Abstract
Purpose
We performed TaqMan genotyping assays of anti-Mullerian hormone (AMH) and anti-Mullerian hormone receptor type II (AMHRII) single nucleotide polymorphisms (SNPs) in order to investigate how their frequency and distribution affect infertility treatment outcome.
Methods
Eighty Japanese women (advanced age: n = 51, endometriosis: n = 18, male infertility as a control: n = 11) who undertook ART were included in the study, and all couples underwent a full infertility investigation protocol. In order to investigate the natural distribution of SNPs, a naturally pregnant group of 28 subjects was recruited from among women who conceived naturally and subsequently delivered in our department. Genomic DNA was extracted from peripheral blood and genotyping was conducted by TaqMan genotyping assay. The relationship of AMH and AMHRII SNPs and treatment outcome in infertile women. Comparison of allele and genotype frequencies of infertile patients with naturally pregnant women.
Results
AMHRII −482 A>G homozygote mutation was complicated with ISV 5–6 C>T homozygote mutation and showed a significantly lower oocyte retrieval rate compared with a wild type. Two of 3 cases of AMHRII −482 A>G homozygote mutation were poor responders, and the distribution and frequency of each allele of naturally pregnant women showed no statistical difference compared with infertile women.
Conclusions
This study revealed the possible involvement of AMHRII −482 A>G polymorphism on the malfunction of follicular development in Japanese women.
Keywords: AMH, AMHRII, Single nucleotide polymorphism, AMHRII −482 A>G, Oocyte retrieval rate
Introduction
There are various known parameters for assessing ovarian reserve, including ovarian volume, antral follicle count, follicle stimulating hormone (FSH) and Inhibin B at the beginning of the menstrual cycle, as well as anti-Mullerian hormone (AMH).
Serum AMH, in particular, has been shown to be a better endocrine indicator of a patient’s follicular response to controlled ovarian stimulation (COS) with gonadotropins [1–5] in assisted reproductive technology. On the other hand, it has been reported that SNPs of AMH genes also affect follicle counts and folliculogenesis, thus leading to infertility [6]. However, what impact these SNPs have on the clinical outcome of infertile treatment remains unknown. To investigate how the frequency and distribution of AMH and AMHRII SNPs in infertile patients affect infertility treatment outcome, we performed TaqMan genotyping assays of AMH and AMHRII.
Material and methods
Patients
We included in this study 80 Japanese women who undertook ART between March 2010 and March 2013 in the Department of Obstetrics and Gynecology at Osaka Medical College. All couples underwent a full infertility investigation protocol, including a laparoscopic examination. Infertile patients were classified into 3 groups: advanced age (35 years old or older), severe endometriosis, and male infertility. In the study, male infertility was allocated as the control group, and unexplained infertility was included in the advanced age group.
Moreover, to investigate the natural distribution of the SNPs, a group of 28 subjects was recruited from among women who conceived naturally and subsequently delivered in our department during the same period. Our institutional review board approved this protocol (No. 71) and its consent form, and we received informed consent for this study from all participants.
Protocol for controlled ovarian hyperstimulation (COH)
Controlled ovarian hyperstimulation, hormone assays, follicle monitoring, oocyte retrieval, insemination, embryo culture, embryo transfers and confirming embryo quality were performed as previously reported [7]. Pregnancy was confirmed during ultrasound examination by the identification of an intrauterine gestational sac.
DNA preparation and TaqMan® genotyping assay
Genomic DNA was extracted from peripheral blood using a High Pure PCR Template Preparation Kit (Roche Diagnostic GmbH, Mannheim, Germany), and genotyping was carried out by TaqMan® genotyping assay.
TaqMan® SNP Genotyping Assay 5′ nuclease technology uses two allele-specific TaqMan® MGB probes and a PCR primer pair to detect the specific SNP target. The probes and primers uniquely align with the genome, enabling TaqMan® genotyping technology to provide unmatched specificity. Genotypes were decided using Taqman allelic discrimination assays. For the AMH and AMHRII polymorphisms, each probe is shown in Table 1. Each PCR reaction contained 2 ng of dried genomic DNA, 1 μl of TaqMan Universal PCR Master Mix 2 ×, 0.025 μl of the 80 × AMH Assay Mix or 0.1 μl of the 20 × AMHRII SNP mix in a total volume of 2 μl.
Table 1.
For investigating the AMH and AMHRII polymorphisms, genotypes were decided using Taqman allelic discrimination assays
| Gene | Base pair change | Position (aa change) | Primers | Base pair | Tm (C0) |
|---|---|---|---|---|---|
| AMH | 146 T>G | Exon1 | F:ACCAGTGGCCTCATCTTCC | 152 | 59 |
| R:AGGAAGGCCTGCTCATAGG | |||||
| AMHR-II | −482 A>G | Promoter | F:GGTAACCTCTAATATGGGCTGTG | 150 | 59 |
| R:TCTCAGGAGGAAACCAATGTG | |||||
| AMHR-II | IVS1+149 T>A | Intron 1 | F:CCCTTTGGAAGAGTGGTGAG | 196 | 56 |
| (−) | R:AGGTGGGAGTGAATGCAGAG | ||||
| AMHR-II | IVS5-6 C>T | Intron 5 | F:AGCTGTGTTTCTCCCAGGTG | 197 | 56 |
| (−) | R:ATGGCAACCAGTTTTCCTTG | ||||
| AMHR-II | IVS10+77 A>G | Intron 10 | F:AAGAGGCCTAGGCTGTTGGT | 241 | 60 |
| (−) | R:CAAGGTTGAGCAGGAGGAAG |
Each SNP assay was designed by Life Technologies (Tokyo, Japan). The TaqMan® genotyping assay was performed in a 48-well StepOne™ Real-Time PCR according to the manufacturer’s instructions.
Statistical analysis
All experiments were performed in duplicate, and statistical analysis was done with StatMate IV (ATMS Co., Ltd.). Genotype distribution was tested for Hardy-Weinberg equilibrium, and the difference in genotype frequencies was determined/calculated by a chi-square test. A P value of <0.05 was considered to be statistically significant.
Results
Comparison of Allele and genotype frequencies of infertile patient with women who delivered following natural conception
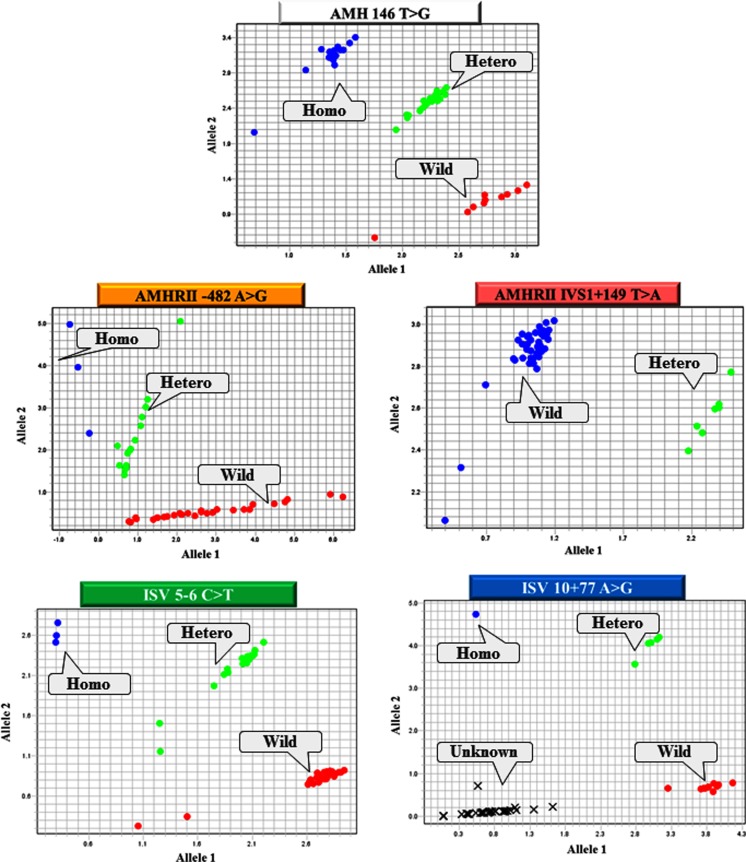
All genotypes, except for IVS10+77 A>G of the AMH and AMHRII genes, were easily confirmed by TaqMan® genotyping assays. In IVS10+77 A>G, there were many cases of unclassifiable genotypes (Fig. 1). Table 2 shows the distribution of each genotype in infertile patients, the male infertility control, and the natural pregnancy group (Table 2). The distribution and frequency of each allele in women who conceived naturally showed no statistical difference compared with infertile women (Table 3).
Fig. 1.
All genotypes, except for IVS10+77 A>G of the AMH and AMHRII genes, were easily confirmed by TaqMan genotyping assays. In IVS10+77 A>G, there were many cases of unclassifiable genotypes
Table 2.
The distribution of each genotype of women with endometriosis, advanced age, and natural pregnancy as control
| AMH 146 T>G | AMHR −482 A>G | AMHR IVS1+149 T>A T>G | AMHR ISV 5-6 C>T | AMHR ISV 10+77 A>G | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homo | Hetero | Wild | Homo | Hetero | Wild | Homo | Hetero | Wild | Homo | Hetero | Wild | Homo | Hetero | Wild | |
| Endometriosis | 3 | 8 | 4 | 3 | 2 | 12 | 0 | 6 | 11 | 3 | 2 | 7 | 2 | 2 | 6 |
| Advanced age | 8 | 26 | 14 | 0 | 27 | 23 | 0 | 9 | 25 | 1 | 25 | 21 | 0 | 13 | 8 |
| Male infertility | 2 | 18 | 21 | 3 | 13 | 18 | 0 | 8 | 27 | 4 | 11 | 17 | 2 | 2 | 6 |
| Natural pregnancy | 1 | 11 | 9 | 3 | 9 | 11 | 0 | 7 | 17 | 3 | 8 | 11 | 2 | 0 | 0 |
Table 3.
The distribution and frequency of each allele in women who conceived naturally showed no statistical difference compared with infertile women
| Infertility | Natural pregnancy | X 2 test (P value) | |
|---|---|---|---|
| AMH 146 T>G | |||
| T:G | 90:54 | 29:13 | NS |
| T/T:T/G:G/G | 25:40:2 | 9:11:1 | NS |
| AMHR −482 A>G | |||
| A:G | 118:38 | 31:15 | NS |
| A/A:A/G:G/G | 43:32:3 | 11:9:3 | NS |
| AMHR IVS1+149 T>A | |||
| T:A | 148:6 | 41:7 | NS |
| T/T:T/A:A/A | 66:16:0 | 17:7:0 | NS |
| AMHR ISV5-6 C>T | |||
| A:G | 57:21 | 28:10 | NS |
| A/A:A/G:G/G | 20:17:2 | 11:8:3 | NS |
| AMHR ISV10+77 A>G | |||
| C:T | 100:40 | 30:14 | NS |
| C/C:C/T:T/T | 35:30:5 | 11:6:0 | NS |
Clinical characteristics of AMH and AMHRII SNP in infertile women
AMHRII −482 A>G homozygote mutation, all of which were accompanied with ISV 5–6 C>T homozygote mutation, showed a statistically lower oocyte retrieval rate (28.5 %) and pregnancy rate (0 %) in comparison with the wild type; however, other homozygote mutations of AMH and AMHRII showed no statistical difference in their oocyte retrieval rate, fertilization rate, and pregnancy rate (Table 4).
Table 4.
AMHRII -482 A>G homozygote mutation, all of which were accompanied with ISV 5-6 C>T homozygote mutation, showed a statistically lower oocyte retrieval rate (28.5 %) and pregnancy rate (0 %) in comparison with the wild type; however, other homozygote mutations of AMH and AMHRII showed no statistical difference in their oocyte retrieval rate, fertilization rate, and pregnancy rate
| Polymorphism (N = 80) | Aspirated follicle | Retrieved oocyte | Oocyte retrieved rate | Fertilization rate (%) | Pregnancy rate (%) | |
|---|---|---|---|---|---|---|
| AMH 146 T>G | Homo (n = 12) | 96 | 48 | 50.0 | 68.8 | 27.2 |
| Wild (n = 27) | 266 | 155 | 58.2 | 72.9 | 29.3 | |
| AMHR -482 A>G | Homo (n = 3) | 21 | 6 | 28.5a | 50.0 | 0b |
| Wild (n = 45) | 503 | 276 | 54.8 | 71.0 | 28.1 | |
| AMHR ISV5-6 C>T | Homo (n = 5) | 49 | 22 | 44.8 | 72.7 | 0b |
| Wild (n = 36) | 436 | 245 | 56.8 | 69.8 | 19.4 | |
| AMHR ISV10+77 A>G | Homo (n = 3) | 17 | 7 | 41.1 | 71.4 | 33.3 |
| Wild (n = 21) | 249 | 126 | 50.6 | 73.0 | 14.2 | |
X 2 test
a,b p < 0.05
As well, there was no statistical difference in the serum hormonal value of AMHRII −482 A>G homozygote mutation compared with the wild type. Additionally, total dose of hMG/FSH to retrieve the oocyte showed no statistical difference between the homozygote mutation and wild types in each SNP (Table 5).
Table 5.
There was no statistical difference in the serum hormonal value of AMHRII -482 A>G homozygote mutation compared with the wild type. Additionally, total dose of hMG/FSH to retrieve the oocyte showed no statistical difference between the homozygote mutation and wild types in each SNP
| AMHR −482 A>G | AMHR −482 A>G | P value | |
|---|---|---|---|
| Homo mutant | Wild type | ||
| N = 3 | N = 45 | ||
| Age(y) | 38.7 ± 4.16 | 38.0 ± 4.65 | NS |
| LH (mIU/ml) | 2.53 ± 0.95 | 7.47 ± 6.4 | NS |
| FSH (mIU/ml) | 3.4 ± 1.3 | 9.4 ± 5.7 | NS |
| Estradiol | 21.4 ± 25.4 | 67.6 ± 107.8 | NS |
| Total dose of hMG/FSH (IU) | 3100.1 ± 3408.5 | 3041.6 ± 1359.0 | NS |
Values presented as mean ± SD
Patients background of AMHRII −482 A>G homozygote mutation
All of AMHRII −482 A>G homozygote mutations were found in the presence of endometriosis, and two of them were complicated with diminished ovarian reserve (DOR). All cases of AMHRII −482 A>G homozygote mutation were also accompanied by ISV 5–6 C>T homozygote mutation. In the natural pregnancy group, a homozygote mutation was confirmed in all SNPs. −482 A>G homozygote mutation cases consisted of two patients with a past history of repeated pregnancy loss (RPL) and one case of fetal growth restriction (FGR). All cases of −482 A>G homozygote mutation were accompanied by a ISV 5–6 C>T homozygote mutation (Table 6).
Table 6.
All of AMHRII -482 A>G homozygote mutations were found in the presence of endometriosis, and two of them were complicated with diminished ovarian reserve (DOR). All cases of AMHRII -482 A>G homozygote mutation were also accompanied by ISV 5-6 C>T homozygote mutation. In the natural pregnancy group, a homozygote mutation was confirmed in all SNPs. -482 A>G homozygote mutation cases consisted of two patients with a past history of repeated pregnancy loss (RPL) and one case of fetal growth restriction (FGR). All cases of -482 A>G homozygote mutation were accompanied by a ISV 5-6 C>T homozygote mutation
| Age | Gravity & Parity | Diagnosis or Obstetric outcome | AMH 146 T>G | AMHR −482 A>G | IVS1+149 T>A | ISV5-6 C>T | ISV10+77 A>G |
|---|---|---|---|---|---|---|---|
| 40 | G0P0 | Endometriosis | Hetero | Homo | Hetero | Homo | Homo |
| poor-responder | |||||||
| 40 | G0P0 | Endometriosis | Hetero | Homo | Hetero | Homo | unknown |
| poor-responder | |||||||
| 34 | G0P0 | Endometriosis | unknown | Homo | Hetero | Homo | unknown |
| 40 | G6P1 | 37 wks 2,948 g F | Hetero | Homo | Hetero | Homo | Homo |
| 40 | G6P1 | 37 wks 3282 g M | Hetero | Homo | Hetero | Homo | unknown |
| 34 | G0P0 | 37 wks 1200 g M | unknown | Homo | Hetero | Homo | unknown |
wks weeks, F female, M male
Discussion
Anti-Mullerrian hormone (AMH), also known as Mullerian inhibiting substance, is a member of the transforming growth factor-β family [8]. During folliculogenesis, AMH expression starts in the granulosa cells of primary follicles, is highest in granulosa cells of preantral and small antral follicles, and gradually diminishes in the subsequent stages of follicular development. As well, AMH is produced by the granulosa cells of early developing follicles in the ovary, and these cells keep expressing AMH until the follicle reaches the appropriate size and differentiation. In this differentiated state, follicles are selected for dominance by the action of FSH [9–11]. Studies of AMH knockout mice have revealed that follicles are recruited at a faster rate than usual and that they become more sensitive to FSH [12]. This indicates that AMH plays an inhibitory role in the recruitment of primordial follicles and that the absence of AMH leads to a prematurely exhausted follicle pool and, subsequently, an earlier cessation of the estrus cycle. Consequently, follicles might be more sensitive to FSH and may be selected for dominance in advance [13]. It has recently been reported that oocytes from early preantral, late preantral, and preovulatory follicles up-regulate AMH mRNA levels in granulosa cells in a manner that is dependent on the development stage of the oocyte itself.
The human AMH gene was cloned in 1986 and is located on the tip of the short arm of chromosome 19p13.3. The length of the human AMH gene is only 2.75kbp and consisted of 5 exons. At the time of this study, 38 different mutations of the AMH gene have been detected. AMH polymorphisms could affect hormone biological activities, which play an important role in controlling follicle recruitment and development. However, in this study, AMH homozygote mutation showed no apparent effect on clinical ART outcomes. AMH binds specifically to AMHR receptor type II (AMHRII) and was cloned in 1994 by two different groups [8, 14]. AMHRII is located on the chromosome 12q13, has a total length of 8.7kbp, and consists of 11 exons. AMHRII is a specific type II receptor, ALK2, 3 and 6 are type I receptors, and Smad 1, 5, 8 are cytoplasmic effectors. Cofactors, coactivators and corepressors for AMH transduction and its relationship with other transduction pathways is not known at the present time.
Most target genes are repressed by AMH action [15], and AMH 146 T>G and AMHRII −482 A>G polymorphisms contribute to the individual variation in the FSH threshold of the ovary [16]. Recent studies in normo-ovulatory women have revealed an association of SNPs of AMH and AMHRII with estradiol levels during the early follicular phase of the menstrual cycle, thus suggesting AMH’s role in the regulation of FSH sensitivity.
Moreover, in patients suffering from normogonadotrophic normo-estrogenic anovulatory infertility, these polymorphisms might become valuable predictors which decide the individual FSH threshold. Distinct differences in the amount of exogenous FSH required to induce an ovarian response might lead to the risk of hyper-response and its subsequent complications such as ovarian hyper-stimulating syndrome (OHSS) and multiple pregnancy [17, 18]. The −482 A>G homozygote mutation could result in diminished AMH signaling [16] and, because the −482 A>G SNP is located at the promoter region, it could possibly cause a disequilibrium with several other SNPs [16]. However, the detail of disequilibrium with other SNPs remains unknown.
The −482 A>G SNP is located at a potential c-Myb and c-Myc transcription factor-binding site and, therefore, may modify promoter activity [19].
In this study, -482 A>G homozygote mutation was associated with ISV5-6 C>T homozygote mutation, and the oocyte retrieval rate of −482 A>G homozygote mutation was statistically lower than in the control group and in other SNPs, despite the result that total dose of hMG/FSH for oocyte retrieval in −482A>G homozygote mutation cases showed no statistical difference compared with the wild type. These results suggest that −482 A>G homozygote mutation could cause poor follicular development. In the normal pregnancy group, all cases of −482 A>G homozygote mutation were associated with ISV5-6 and showed poor obstetric outcome. The inclusion of more cases is necessary to determine whether endometriosis and advanced age affect the frequencies of AMH and AMHR SNPs and whether obstetric outcome is affected by these SNPs.
In conclusion, additional replication and functional studies are necessary to secure definite conclusions as to the effect of AMHRII −482 A>G polymorphism on infertility and obstetric outcome. However, this study revealed the possible involvement of AMHRII −482 A>G polymorphism on the malfunction of follicular development in Japanese women.
Footnotes
Capsule
AMHRII -482A>G polymorphism may have the potential to cause a malfunction in follicular development
References
- 1.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatmen. Hum Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 3.Nakhuda GS, Chu MC, Wang JG, Sauer MV, Lobo RA. Elevated serum müllerian-inhibiting substance may be a marker for ovarian hyperstimulation syndrome in normal women undergoing in vitro fertilization. Fertil Steril. 2006;85:1541–1543. doi: 10.1016/j.fertnstert.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 4.La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–771. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 5.Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles–implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 6.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27:594–608. doi: 10.1093/humrep/der391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karita M, Yamashita Y, Hayashi A, Yoshida Y, Hayashi M, Yamamoto H, et al. Influence of severe endometriosis on gene expression of progesterone receptor in granulosa cells in vitro fertilization-embryo transfer. Fertil Steril. 2011;95:889–894. doi: 10.1016/j.fertnstert.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 8.di Clemente N, Josso N, Gouédard L, Belville C. Components of the anti-Müllerian hormone signaling pathway in gonads. Mol Cell Endocrinol. 2003;211:9–14. doi: 10.1016/j.mce.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Müllerian inhibiting substance signaling. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 10.Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 12.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- 13.Kevenaar ME, Thermmen APN, Rivadeneria F, Uitterlinden AG, Laven JSE, van Schoor NM, et al. A polymorphism in the AMH type II receptor gene is associated with age at menopause in interaction with parity. Hum Reprod. 2007;22:2382–2388. doi: 10.1093/humrep/dem176. [DOI] [PubMed] [Google Scholar]
- 14.Grootegoed JA, Baarends WM, Themmen AP. Welcome to the family: the anti-müllerian hormone receptor. Mol Cell Endocrinol. 1994;100:29–34. doi: 10.1016/0303-7207(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 15.Josso N, Rey R, Picard JY. Testicular anti-Müllerian hormone: clinical applications in DSD. Semin Reprod Med. 2012;5:364–373. doi: 10.1055/s-0032-1324719. [DOI] [PubMed] [Google Scholar]
- 16.Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22:1547–1554. doi: 10.1093/humrep/dem036. [DOI] [PubMed] [Google Scholar]
- 17.Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18(1):71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- 18.Mulders AG, Laven JS, Imani B, Eijkemans MJ, Fauser BC. IVF outcome in anovulatory infertility (WHO group 2)–including polycystic ovary syndrome–following previous unsuccessful ovulation induction. Reprod Biomed Online. 2003;7:50–58. doi: 10.1016/S1472-6483(10)61728-2. [DOI] [PubMed] [Google Scholar]
- 19.Schug J, Overton GC. Modeling transcription factor binding sites with Gibbs Sampling and Minimum Description Length encoding. Proc Int Conf Intell Syst Mol Biol. 1997;5:2. [PubMed] [Google Scholar]