi: 10.1134/S0026893312010207. [DOI] [PubMed] [Google Scholar]
- 34.Zheng HY, Tang Y, Niu J, Li P, Ye DS, Chen X, et al. Aberrant DNA methylation of imprinted loci in human spontaneous abortions after assisted reproduction techniques and natural conception. Hum Reprod. 2013;28(1):265–273. doi: 10.1093/humrep/des358. [DOI] [PubMed] [Google Scholar]
- 35.Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod. 2005;20(4):950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 36.Lidegaard Ø, Pinborg A, Andersen AN. Imprinting disorders after assisted reproductive technologies. Curr Opin Obstet Gynecol. 2006;18(3):293–296. doi: 10.1097/01.gco.0000193006.42910.ee. [DOI] [PubMed] [Google Scholar]
- 37.Grace KS, Sinclair KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27(5):409–416. doi: 10.1055/s-0029-1237429. [DOI] [PubMed] [Google Scholar]
- 38.Nelissen EC, Dumoulin JC, Daunay A, Evers JL, Tost J, van Montfoort AP. Placentas from pregnancies conceived by IVF/ICSI have a reduced DNA methylation level at the H19 and MEST differentially methylated regions. Hum Reprod. 2013;28(4):1117–1126. doi: 10.1093/humrep/des459. [DOI] [PubMed] [Google Scholar]