Abstract
Purpose
To identify the independent predictors of live birth following IVF, and to assess the role of cohort-specific parameters, including antral follicle count (AFC), the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos, in fresh IVF cycles.
Methods
A retrospective cohort study of 2,525 infertile women undergoing IVF between 2002 and 2007. The hypothesis that the number and quality of embryos transferred capture the effects previously attributed to cohort-specific variables was examined using mediation analysis and spline analysis. Independent predictors were identified by a bootstrap algorithm. Multivariable logistic regression was performed and the proportion of explained variation was measured to compare the relative importance of transfer-specific vs. cohort-specific predictors.
Results
The number of good-quality embryos transferred and progesterone level on the day of hCG administration ranked as the two most important predictors of live birth. Prospects of pregnancy started to decrease after progesterone level exceeded 0.6 ng/ml. The achievement of live birth in a fresh IVF cycle is primarily determined by the number and quality of embryos transferred, rather than by embryo cohort-specific variables.
Conclusions
The associations between cohort-specific variables and live birth in a fresh IVF cycle are completely mediated by the quality of embryos transferred. Progesterone level on the day of hCG administration is an independent predictor of pregnancy and merits further investigation.
Keywords: IVF, Live birth, Progesterone, Embryo quality
Introduction
Since the advent of IVF, many studies have been performed to search for predictors of IVF outcomes. Some have focused on factors predicting pregnancy after fresh embryo transfer [1], whereas others have focused on predicting the cumulative outcome of a completed IVF cycle, which includes a fresh cycle and/or subsequent frozen cycles [2]. Very few previous works have examined whether predictors of cumulative outcome still retain their predictive value in a fresh cycle or vice versa. Such uncertainty may contribute to the conflicting conclusions in the existing literature, even for the most recognized prognostic factors, such as embryo quality.
Cai et al. found that the size and quality of the embryo cohort are the strongest predictors of cumulative outcome in a completed treatment cycle [2]. Jun et al. concluded that embryo cohort-specific variables are more informative than any measures of individual transferred embryos in a fresh cycle [3]. Many studies have ignored the effects of the number and quality of embryos transferred when investigating the prognostic values of cohort-specific parameters including antral follicle count (AFC), the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos, in predicting the IVF outcomes of fresh cycles [1, 3–9].
In a typical IVF cycle, controlled ovarian hyperstimulation (COH) is used to produce a large number of eggs with the intent of creating a cohort of embryos for both fresh transfer and subsequent frozen cycles. The current practice is to transfer no more than two or three embryos in a fresh cycle to minimize the risk of multiple gestation. This leads to an important question: Does the quality of all embryos from a stimulated cycle contribute to a successful preganancy in a fresh IVF cycle, or only that of those actually transferred to the woman’s uterus in that cycle?
Randomized studies revealed that double embryo transfer (DET) led to significantly higher pregnancy prospects than single embryo transfer (SET) [10–12] and hereby established the causal relationship between the number and quality of embryos transferred and pregnancy after fresh embryo transfer. Our recent study has shown that the association between the number of oocytes retrieved and pregnancy after fresh embryo transfer was mediated entirely by the number and quality of embryos transferred. When similarly-aged patients have the same number and quality of embryos transferred, their chances of success have no correlation with the number of eggs retrieved [13]. Thus, it is reasonable to hypothesize that cohort-specific parameters have no direct effects on IVF outcomes in fresh cycles, only indirect effects mediated by the number and quality of embryos transferred. Understanding this relationship may aid in making more informed decisions on ovarian stimulation protocols and embryo transfer strategies, especially when there is a trend to suggest that the best outcome could be reached by tuning the number of oocytes retrieved via specified ovarian stimulation [8, 9]. In addition, there is still much debate as to the contribution of other factors, such as serum progesterone level on the day of human chorionic gonadotropin (hCG) administration, as independent predictors of IVF outcomes [14, 15].
In view of these issues, the present work utilized data from a large cohort of patients [2] to identify the independent predictors of live birth in a fresh IVF cycle. Specifically, the study intended to clarify the role of cohort-specific parameters including AFC, the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos, in pregnancy prognosis after fresh embryo transfer, and to verify the status of potential predictors still under debate.
Materials and methods
Patient population
We retrospectively studied the first fresh cycles of 2,525 patients undergoing IVF/ICSI at the Center for Reproductive Medicine at the Women and Children’s Hospital of Guangdong Province, P. R. China between January 2002 and December 2007. Because of the retrospective nature of this study and the fact that there were no interventions other than standard IVF preparation and treatment, this study was exempt from Institutional Review Board approval. Cycles with the following characteristics were excluded from this analysis: cycles arising from oocyte or sperm donation, in vitro maturation, unstimulated cycles, cycles utilizing frozen embryos, and cycles that did not lead to embryo transfer. In addition, patients with blastocyst embryo transfer (n = 80) were also removed from consideration because the blastocyst embryo grading system is not comparable to that of day-3 embryos.
In vitro fertilization
The IVF procedures employed in our IVF unit have been described elsewhere [2]. Briefly, all female partners underwent COH with gonadotropins, usually in concert with down regulation with gonadotropin-releasing hormone (GnRH) agonist (n = 2439) or, in a few cases, with GnRH antagonist (n = 86). Hormonal measurement of oestradiol, luteinizing hormone (LH), and progesterone, along with ultrasound assessment of follicular growth and endometrium were performed at the initiation of stimulation, on day 5, and on day 8 of stimulation, and then daily up to and including the day of hCG administration. Final oocyte maturation was achieved with 10,000 IU of hCG after three or more follicles reached a mean diameter of 18 mm. Transvaginal oocyte retrieval was performed 34–36 h later. After routine IVF/ICSI procedure, embryos were cultured in Quinnes sequential culture media and placed in a CO2 incubator.
Embryo quality was assessed morphologically by considering the number of blastomeres, day of transfer, and the degree of fragmentation. Good-quality embryos were defined as those with a normal cleavage rate (4 cells on day 2 and 6–8 cells on day 3) and <20 % fragmentation [4, 16]. In accordance with the Code of Practice for Assisted Reproductive Technology formulated by the Ministry of Health of P. R. China, a maximum of three embryos were transferred on either day 2 or day 3. Two days following retrieval, 20–40 mg progesterone in oil was given for luteal support.
Progesterone measurement
Serum progesterone level was measured on the day of hCG administration. Samples were tested with radioimmunoassay (Tianjin Depu Diagnostic Product Co.). The intra-assay and interassay coefficients of variation were 7.0 % and 9.6 %, respectively. The sensitivity for progesterone was 0.1 ng/ml, and the range of measurement was 0.1–40 ng/ml. This assay was used for the duration of the study.
Outcome measures
A live birth was defined as any birth event in which at least one baby was born alive and survived for more than 1 month.
Statistical analysis
A restricted cubic spline analysis was used to explore the functional forms of the relationships between live birth and important covariates including maternal age, duration of infertility, AFC, oestradiol level on the day of hCG administration, progesterone level on the day of hCG administration, endometrial thickness, the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos.
Mediation analysis was implemented in order to test the hypothesis that the quality of embryos transferred completely mediates the association between live birth in fresh cycles and the following cohort-specific parameters: AFC, the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos [17, 18]. The complete mediation model predicts that cohort-specific variables influence the “mediator”, i.e., the quality of embryos transferred, which in turn influences live birth in fresh cycles. However, there is no direct causal relationship between cohort-specific variables and IVF outcomes in fresh cycles. Complete mediation is established when after controlling for the quality of embryos transferred, a woman’s chance of pregnancy in a fresh cycle is not associated with the cohort-specific variables.
We used a two-step regression analysis to test our mediation hypothesis [18]. First, we modeled the outcome variable, live birth, against each of the cohort-specific independent variables (separately). This step tested whether the cohort-specific variables have any effect (indirect plus direct effects) on live birth. Second, we regressed the live birth outcome variable against both the cohort-specific independent variable and the proposed mediator: the quality of embryos transferred. This step tested whether any direct effect of cohort-specific variables remains after controlling for the mediator. We used the number of embryos transferred and the number of good-quality embryos transferred as a proxy for the quality of embryos transferred. Because morphology may inadequately measure true embryo quality, and because embryos from younger women tend to be better than those from older women even when the embryo morphology grades are same [19], we included age along with the two mediator variables as a separate scenario. Thus, for each cohort specific variable, three logistic regressions were implemented. To examine the robustness of the conclusion against possible confounding, we performed a sensitivity analysis by adjusting for several covariates (progesterone level, oestradiol level, endometrial thickness, and LH level on hCG day, plus basal oestradiol level and duration of infertility) in the three regression models. Under our hypothesis of complete mediation, we expected to observe significant effects for the cohort-specific variables in the first models (with no mediators), but non-significant effects in the second models where we controlled for the quality of embryos transferred.
A bootstrap variable selection algorithm was used to construct the final prediction model [20]. We generated 5000 bootstrap samples of the same size from the original data. Within each bootstrap sample, we performed stepwise logistic regression with thresholds of p = 0.10 for selection and variable elimination. Predictors present in at least 3250 runs (65 %) were entered in a final logistic regression model using the full original data. Relative importance of the predictors retained in the final model was compared using the proportion of explained variation (PEV), commonly known as R-squared [21, 22]. Marginal PEV measures the explained variation contributed individually by a predictor and partial PEV measures the decline in the explained variation after removing a predictor from the full prediction model.
The statistical analyses and plotting graphics were performed using the SAS 9.2 statistical package (SAS Inc., Cary, NC) and R statistical package (www.r-project.org).
Results
Characteristics of the 2,525 patients by live birth are shown in Table 1. The average age of the patients was 31.2 ± 4.0 years. The primary indication for infertility was tubal factor (55.29 %) and conventional IVF was used in the majority of cycles (66.50 %). For patients in the four age groups (<28, 28–30, 31–34, ≥35), the mean number of embryos transferred was 2.2 ± 0.5, 2.3 ± 0.5, 2.2 ± 0.5, and 2.7 ± 0.6, respectively, and the mean number of good-quality embryos transferred was 1.7 ± 0.7, 1.7 ± 0.7, 1.7 ± 0.7, and 1.8 ± 1.1, respectively. The overall live birth rate per transfer was 37.8 %.
Table 1.
Patient and Cycle Characteristics by Live Birth
| Variables | Live birth | Not live birth | P-value |
|---|---|---|---|
| (n = 880) | (n = 1645) | ||
| Protocol | |||
| Long protocol | 630(71.59 %) | 1047(63.65 %) | <0.0001a |
| Short protocol | 218(24.77 %) | 544(33.07) | |
| GnRH antagonist protocol | 32(3.64 %) | 54(3.28) | |
| Insemination method | |||
| IVF | 603(68.52 %) | 1076(65.41 %) | 0.345a |
| ICSI | 238(27.04 %) | 489(29.73 %) | |
| 50%IVF-50%ICSI | 39(4.43 %) | 80(4.86 %) | |
| Diagnosis of infertility | |||
| Tubal factor | 507(57.61 %) | 889(54.04 %) | 0.160a |
| Male factor | 175(19.89 %) | 349(21.22 %) | |
| Endometriosis | 25(2.84 %) | 49(2.98 %) | |
| Ovarian factor | 23(2.61 %) | 75(4.56 %) | |
| Unexplained | 49(5.57 %) | 102(6.20 %) | |
| Other reasons | 101(11.48 %) | 181(11.0 %) | |
| Type of infertility | |||
| Primary infertility | 445(50.57 %) | 796(48.42 %) | 0.303a |
| Secondary infertility | 435(49.43 %) | 848(51.58 %) | |
| Age(year) | 30.61 ± 3.68 | 31.62 ± 4.17 | <0.0001b |
| Antral follicle count | 10(1–45) | 9(1–40) | <0.0001c |
| Body mass index(kg/m2) | 20.98 ± 2.90 | 21.16 ± 2.81 | 0.142b |
| Basal serum FSH level (mIU/ml) | 6.40 ± 1.96 | 6.46 ± 2.71 | 0.531b |
| Basal serum E2 level (pg/ml) | 36.82 ± 27.57 | 37.48 ± 20.53 | 0.496b |
| Duration of infertility (year) | 4(0.1–17) | 4.5(0.4–20) | <0.0001c |
| Mean ovarian volume(cm3) | 5.55 ± 2.53 | 5.48 ± 2.92 | 0.533b |
| Total ovarian volume(cm3) | 11.10 ± 5.06 | 10.95 ± 5.84 | 0.533b |
| E2 on hCG injection day(pg/ml) | 3045.20 ± 1709.81 | 3138.55 ± 1912.16 | 0.226b |
| Progesterone on hCG injection day(ng/ml) | 1.10 ± 0.63 | 1.28 ± 0.75 | <0.0001b |
| LH on hCG injection day(mIU/ml) | 1(0.2–19) | 1.1(0.2–13.10) | 0.500c |
| E2 on the day after hCG injection (pg/ml) | 3752.90 ± 2059.67 | 4103.82 ± 2416.70 | 0.001b |
| Number of follicles[10,14 mm) | 3(0–17) | 3(0–27) | 0.367c |
| Number of follicles[14,18 mm) | 7(0–22) | 6(0–21) | 0.285c |
| Number of follicles ≥18 mm | 3(0–11) | 2(0–13) | 0.077c |
| Number of follicles <10 mm | 6(0–30) | 5(0–25) | 0.001c |
| Number of follicles≥14 mm | 9(1–27) | 9(1–29) | 0.106c |
| Endometrial thickness(mm) | 11.13 ± 5.15 | 10.37 ± 2.09 | <0.0001b |
| Number of oocytes retrieved | 14.74 ± 6.50 | 14.25 ± 6.85 | 0.082b |
| Number of oocytes fertilized | 11.99 ± 5.93 | 11.23 ± 5.98 | 0.088b |
| Fertilization rate | 0.74 ± 0.20 | 0.69 ± 0.22 | <0.0001b |
| Number of embryos transferred | 2.30 ± 0.48 | 2.31 ± 0.55 | 0.201b |
| Number of good-quality embryos transferred | 1.84 ± 0.67 | 1.60 ± 0.86 | <0.0001b |
| Total number of embryos | 10.35 ± 5.39 | 9.37 ± 5.56 | <0.0001b |
| Total number of good-quality embryos | 5.14 ± 3.51 | 4.26 ± 3.72 | <0.0001b |
aPearson Chi-square
bTwo sample T test
cTwo sample Wilcoxon test
The results of the spline analysis are presented in Figs. 1 and 2. The chance of live birth rose with increasing oestradiol level up to 3,500 pg/ml but steadily declined afterwards (Fig. 1a). There was clearly an increasing trend in live birth with increasing endometrial thickness (Fig. 1b). The chance of achieving a live birth decreased as age and duration of infertility increased (Fig. 1c and d), and in particular, there was more rapid decline after age 35. Figure 1e reveals a decreasing trend in the odds of achieving a live birth with increasing progesterone level on the day of hCG injection after the threshold point of 0.6 ng/ml.
Fig. 1.
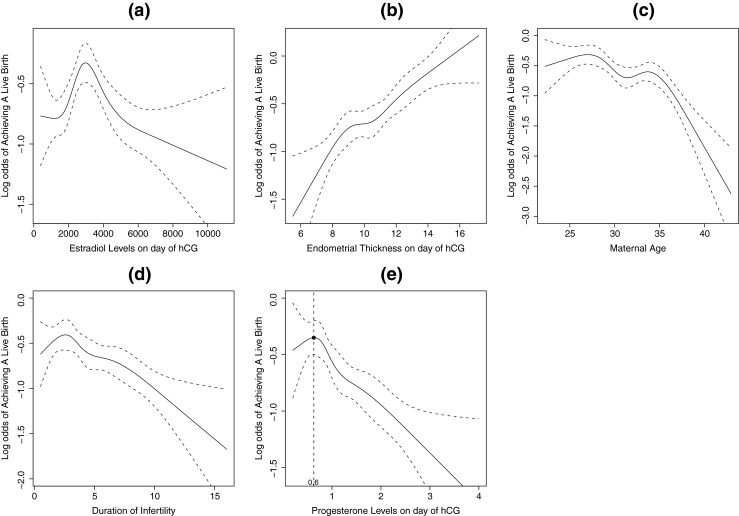
a Restricted cubic spline curve and 95 % confidence levels between oestradiol level on the day of hCG administration and log odds of live birth; b Restricted cubic spline curve and 95 % confidence levels between endometrial thickness on the day of hCG administration and log odds of live birth; c Restricted cubic spline curve and 95 % confidence levels between maternal age and log odds of live birth; d Restricted cubic spline curve and 95 % confidence levels between duration of infertility and log odds of live birth; e Restricted cubic spline curve and 95 % confidence levels between progesterone level on the day of hCG administration and log odds of live birth; the threshold point at 0.6 ng/ml is plotted
Fig. 2.
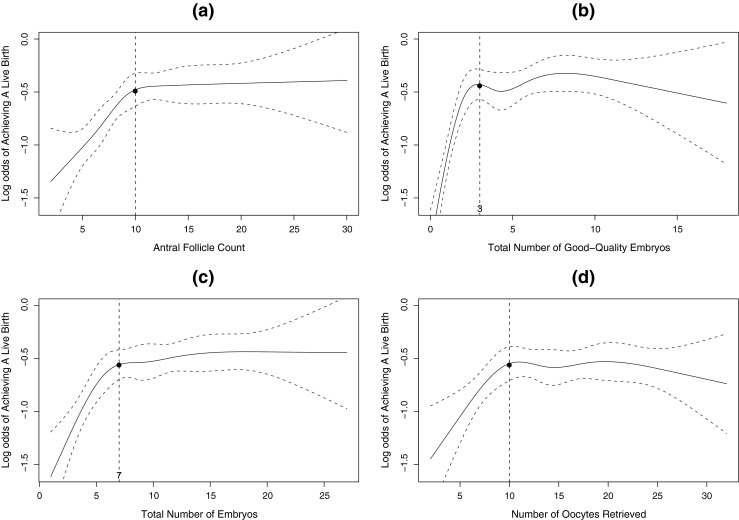
a Restricted cubic spline curve and 95 % confidence levels between antral follicle count and log odds of live birth; b Restricted cubic spline curve and 95 % confidence levels between the total number of good-quality embryos and log odds of live birth; c Restricted cubic spline curve and 95 % confidence levels between the total number of embryos and log odds of live birth; d Restricted cubic spline curve and 95 % confidence levels between the number of oocytes retrieved and log odds of live birth
Figure 2 demonstrates that there are similar non-linear trends in the associations between live birth, and AFC, the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos. The chance of achieving a live birth initially rose with increasing number of each of these four cohort-specific variables, but then plateaued, indicating that these variables have no association with pregnancy once they are greater than the threshold levels, (e.g. 10 oocytes retrieved or three good-quality embryos available) as shown by the horizontal line within the 95 % confidence bands. The chance of achieving a live birth stopped rising when the total number of good-quality embryos was greater than three (Fig. 2b), which is the maximum number of embryos allowable for replacement in our data. In particular, the cutoff of 10 oocytes and the cutoff of three good-quality embryos approximately reflect the fact that on average, 50 %–75 % of the eggs will fertilize and half of the fertilized eggs will develop into embryos of sufficient quality to transfer or freeze.
Table 2 reports the mediation analyses. All four cohort-specific variables had highly significant associations with live birth (P < 0.05) when the number and quality of embryos transferred were not included in the model. In particular, for each of the four variables, the medium-yield and high-yield groups had significantly higher chances of achieving a live birth than the low-yield group. However, once we controlled for the number and quality of embryos transferred and maternal age, there was no longer a significant association between prospects of pregnancy, and the number of oocytes retrieved, the total number of embryos, and the total number of good-quality embryos (P > 0.1). In fact, the odds ratios were all close to 1, suggesting no practical differences in the chance of live birth among the three groups (low-, medium- and high-yield group). In contrast, AFC had a marginally significant association with pregnancy even after controlling for the number and quality of embryos transferred (P < 0.1), However, it also became non-significant once we controlled for maternal age. The sensitivity analysis produced similar results (data not shown).
Table 2.
Mediation analysis of the associations between live birth, and number of oocytes retrieved, total number of embryos, total number of good-quality embryos, and AFC using logistic regression
| No. | Variable | Model Output | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratioa | P-valuea | Odds Ratiob | P-valueb | Odds Ratioc | P-valuec | |||
| 1 | Number of oocytes | 0.003* | 0.387* | 0.334* | ||||
| Low | ≤6 | 1 | 1 | 1 | ||||
| Medium | 7–14 | 1.62 | 0.002 | 1.22 | 0.233 | 1.06 | 0.721 | |
| High | ≥15 | 1.69 | 0.001 | 1.12 | 0.511 | 0.93 | 0.668 | |
| 2 | Total number of embryos | <0.0001* | 0.873* | 0.989* | ||||
| Low | ≤5 | 1 | 1 | 1 | ||||
| Medium | 6–9 | 1.46 | 0.001 | 1.04 | 0.750 | 0.98 | 0.884 | |
| High | ≥10 | 1.73 | 0.001 | 1.07 | 0.604 | 0.99 | 0.912 | |
| 3 | Total number of good-quality embryos | <0.0001* | 0.770* | 0.911* | ||||
| Low | ≤3 | 1 | 1 | 1 | ||||
| Medium | 4–6 | 1.62 | <0.0001 | 1.09 | 0.470 | 1.03 | 0.780 | |
| High | ≥7 | 1.66 | <0.0001 | 1.05 | 0.679 | 0.99 | 0.904 | |
| 4 | AFC | <0.0001* | 0.057* | 0.313* | ||||
| Low | ≤6 | 1 | 1 | 1 | ||||
| Medium | 7–10 | 1.43 | 0.003 | 1.20 | 0.136 | 1.12 | 0.353 | |
| High | ≥11 | 1.80 | <0.0001 | 1.35 | 0.017 | 1.22 | 0.131 | |
aLogistic regression model with only cohort-specific variable included
bLogistic regression model with cohort-specific variable, number of good-quality embryos transferred, and number of embryos transferred
cLogistic regression model with cohort-specific variable, number of good-quality embryos transferred, number of embryos transferred, and maternal age
*P-value of testing overall effects (df = 2)
Nine variables were selected in at least 65 % of 5000 bootstrap samples: 1) the number of good-quality embryos transferred (100 %); 2) progesterone level on the day of hCG administration (100 %); 3) endometrial thickness on the day of hCG administration (100 %); 4) maternal age (99 %); 5) oestradiol level on the day of hCG administration (94 %); 6) LH level on the day of hCG administration (79 %); 7) basal oestradiol level (78 %); 8) duration of infertility (67 %); and 9) the number of embryos transferred (65 %).
The following statistics from the multivariable prediction model are listed in Table 3: p-values, odds ratios with corresponding 95 % CIs, and relative importance of nine predictors in terms of marginal and partial PEVs. The marginal PEV for the number of good-quality embryos transferred was 3.57 % and partial PEV was 1.89 %, both of which were significantly higher than the corresponding PEVs of the other eight predictors (P < 0.05).
Table 3.
Multivariable logistic regression model for predicting live birth and relative importance of predictors
| Variable Name | Odds ratios (95%CI) | P-value | Marginal PEV (%) | Partial PEV (%) |
|---|---|---|---|---|
| Number of good-quality embryos transferred | <0.0001a | 3.03 | 1.87 | |
| 3 | 2.89[1.83–4.56] | <0.0001b | ||
| 2 | 2.77[1.97–3.91] | <0.0001b | ||
| 1 | 1.57[1.07–2.30] | 0.021b | ||
| 0 | 1 | |||
| Progesterone on the day of hCG injection | 1.41* | 1.00* | ||
| ≥1.2 | 0.59[0.48–0.73] | <0.0001 | ||
| <1.2 | 1 | |||
| Endometrial thickness | <0.0001a | 1.37* | 0.86* | |
| ≥13 | 1.92[1.46–2.52] | <0.0001b | ||
| [10,13) | 1.34[1.11–1.63] | 0.002b | ||
| <10 | 1 | |||
| Age | <0.0001a | 1.76* | 0.76* | |
| ≥35 | 0.48[0.33–0.69] | <0.0001b | ||
| [30,35) | 0.81[0.64–1.03] | 0.084b | ||
| [28,30) | 1.07[0.82–1.39] | 0.640b | ||
| <28 | 1 | |||
| E2 on the day of hCG injection | <0.0001a | 0.77*** | 0.52* | |
| ≥4000 | 0.80[0.63–1.01] | 0.064b | ||
| [2500,4000) | 1.27[1.04–1.57] | 0.022b | ||
| <2500 | 1 | |||
| Duration of infertility | 0.097a | 0.76*** | 0.27** | |
| ≥8 | 0.72[0.53–0.98] | 0.036b | ||
| [5,8) | 0.90[0.70–1.16] | 0.431b | ||
| [2.5,5) | 1.02[0.81–1.30] | 0.840b | ||
| <2.5 | 1 | |||
| Basal serum E2 level | ||||
| ≥35 | 0.81[0.68–0.96] | 0.016 | 0.25*** | 0.21** |
| <35 | 1 | |||
| Number of embryos transferred | 0.087a | 1.35** | 0.15** | |
| 3 | 2.23[1.05–4.73] | 0.036b | ||
| 2 | 1.94[0.92–4.11] | 0.083b | ||
| 1 | 1 | |||
| LH on the day of hCG injection | ||||
| ≥2 | 1.23[0.98–1.54] | 0.071 | 0.25*** | 0.11** |
| <2 | 1 | |||
a P-value of type 3 chi-square test for each variable’s overall effects after adjusting for the other variables
b P-value of chi-square test between each variable’s subgroups and reference group
***Significantly different from relative importance of number of good-quality embryos transferred at α = 0.001 level
**Significantly different from relative importance of number of good-quality embryos transferred at α = 0.01 level
*Significantly different from relative importance of number of good-quality embryos transferred at α = 0.05 level
Discussion
This retrospective study identified nine independent predictors of live birth in a fresh IVF cycle. The number of good-quality embryos transferred and progesterone level on the day of hCG administration were the two most important predictors. Moreover, our study also supports the hypothesis that the associations between live birth in a fresh IVF cycle and cohort-specific variables are completely mediated by the quality of embryos transferred.
The age-related decline in fertility, as shown in this study and many others, is generally believed to be due to a decrease in oocyte quality and quantity [23]. However, in the present study, women older than 35 years old had, on average, slightly more embryos transferred and more morphologically good-quality embryos transferred in fresh cycles than younger women. Some studies have found that the success of donor egg therapy is unaffected by recipient age up to the later 40s, which suggests that the decrease in endometrial receptivity may not be a major factor in age-related decline in fertility [24–26]. Thus, the sharp decline in the chance of achieving a live birth after age 35 suggests that poor embryo quality is the primary reason for the age-related decline in fertility, and that embryo morphology may not be a consistent measure of true embryo quality, with poorer performance for women older than 35 years old [2]. Despite this inadequacy, the number of good-quality embryos transferred, as assessed by morphological score, still possessed the highest predictive value among all predictors identified in this study.
The prognostic value of cohort-specific variables has been studied extensively. The total number of good-quality embryos has been shown to be the most important predictor of cumulative outcome in a completed IVF cycle [2]. Some studies have also reported that AFC, the number of oocytes, the total number of embryos, and the total number of good-quality embryos predict pregnancy after fresh embryo transfer, with higher numbers of each associated with better chance of success [1, 3–9]. Only a few researchers have explored the functional form of the associations of these parameters with IVF outcomes specifically in fresh cycles. Van der Gaast et al. and Sunkara et al. reported a nonlinear association between the number of oocytes and pregnancy graphically. The optimal number of eggs, which corresponded with the highest pregnancy rate, was derived and ovarian stimulation protocols were suggested to target these “ideal” egg totals [8, 9]. This approach inherently assumed the optimal number of eggs leads to the best pregnancy chance. However, we must use caution when making causal interpretations from association analyses performed in observational studies. The conclusions from these observational studies were hampered by the fact that the number and quality of embryos transferred (as opposed to those in the overall cohort) were ignored in the analyses. In contrast to previous studies, our recent study found that the patients, from whom the “optimal” number of oocytes were retrieved, were not only younger but also had the highest probability of having two good-quality embryos replaced, which we proposed was the primary cause behind the highest rate of pregnancy success. The present study not only confirmed the previous findings with data from a different center, but also further revealed that the same increasing-then-plateauing shape of the association exists for other cohort-specific parameters (Fig. 2).
The mediation analysis revealed that the number or quality of embryos in the cohort or the number of oocytes retrieved had no direct effects on IVF outcome in fresh cycles. When similarly-aged patients have a similar number and quality of embryos transferred in fresh cycles, their prospects of pregnancy are similar, regardless of how many embryos or oocytes are available in the cohort. Collectively, these results from the spline and mediation analyses suggest that patients have a similar probability of having two or three good-quality embryos transferred in fresh cycles once certain thresholds are exceeded (i.e. 10 oocytes or three good embryos), and thus their chances of achieving a live birth are approximately equal and maximized. This explains why we observed the increasing-then-plateauing trend in the spline curves (Fig. 2).
Thus, while it is true that cohort-specific parameters are correlated with the IVF outcome in fresh cycles, the only source of such correlation is the fact that these variables are themselves correlated with the number and quality of embryos transferred, which in reality determines the IVF outcome. We could also verify this conclusion via randomized studies comparing treatment effectiveness of DET vs. SET. Once the randomization delinked the association between the cohort-specific variables and the quality of embryos transferred, the cohort-specific variables were no longer associated with pregnancy in fresh cycles [27, 28]. In clinical practice, embryo transfer strategies are typically determined by national and center policies, or even physicians’ preferences and experiences, rather than by the quantity of cohort-specific variables. For this reason, we cannot assume that patients with the “optimal” number of eggs or embryos in their cohort have the best pregnancy prospects in a fresh cycle without considering the number and quality of embryos transferred. It is not the optimal egg number or embryo cohort that maximizes IVF outcomes in fresh cycles, but rather the quality of the embryos transferred. Using the optimal egg or embryo number to guide ovarian stimulation to maximize the chance of pregnancy may not be the best medical decision for the general patient population and could unnecessarily place pressures upon poor responders [13]. We should resort to more flexible ovarian stimulation protocols, based on evaluation of an individual’s ovarian reserve, to retrieve the desired number of good embryos and thus maximize the chance of pregnancy for each woman.
Our previous study confirms that embryo cohort-specific variables are the most important predictors of cumulative outcome in a complete treatment cycle [2]. The current study demonstrates that what matters to the prospect of pregnancy after fresh embryo transfer is the quality and number of the embryos replaced, not the cohort-specific parameters. The combined findings from these two studies imply that the quality and size of the embryo cohort enables the selection of good-quality embryos for transfer in fresh and subsequent frozen cycles. The optimal performance of a complete treatment cycle relies on the quality and quantity of the embryos transferred in each individual cycle.
The influence of serum progesterone level on the day of hCG administration on IVF outcomes has long been a matter of debate. A recent meta-analysis concluded that there was no statistically significant association between progesterone elevation and the probability of live birth [14]. However, Bostch et al. found that elevated progesterone level on the day of hCG administration was associated with reduced ongoing pregnancy rates [15]. Our study showed that progesterone level on the day of hCG administration was as predictive as maternal age, was selected 100 % of the time during the 5000 bootstrapping runs, and in fact ranked second in terms of predictive value in a fresh IVF cycle among all predictors identified (Table 3). The contradictory conclusions from existing studies on this issue might be explained by the different timing of hCG administration used and the relatively small sample sizes employed in some studies. In contrast, progesterone level on the day of hCG administration was a weak predictor of cumulative outcome [2]. These findings collectively suggest that the negative effect of elevated progesterone level on pregnancy with fresh embryo transfer is not a result of an adverse effect on the oocytes. This conclusion is in accordance with previous studies [29–31].
Although the precise mechanism of the deleterious effect of elevated progesterone is unclear, evidence suggests that elevated progesterone could initiate premature secretory transformation of the endometrium, leading to a premature closure of the implantation window before embryos are available for transfer [32]. This state of asynchronous embryo-endometrium cross-dialogue may adversely affect implantation and consequently result in a reduced pregnancy rate [33, 34].
In summary, relying on cohort-specific variables to predict outcomes in fresh IVF cycles could lead to a biased interpretation if the information on the embryos transferred is ignored. Ovarian stimulation protocols designed to retrieve an “optimal” quantity of oocytes in order to maximize pregnancy rates during fresh cycles may not be the best medical decision. Serum progesterone level on the day of hCG administration merits further investigation. Other predictors identified could be useful for patient counseling in clinical practice.
Acknowledgments
All authors report no conflicts of interest and no financial support.
Conflict of interest and sources of funding statement
All authors report no financial and other conflict of interest and no funding support relevant to the subject of this article.
Footnotes
Capsule This retrospective study identified nine predictors of live birth in fresh IVF cycles and determined that the two most important predictors were the number of good-quality embryos transferred and progesterone level on the day of hCG administration.
Authors’ roles
Qianfang Cai: substantial contributions to conception and design, acquisition of data, drafting and revising the article and final approval of the version to be published.
Fei Wan: substantial contributions to analysis and interpretation of data, manuscript drafting and final approval of the version to be published.
Dina Appleby: substantial contributions to revising the article critically for important intellectual content and final approval of the version to be published.
Linli Hu: substantial contributions to revising the article critically for important intellectual content and final approval of the version to be published.
Hanwang Zhang: substantial contributions to revising the article critically for important intellectual content and final approval of the version to be published.
References
- 1.Van Loendersloot LL, van Wely M, Limpens J, Bossuyt PM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–589. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 2.Cai QF, Wan F, Huang R, Zhang HW. Factors predicting the cumulative outcome of IVF/ICSI treatment: a multivariable analysis of 2450 patients. Hum Reprod. 2011;26:2532–2540. doi: 10.1093/humrep/der228. [DOI] [PubMed] [Google Scholar]
- 3.Jun SH, Choi B, Shahine L, Westphal LM, Behr B, Reijo Pera RA, et al. Defining human embryo phenotypes by cohort-specific prognostic factors. PLoS One. 2008;3:e2562. doi: 10.1371/journal.pone.0002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strandell A, Bergh C, Lundin K. Selection of patients suitable for one-embryo transfer may reduce the rate of multiple births by half without impairment of overall birth rates. Hum Reprod. 2000;15:2520–2525. doi: 10.1093/humrep/15.12.2520. [DOI] [PubMed] [Google Scholar]
- 5.Opsahl MS, Blauer KL, Black SH, Lincoln SR, Thorsell L, Sherins RJ. The number of embryos available for transfer predicts successful pregnancy outcome in women over 39 years with normal ovarian hormonal reserve testing. J Assist Reprod Genet. 2001;18:551–556. doi: 10.1023/A:1011906024170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpes A, Sammartano F, Coffaro F, Mistretta V, Scaglione P, Allegra A. Number of good quality embryos on day 3 is predictive for both pregnancy and implantation rates in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82:1330–1336. doi: 10.1016/j.fertnstert.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. The antral follicle count is a better marker than basal follicle-stimulating hormone for the selection of older patients with acceptable pregnancy prospects after in vitro fertilization. Fertil Steril. 2005;83:811–814. doi: 10.1016/j.fertnstert.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13:476–480. doi: 10.1016/S1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 9.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 10.Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 11.Baruffi RL, Mauri AL, Petersen CG, Nicoletti A, Pontes A, Oliveira JB, et al. Single-embryo transfer reduces clinical pregnancy rates and live births in fresh IVF and Intracytoplasmic Sperm Injection (ICSI) cycles: a meta-analysis. Reprod Biol Endocrinol. 2009;7:36. doi: 10.1186/1477-7827-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandian Z, Templeton A, Serour G, Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane review. Hum Reprod. 2005;20:2681–2687. doi: 10.1093/humrep/dei153. [DOI] [PubMed] [Google Scholar]
- 13.Cai Q, Wan F, Huang K, Zhang H. Does the number of oocytes retrieved influence pregnancy after fresh embryo transfer? PLoS ONE. 2013;8(2):e56189. doi: 10.1371/journal.pone.0056189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13:343–355. doi: 10.1093/humupd/dmm007. [DOI] [PubMed] [Google Scholar]
- 15.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–2100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Chen CD, Tsai YY, Chang LJ, Ho HN, Yang YS. Embryo quality is more important for younger women whereas age is more important for older women with regard to in vitro fertilization outcome and multiple pregnancy. Fertil Steril. 2006;86:64–69. doi: 10.1016/j.fertnstert.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 17.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York: Erlbaum; 2008. [Google Scholar]
- 18.Kenny DA. Mediation. Available at: http://davidakenny.net/cm/mediate.htm#SE. Accessed Mar 23, 2013.
- 19.Munné S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online. 2007;14:628–634. doi: 10.1016/S1472-6483(10)61057-7. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131–137. doi: 10.1198/0003130043277. [DOI] [Google Scholar]
- 21.Schemper M. The relative importance of predictors in studies of survival. Stat Med. 1993;12:2377–2382. doi: 10.1002/sim.4780122413. [DOI] [PubMed] [Google Scholar]
- 22.Mittlbock M, Schemper M. Explained variation for logistic regression. Stat Med. 1996;15:1987–1997. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1987::AID-SIM318>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Wong IL, Paulson RJ, Lobo RA, Sauer MV. Recipient’s age does not adversely affect pregnancy outcome after oocyte donation. Am J Obstet Gynecol. 1995;172:96–100. doi: 10.1016/0002-9378(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 25.Stolwijk AM, Zielhuis GA, Sauer MV, Hamilton CJ, Paulson RJ. The impact of the woman’s age on the success of standard and donor in vitro fertilization. Fertil Steril. 1997;67:702–710. doi: 10.1016/S0015-0282(97)81370-2. [DOI] [PubMed] [Google Scholar]
- 26.Toner JP, Grainger DA, Frazier LM. Clinical outcomes among recipients of donated eggs: an analysis of the U.S. national experience, 1996–1998. Fertil Steril. 2002;78:1038–1045. doi: 10.1016/S0015-0282(02)03371-X. [DOI] [PubMed] [Google Scholar]
- 27.Thurin A, Hardarson T, Hausken J, Jablonowska B, Lundin K, Pinborg A, et al. Predictors of ongoing implantation in IVF in a good prognosis group of patients. Hum Reprod. 2005;20:1876–1880. doi: 10.1093/humrep/deh872. [DOI] [PubMed] [Google Scholar]
- 28.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanchin R, Righini C, Olivennes F, Taieb J, de Ziegler D, Frydman R. Computerized assessment of endometrial echogenicity: clues to the endometrial effects of premature progesterone elevation. Fertil Steril. 1999;71:174–181. doi: 10.1016/S0015-0282(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 30.Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21:3217–3227. doi: 10.1093/humrep/del284. [DOI] [PubMed] [Google Scholar]
- 31.Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohi J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod. 2006;21:1503–1507. doi: 10.1093/humrep/dei474. [DOI] [PubMed] [Google Scholar]
- 32.Marchini M, Fedele L, Bianchi S, Losa GA, Ghisletta M, Candiani GB. Secretory changes in preovulatory endometrium during controlled ovarian hyperstimulation with buserelin acetate and human gonadotropins. Fertil Steril. 1991;55:717–721. doi: 10.1016/s0015-0282(16)54236-8. [DOI] [PubMed] [Google Scholar]
- 33.Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. 2003;9:515–522. doi: 10.1093/humupd/dmg045. [DOI] [PubMed] [Google Scholar]
- 34.Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, et al. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril. 2009;91:949–952. doi: 10.1016/j.fertnstert.2006.12.064. [DOI] [PubMed] [Google Scholar]


