Abstract
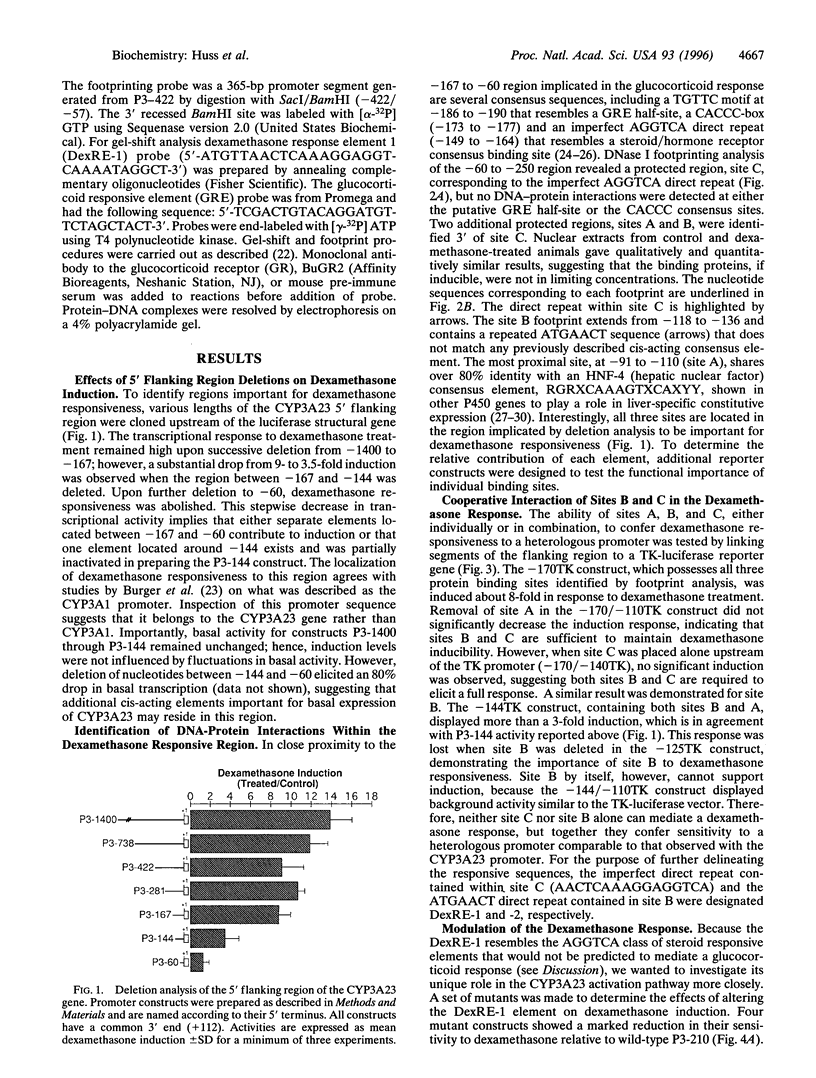
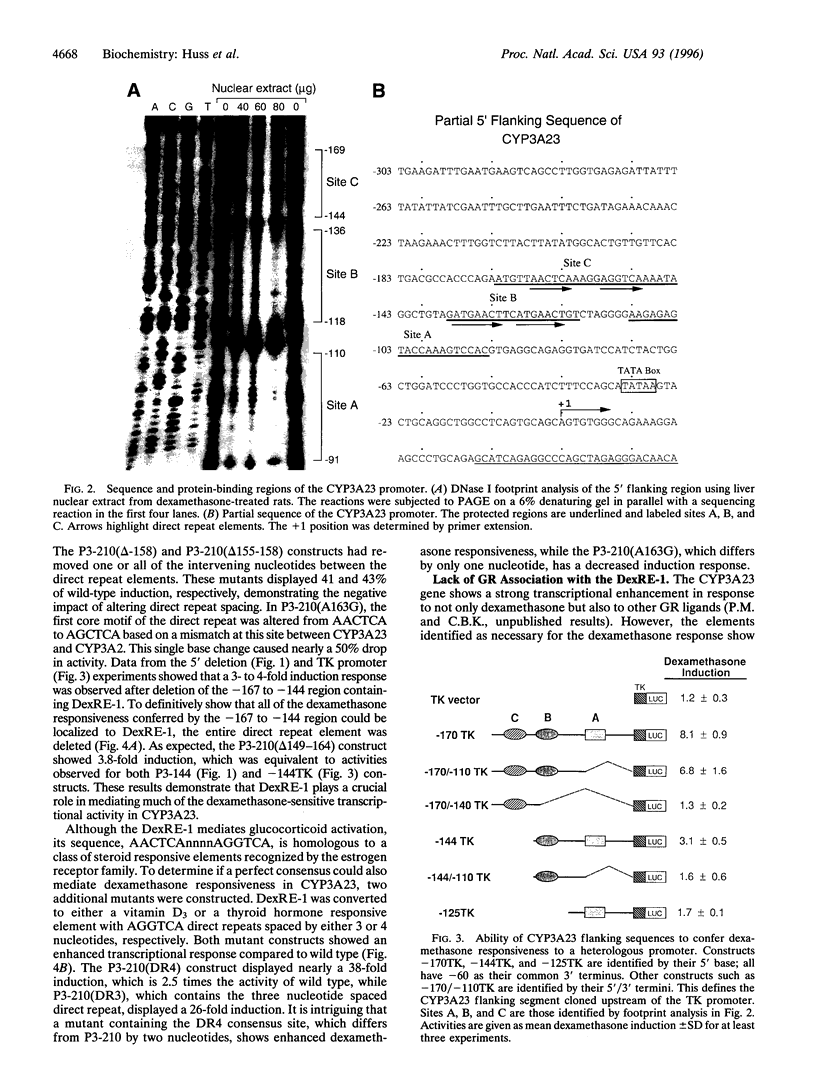
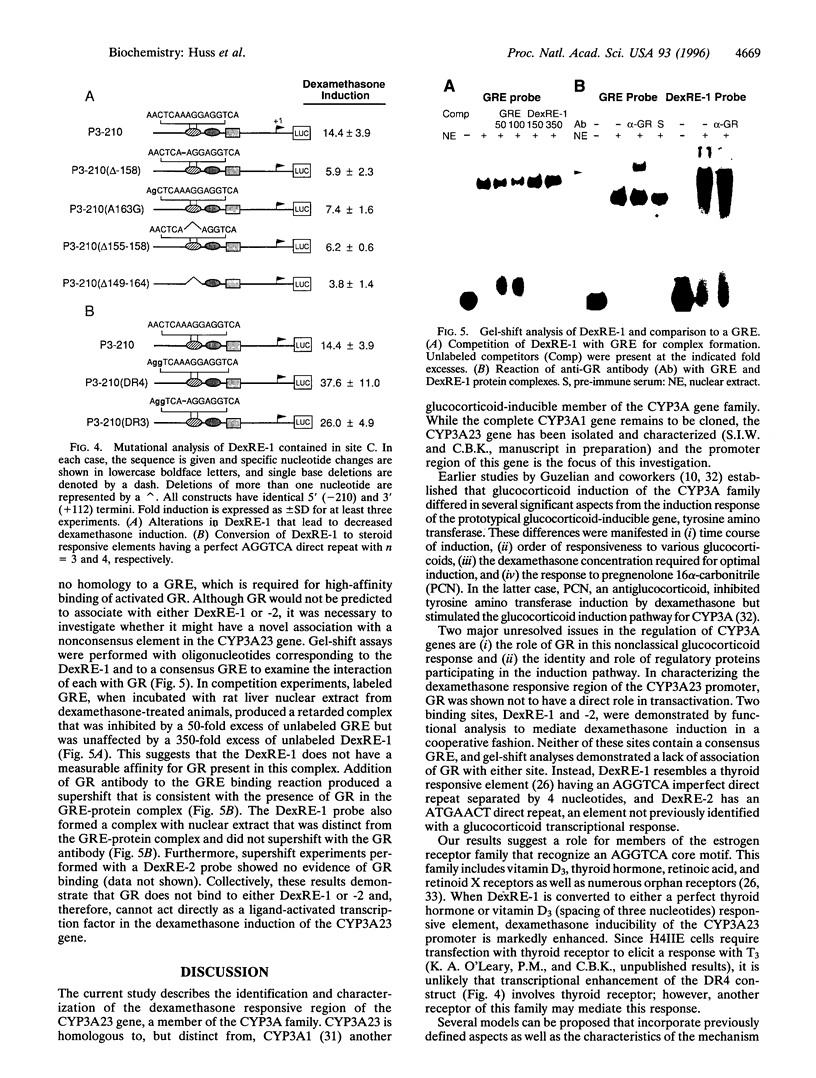
Elements responsible for dexamethasone responsiveness of CYP3A23, a major glucocorticoid-inducible member of the CYP3A gene family, have been identified. DNase I footprint analysis of the proximal promoter region revealed three protected sites (sites A, B, and C) within the sequence defined by -167 to -60. Mutational analysis demonstrated that both sites B and C were necessary for maximum glucocorticoid responsiveness and functioned in a cooperative manner. Interestingly, neither site contained a glucocorticoid responsive element. Embedded in site C was an imperfect direct repeat (5'-AACTCAAAGGAGGTCA-3'), showing homology to an AGGTCA steroid receptor motif, typically recognized by the estrogen receptor family, while site B contained an ATGAACT direct repeat; these core sequences were designated dexamethasone response elements 1 and 2 (DexRE-1 and -2), respectively. Neither element has previously been associated with a glucocorticoid-activated transcriptional response. Conversion of the DexRE-1 to either a perfect thyroid hormone or vitamin D3 responsive element further enhanced induction by dexamethasone. Gel-shift analysis demonstrated that glucocorticoid receptor did not associate with either DexRE-1 or -2; hence, glucocorticoid receptor does not directly mediate glucocorticoid induction of CYP3A23. These unusual features suggest an alternate pathway through which glucocorticoids exert their effects.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell P. A., Falany C. N., McQuiddy P., Kasper C. B. Glucocorticoid repression and basal regulation of the epoxide hydrolase promoter. Arch Biochem Biophys. 1990 Jun;279(2):363–369. doi: 10.1016/0003-9861(90)90503-q. [DOI] [PubMed] [Google Scholar]
- Brooks B. A., McBride O. W., Dolphin C. T., Farrall M., Scambler P. J., Gonzalez F. J., Idle J. R. The gene CYP3 encoding P450pcn1 (nifedipine oxidase) is tightly linked to the gene COL1A2 encoding collagen type 1 alpha on 7q21-q22.1. Am J Hum Genet. 1988 Sep;43(3):280–284. [PMC free article] [PubMed] [Google Scholar]
- Burger H. J., Schuetz J. D., Schuetz E. G., Guzelian P. S. Paradoxical transcriptional activation of rat liver cytochrome P-450 3A1 by dexamethasone and the antiglucocorticoid pregnenolone 16 alpha-carbonitrile: analysis by transient transfection into primary monolayer cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2145–2149. doi: 10.1073/pnas.89.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Lepar G., Kemper B. A transcriptional regulatory element common to a large family of hepatic cytochrome P450 genes is a functional binding site of the orphan receptor HNF-4. J Biol Chem. 1994 Feb 18;269(7):5420–5427. [PubMed] [Google Scholar]
- Chen D., Park Y., Kemper B. Differential protein binding and transcriptional activities of HNF-4 elements in three closely related CYP2C genes. DNA Cell Biol. 1994 Jul;13(7):771–779. doi: 10.1089/dna.1994.13.771. [DOI] [PubMed] [Google Scholar]
- Cooper K. O., Reik L. M., Jayyosi Z., Bandiera S., Kelley M., Ryan D. E., Daniel R., McCluskey S. A., Levin W., Thomas P. E. Regulation of two members of the steroid-inducible cytochrome P450 subfamily (3A) in rats. Arch Biochem Biophys. 1993 Mar;301(2):345–354. doi: 10.1006/abbi.1993.1154. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by pregnenolone-16 alpha-carbonitrile in primary monolayer cultures of adult rat hepatocytes and in a cell-free translation system. J Biol Chem. 1981 Jun 25;256(12):6060–6068. [PubMed] [Google Scholar]
- Elshourbagy N. A., Guzelian P. S. Separation, purification, and characterization of a novel form of hepatic cytochrome P-450 from rats treated with pregnenolone-16 alpha-carbonitrile. J Biol Chem. 1980 Feb 25;255(4):1279–1285. [PubMed] [Google Scholar]
- Glass C. K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994 Jun;15(3):391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Gonzalez F. J., Schmid B. J., Umeno M., Mcbride O. W., Hardwick J. P., Meyer U. A., Gelboin H. V., Idle J. R. Human P450PCN1: sequence, chromosome localization, and direct evidence through cDNA expression that P450PCN1 is nifedipine oxidase. DNA. 1988 Mar;7(2):79–86. doi: 10.1089/dna.1988.7.79. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Song B. J., Hardwick J. P. Pregnenolone 16 alpha-carbonitrile-inducible P-450 gene family: gene conversion and differential regulation. Mol Cell Biol. 1986 Aug;6(8):2969–2976. doi: 10.1128/mcb.6.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kirita S., Matsubara T. cDNA cloning and characterization of a novel member of steroid-induced cytochrome P450 3A in rats. Arch Biochem Biophys. 1993 Dec;307(2):253–258. doi: 10.1006/abbi.1993.1587. [DOI] [PubMed] [Google Scholar]
- Komori M., Oda Y. A major glucocorticoid-inducible P450 in rat liver is not P450 3A1. J Biochem. 1994 Jul;116(1):114–120. doi: 10.1093/oxfordjournals.jbchem.a124482. [DOI] [PubMed] [Google Scholar]
- Miyata M., Nagata K., Yamazoe Y., Kato R. A gene structure of testosterone 6 beta-hydroxylase (P450IIIA). Biochem Biophys Res Commun. 1991 May 31;177(1):68–73. doi: 10.1016/0006-291x(91)91949-d. [DOI] [PubMed] [Google Scholar]
- Miyata M., Nagata K., Yamazoe Y., Kato R. Transcriptional elements directing a liver-specific expression of P450/6 beta A (CYP3A2) gene-encoding testosterone 6 beta-hydroxylase. Arch Biochem Biophys. 1995 Apr 1;318(1):71–79. doi: 10.1006/abbi.1995.1206. [DOI] [PubMed] [Google Scholar]
- Muller M., Renkawitz R. The glucocorticoid receptor. Biochim Biophys Acta. 1991 Feb 16;1088(2):171–182. doi: 10.1016/0167-4781(91)90052-n. [DOI] [PubMed] [Google Scholar]
- Nagata K., Gonzalez F. J., Yamazoe Y., Kato R. Purification and characterization of four catalytically active testosterone 6 beta-hydroxylase P-450s from rat liver microsomes: comparison of a novel form with three structurally and functionally related forms. J Biochem. 1990 May;107(5):718–725. doi: 10.1093/oxfordjournals.jbchem.a123115. [DOI] [PubMed] [Google Scholar]
- Ribeiro V., Lechner M. C. Cloning and characterization of a novel CYP3A1 allelic variant: analysis of CYP3A1 and CYP3A2 sex-hormone-dependent expression reveals that the CYP3A2 gene is regulated by testosterone. Arch Biochem Biophys. 1992 Feb 14;293(1):147–152. doi: 10.1016/0003-9861(92)90377-9. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J Biol Chem. 1984 Feb 10;259(3):2007–2012. [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate de novo synthesis of a common form of cytochrome P-450 in cultures of adult rat hepatocytes and in the liver in vivo. J Biol Chem. 1984 Feb 10;259(3):1999–2006. [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Lalley P. A., Kasper C. B. Chromosomal assignments of genes coding for components of the mixed-function oxidase system in mice. Genetic localization of the cytochrome P-450PCN and P-450PB gene families and the nadph-cytochrome P-450 oxidoreductase and epoxide hydratase genes. J Biol Chem. 1985 Jan 10;260(1):515–521. [PubMed] [Google Scholar]
- Simmons D. L., McQuiddy P., Kasper C. B. Induction of the hepatic mixed-function oxidase system by synthetic glucocorticoids. Transcriptional and post-transcriptional regulation. J Biol Chem. 1987 Jan 5;262(1):326–332. [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Telhada M. B., Pereira T. M., Lechner M. C. Effect of dexamethasone and phenobarbital on run-on transcription rate and CYP3A mRNA concentration in rat liver: changes during development. Arch Biochem Biophys. 1992 Nov 1;298(2):715–725. doi: 10.1016/0003-9861(92)90471-8. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]







