Abstract
Purpose
We assessed the utility of using anti-Müllerian hormone (AMH) and clinical features of polycystic ovary syndrome (PCOS), polycystic ovarian morphology (PCOM), oligo/amenorrhea (OA), and hyperandrogenism (HA) for diagnosing PCOS, and compared their diagnostic accuracy with those of classical diagnostic systems.
Methods
A total of 606 females were admitted to a university hospital with menstrual irregularities or symptoms of hyperandrogenism were enrolled in this cross-sectional study. Fasting blood samples were collected. Pelvic and/or abdominal ultrasonography and clinical examination were performed. Patients were evaluated for the presence of PCOS according to conventional diagnostic criteria. The diagnostic performance of using serum AMH levels alone and in various combinations with the clinical features of PCOM, OA, and HA were investigated.
Results
For the diagnosis of PCOS, the combination of OA and/or HA with AMH showed 83 % sensitivity and 100 % specificity according to the Rotterdam criteria; 83 % sensitivity and 89 % specificity according to the National Institutes of Health (NIH) criteria; and 82 % sensitivity and 93.5 % specificity according to the Androgen Excess Society (AES) criteria.
Conclusions
The serum AMH level is a useful diagnostic marker for PCOS and is correlated with conventional diagnostic criteria. The combination of AMH level with OA and/or HA markedly increased the clinical scope for PCOS diagnosis and can be introduced as a possible objective criterion for the diagnosis of this disease.
Keywords: AMH, PCOS, Hyperandrogenism, Oligo/amenorrhea, Rotterdam criteria, Androgen Excess Society, NIH
Introduction
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism and ovulatory dysfunction. Approximately 6.6–8 % of women of reproductive age are affected, and it is the main cause of anovulatory infertility [1,2].
Anti-Müllerian hormone (AMH) is a member of the transforming growth factor-beta (TGF-β) superfamily. It is secreted by the granulosa cells of small antral and pre-antral follicles for the regulation of early follicular development.
Since the relationship between the number of primordial follicles and their rate of activation, which is reflected by the number of growing follicles, was documented, the utility of this relationship as a marker for ovarian reserve has become widely accepted [3–5]. In addition, the AMH level reflects the quantity of remaining oocytes. It also has minimal inter- and intracycle variability [6,7]. In clinical practice, it is useful for the prediction of poor responders [8] and ovarian hyperstimulation syndrome (OHSS) [9]. Moreover, it is positively correlated with pregnancy rates [10,11].
Levels of AMH in the serum are closely correlated with the number of early antral follicles in both healthy women and women with PCOS [12–14]. Thus, AMH may be a suitable hormonal marker of the ovarian follicle count [15]. Some investigators have suggested that increased AMH levels result from the stimulatory effect of androgens in early follicular growth [16], and others have concluded that AMH can be utilized as a diagnostic marker for ovarian hyperandrogenism [17]. Most researchers agree that AMH should be considered a marker for increased ovarian reserve [18].
Impaired folliculogenesis may result in excess accumulation of pre-antral and small antral follicles, which may ultimately cause the increased AMH levels associated with PCOS [19]. This phenomenon might be the result of an intrinsic abnormality in the ovarian follicles of patients with PCOS, which could contribute to disordered folliculogenesis [20].
Three main diagnostic criteria systems are currently accepted for PCOS: the Rotterdam (ROT), Androgen Excess Society (AES), and National Institutes of Health (NIH) systems. They all consider polycystic ovarian morphology (PCOM), oligo/amenorrhea (OA), and hyperandrogenism (HA) [21–25]. However, each system has recently changed its definition of what constitutes a polycystic appearance [26,27]. Moreover, morphological evaluation of the ovaries takes time and is associated with some technical difficulties. The technical capacities of ultrasound devices are far from standardized, and interobserver variability in antral follicle assessment is inevitable. The presence of various PCOS phenotypes complicates the diagnosis even further [28]. In addition, a vast majority of patients in the target population are teenagers, including virgins that cannot receive transvaginal ultrasonographic evaluation [28]. In the obese population, suboptimal visualization of the ovaries via abdominal ultrasonography is also a limitation.
Therefore, a well-defined objective diagnostic marker for PCOS has yet to be established. In the present study, we assessed whether AMH can be utilized for diagnosing PCOS either alone or in combination with the aforementioned clinical features.
Materials and methods
Between January 2011 and September 2012, a total of 606 women admitted to the gynecologic endocrinology department of our university hospital with menstrual irregularities or symptoms of HA were included in this cross-sectional study.
To evaluate the diagnostic value of AMH for PCOS, we integrated HA and OA symptoms with AMH to construct four new groups of criteria; serum AMH level and the presence of HA, serum AMH level and the presence of OA, serum AMH level and the presence of either OA or HA, and the presence of at least two of the three abovementioned parameters (serum AMH level/OA/HA). First, all patients were evaluated according to the ROT 2003, AES, and NIH criteria separately. We ultimately reevaluated the patients for PCOS according to the newly formed criteria. The analyses are explained in detail in the Results section. To integrate AMH into these new criteria, we evaluated the diagnostic potential of AMH for ovarian morphology and selected the optimum AMH value as 3.8 ng/mL Receiver Operating Characteristic analysis (ROC). First, we used AMH alone to diagnose PCOS, and then we integrated AMH as an element of the newly formed criteria.
The 3.8 ng/mL cutoff was chosen using an ROC curve as the optimal diagnostic threshold of AMH, providing >80 % sensitivity and >80 % specificity for all three diagnostic systems (ROT, AED, and NIH). No other statistical tool reached these sensitivity and specificity levels for all three diagnostic systems (this could be clearly seen in the ROC curves).
Diagnosis of PCOS according to the ROT criteria was based on the presence of at least two of the following three clinical features: OA, HA, and PCOM [23]. The NIH criteria were strict and included only OA and HA [25]. The AES proposed that HA should be a mandatory criterion for the diagnosis, and the presence of either OA or PCOM is required because they are defined as two manifestations of ovarian dysfunction [24]. OA was defined as fewer than eight menstrual cycles during the previous 12 months or a menstrual interval of >35 days. Amenorrhea was defined as the absence of menstruation for >90 days. HA was defined either clinically by hirsutism (modified Ferriman–Gallwey score of >6), severe acne, or seborrhea, or biologically based on a free serum testosterone level of >2.7 pg/mL and/or total testosterone level of >80 ng/dL.
PCOM was identified by pelvic or abdominal ultrasonography and defined as the presence of ≥12 follicles in each ovary measuring 2–9 mm in diameter and/or an increased ovarian volume of >10 mL. The blood samples for AMH measurement were collected in a lithium heparin tube and stored at −80 °C. AMH concentrations were measured with an enzymatically amplified two-sided immunoassay (AMH Gen II ELISA; Beckman Coulter Inc., Brea, CA). The theoretical sensitivity of this method was 0.006 ng/mL, the intra-assay coefficient of variation for high values was 3.3 %, and the interassay coefficient of variation for high values was 6.7 %.
Informed consent was obtained from all women. The study was approved by the Human Ethics Committee of Istanbul University. On days 2–4 of the menstrual cycle, a routine gynecological examination, including an evaluation of basal fasting hormones (follicle-stimulating hormone [FSH], luteinizing hormone [LH], total testosterone [total T], free testosterone [free T], AMH, prolactin [PRL], thyroid-stimulating hormone [TSH], and cortisone) and blood glucose levels, was performed. Transvaginal ultrasonographic evaluation was performed by experienced sonographers with a 7-MHz transvaginal transducer (Sonoline Elegra; Siemens SAS, Saint-Denis, France). A normal fasting blood glucose level was enough to exclude the presence of diabetes. An ACTH stimulation test was performed to exclude the presence of adrenal hyperplasia; in suspected cases, the 17OH progesterone level was measured. In cases of extremely high levels of androgens in serum, androgen-secreting tumors were suspected and MRI scans were performed. Cushing syndrome was excluded based on both clinical symptoms and serum cortisone levels. A dexamethasone suppression test was performed when needed. Body weight and height were measured; body mass index (BMI) was calculated with an electronic digital scale (Mercury; AMZ 14, Tokyo, Japan) in light clothes, and height was measured barefoot with a stadiometer (G-Tech International Co. Ltd., Kyonggi Province, Korea). None of the participants had hyperprolactinemia, thyroid dysfunction, diabetes mellitus, adrenal hyperplasia, Cushing syndrome, an androgen-secreting tumor, or any other significant pathology that could possibly affect reproductive physiology. The participants had not taken any medications known to affect sex steroids in the plasma or metabolic parameters during the previous 6 months.
All statistical analyses were performed with SPSS version 17.0 for Windows (SPSS Inc., USA). Groups were compared using independent-samples t-tests with Bonferroni correction. Values are given as mean ± standard deviation (SD) or absolute number and percentage in parentheses. Relationships between the AMH and the other variables were evaluated using Pearson’s correlation coefficient. A P-value of < 0.05 was considered to be statistically significant.
To determine the diagnostic potential of AMH for PCOM, an ROC curve was constructed. Following selection of the threshold value of AMH as 3.8 ng/mL, new ROC curves were constructed to evaluate the diagnostic value of AMH, alone and as a part of the newly formed criteria, for identifying PCOS. Sensitivity against specificity was plotted at each threshold level, and the area under the curve (AUC) was computed. The AUC represented the probability of diagnosing PCOS, and a value of 0.5 indicated that the probability was no better than chance. ROC curves were constructed using a threshold serum AMH level of 3.8 ng/mL alone and the presence of at least two of the three parameters (serum AMH level of >3.8 ng/mL, OA, or HA) with the original PCOS-ROT, PCOS-AES, and PCOS-NIH diagnoses as references.
Results
The mean age, BMI, and serum TSH values between the PCOS and non-PCOS groups were evaluated according to the three different criteria, and the results were not statistically different. The mean serum AMH, FSH, LH, and PRL levels between the PCOS and non-PCOS groups for each criteria were significantly different (Table 1). Pearson’s correlation analysis revealed negative correlations between AMH and age (r = −0.42) and between AMH and FSH (r = −0.32). There was a positive correlation between AMH and LH (r = 0.4). We found no correlation between AMH and BMI.
Table 1.
Demographic properties of the participants according to the three different diagnostic criteria for PCOS
| Age (years) | BMI (kg/m2) | FSH (IU/mL) | LH (IU/mL) | AMH (ng/mL) | TSH (mIU/L) | PRL (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|
| Rotterdam | PCOS | 27.4 ± 5.4 | 25.5 ± 4.6 | 4.9 ± 1.6a | 6.1 ± 4.3a | 8.6 ± 6.5a | 1.7 ± 0.9 | 18.7 ± 8.5a |
| Non-PCOS | 31.2 ± 4.9 | 26.3 ± 6.4 | 7.1 ± 3.1a | 3.8 ± 1.6a | 2.3 ± 1.6a | 1.8 ± 0.9 | 17.9 ± 7.3a | |
| Androgen Excess Society | PCOS | 27.1 ± 4.9 | 25.7 ± 4.6 | 4.9 ± 1.6a | 5.9 ± 4.3a | 8.8 ± 6.7a | 1.8 ± 0.8 | 19.1 ± 8.7a |
| Non-PCOS | 31.1 ± 5.1 | 26.2 ± 7.5 | 7.1 ± 3.1a | 4.0 ± 2.1a | 2.6 ± 2.3a | 1.8 ± 0.9 | 16.8 ± 7.9a | |
| National Institutes of Health | PCOS | 26.2 ± 5.2 | 25.9 ± 4.6 | 4.8 ± 1.6a | 5.9 ± 4.2a | 9.2 ± 7.1a | 1.7 ± 0.9 | 19.6 ± 8.9a |
| Non-PCOS | 30.1 ± 5.1 | 26.1 ± 6.9 | 6.9 ± 3.1a | 4.1 ± 2.3a | 2.8 ± 2.6a | 1.8 ± 0.9 | 16.8 ± 7.8a | |
Bonferroni test for multiple comparison
aSignificant (p <0.05) within each evaluation system
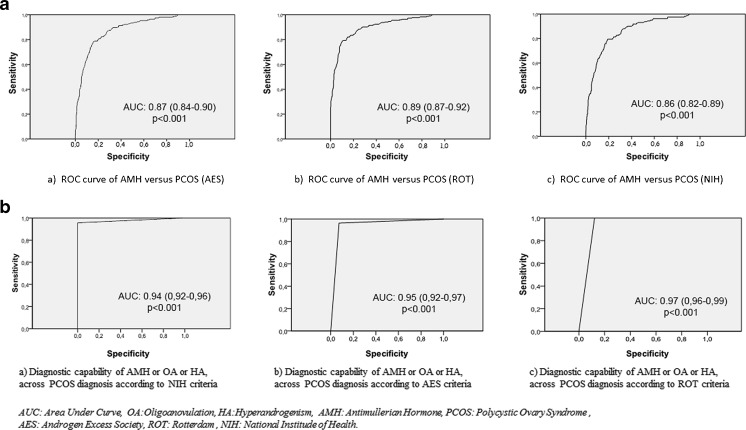
The AMH cutoff value of 3.8 ng/mL had 83 % sensitivity and 87 % specificity for PCOM (Fig. 1). Of the 606 participants, 195 were diagnosed with PCOS according to the AES criteria (Table 2). When the cutoff value was accepted as 3.8 ng/mL, ROC analysis showed that AMH alone had 80 % sensitivity and 80.2 % specificity for the diagnosis of PCOS (AES criteria) (Fig. 2). According to the ROT criteria, 228 patients were diagnosed with PCOS, and the 3.8 ng/mL AMH cutoff value had 81.6 % sensitivity and 85.1 % specificity (Fig. 2). According to the NIH consensus, 164 of 606 patients were diagnosed with PCOS, and the 3.8 ng/mL AMH cutoff value had 80.7 % sensitivity and 74.7 % specificity for the diagnosis (Fig. 2). As shown in the ROC curves, AMH alone had limited sensitivity and specificity for the diagnosis of PCOS compared to each of the other parameters (Fig. 2). After reassessing the participants with our new method using the 3.8 ng/mL AMH cutoff, the diagnostic criteria of the fourth group (presence of two of the three parameters: OA, HA, or AMH) was found to have 100 % specificity and 96 % sensitivity for the diagnosis of PCOS among the patients diagnosed with PCOS according to the ROT criteria (Fig. 2). The 3.8 ng/mL cutoff AMH value in the fourth group resulted in 100 % sensitivity and 88 % specificity for the diagnosis of PCOS according to the NIH criteria and 96 % sensitivity and 93 % specificity for the diagnosis of PCOS according to the AES criteria.
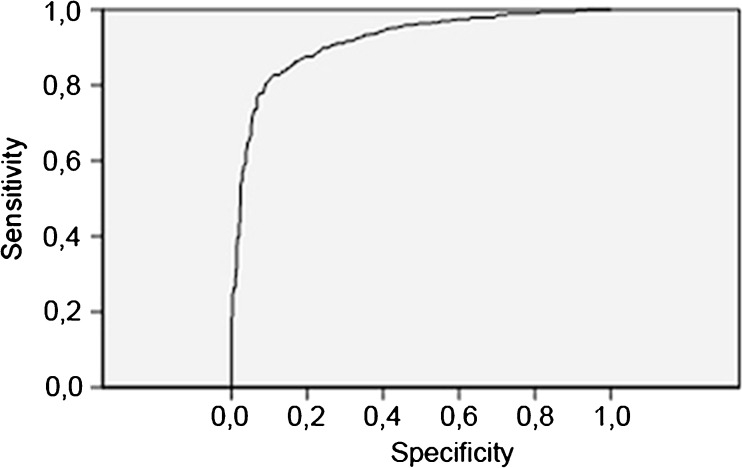
Fig. 1.
ROC curve of AMH versus PCOM. Area under the curve: 0.92 (0.90–0.93) (p < 0.001)
Table 2.
Patients diagnosed with PCOS with three conventional criteria were re-evaluated with the four suggested diagnostic criteria for PCOS, namely the combination of the serum AMH level (3.8 ng/mL) with oligo/amenorrhea (OA) and hyperandrogenism (HA). Distribution of patients before and after the re-evaluation process is depicted
| AMH + OA | AMH + HA | AMH + OA or HA | Two of (AMH/OA/HA) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCOS | Non-PCOS | PCOS | Non-PCOS | PCOS | Non-PCOS | PCOS | Non-PCOS | ||
| ROT | PCOS | 158 | 70 | 166 | 62 | 190 | 38 | 218 | 10 |
| Non-PCOS | 1 | 377 | 1 | 377 | 0 | 378 | 0 | 378 | |
| AES | PCOS | 132 | 63 | 161 | 34 | 160 | 35 | 188 | 7 |
| Non-PCOS | 27 | 384 | 6 | 405 | 30 | 381 | 30 | 381 | |
| NIH | PCOS | 131 | 33 | 137 | 27 | 136 | 28 | 164 | 0 |
| Non-nCOS | 28 | 414 | 30 | 412 | 54 | 388 | 54 | 388 | |
Distribution of patients before and after the re-evaluation process is depicted
Fig. 2.
First row a ROC curves for the diagnostic capability of AMH for PCOS diagnosis in the patient groups previously diagnosed with PCOS according to conventional criteria (AES, ROT, or NIH). Second row b ROC curves for the diagnostic capability of the presence of two of the following features: AMH, HA, or OA
Discussion
In a previous study [29], the highest AMH levels were found in cases in which all three main diagnostic criteria (PCOM, OA, and HA) were used. AMH levels correlate best with PCOM, and OA contributes to increased AMH levels. In that study, HA was found to have less influence on AMH levels, which seem to well reflect the severity of PCOS. Evaluation of PCOM for the diagnosis of PCOS has high interobserver variability. Even with the most technically advanced ultrasonography devices, it can be difficult to count antral follicles transabdominally in virgins or the obese. Thus, there is a need for objective parameters, and the serum AMH level could serve as such for the diagnosis of PCOS.
When AMH was evaluated among the patients diagnosed with PCOS according to all three diagnostic criteria as a single screening tool, it had relatively low sensitivity and specificity for the diagnosis of PCOS. The sensitivity and specificity of AMH with different cutoff values were even lower in the AES and NIH systems than in the ROT system.
We suggest that satisfactory diagnostic potential can be achieved by combining the AMH level with other clinical symptoms. The combination of AMH levels (cutoff value = 3.8 ng/mL) with the presence of HA was found to have 73 % sensitivity and 99 % specificity for diagnosing PCOS among patients previously diagnosed with PCOS according to the ROT criteria (Table 3). Combined with OA, the system showed 69 % sensitivity and 99 % specificity, and combined with either OA or HA resulted in 83 % sensitivity and 100 % specificity. The fourth set of criteria (presence of two of three parameters: serum AMH levels, HA, and OA) gave similar results as the ROT criteria and can be explained as a modification of ROT criteria by replacing serum AMH levels with PCOM. When the AMH cutoff value was set as 3.8 ng/mL, those criteria eventually provided maximum sensitivity and specificity (96 % and 100 %, respectively) for the diagnosis of PCOS.
Table 3.
Diagnostic ability of the four new criteria with a serum AMH cutoff value of 3.8 ng/mL among the patients diagnosed with PCOS according to each of the present diagnostic criteria
| Sensitivity% | Specificity% | AUC | p | ||
|---|---|---|---|---|---|
| Rotterdam | AMH and OA | 69 | 99 | 0.85 (0.84–0.88) | <0.001 |
| AMH and HA | 73 | 99 | 0.86 (0.83–0.89) | <0.001 | |
| AMH and (OA or HA) | 83 | 100 | 0.92 (0.88–0.94) | <0.001 | |
| Two of (AMH/OA/HA) | 96 | 100 | 0.97 (0.96–0.99) | <0.001 | |
| Androgen Excess Society | AMH + OA | 67 | 93 | 0.80 (0.76–0.85) | <0.001 |
| AMH + HA | 83 | 98 | 0.90 (0.87–0.94) | <0.001 | |
| AMH + (OA or HA) | 83 | 93 | 0.87 (0.84–0.90) | <0.001 | |
| Two of (AMH/OA/HA) | 96 | 93 | 0.95 (0.92–0.97) | <0.001 | |
| National Institutes of Health | AMH + OA | 79 | 94 | 0.86 (0.82–0.90) | <0.001 |
| AMH + HA | 83 | 93 | 0.88 (0.84–0.92) | <0.001 | |
| AMH + (OA or HA) | 83 | 88 | 0.86 (0.82–0.89) | <0.001 | |
| Two of (AMH/OA/HA) | 100 | 88 | 0.94 (0.92–0.96) | <0.001 | |
OA oligo/amenorrhea, HA hyperandrogenism
In the AES criteria, HA is an obligatory criterion for PCOS diagnosis, and the combination of AMH level with HA as a diagnostic tool for PCOS reached 98 % specificity and 83 % sensitivity among the patients diagnosed with AES. This relatively low sensitivity increased to 96 % in the fourth group of patients diagnosed using the AES criteria. If attempting to identify the optimum combination for sensitivity and specificity, these values have an advantage over the other combinations (Table 3).
We also found that increased AMH level was not correlated with BMI, in agreement with numerous recent studies [30–33]. Although reproductive function may improve with weight loss in the obese, Thompson et al. [34] did not observe any changes in AMH levels following weight loss in women with PCOS. We previously found a three-fold increase in the serum AMH level in patients with PCOS compared to healthy women, independent of BMI [33]. Numerous associations between plasma AMH levels and other hormonal parameters in women with PCOS have recently been reported [15]. In the present study, we also found a positive correlation between AMH and LH levels, in agreement with a previous study on a group of regularly menstruating females [35]. Pigny et al. [15] found an inverse correlation between circulating AMH and FSH levels in women with PCOS. This correlation was also significant in the present study. Patients with PCOS according to the ROT criteria and without HA had a mean AMH value of 6.83 ± 4.80 ng/mL, significantly lower than that of the remaining 186 patients with HA, who had a mean AMH value of 8.81 ± 6.77 ng/mL (p = 0.3).
Because PCOM is not considered a diagnostic parameter in the NIH criteria, our fourth group successfully covered all patients with PCOS while the PCOS-NIH was used (100 % sensitivity). However, the specificity was found to be relatively low (88 %), as expected.
For patients with an uncertain history of menstruation, a combination of AMH levels only with HA may be useful for diagnosis. This combination had higher sensitivity when both AES and ROT were considered to be gold standards. The combination of AMH only with OA, on the other hand, had relatively lower sensitivity and specificity in all three classical systems than did the combination of AMH and HA.
Li et al. [36] concluded that serum AMH level was not a useful tool for diagnosing all types of PCOS, but rather was only suitable for some specific subtypes such as in patients with HA. They also found that its diagnostic accuracy was very limited when used without HA. Our results are not in agreement, as our data showed that AMH was also with OA, as shown in Table 3. However, both the sensitivity and specificity of the combination of AMH and OA was lower than that of AMH and HA together. Neither combination reached optimum sensitivity and specificity, although both showed sufficient diagnostic capacity compared to the AES and ROT criteria.
We found that AMH levels were significantly higher in PCOS patients with HA than without HA, consistent with previous studies [36,37], indicating that HA is associated with an extra increase in AMH. This may reflect the severity of disruption of folliculogenesis in patients with HA. Serum AMH levels may be related to the severity of the syndrome because they have been observed to be higher in women with insulin-resistant PCOS than in patients with normal insulin sensitivity [38]. According to Wang et al. [19], increased plasma AMH concentrations in women with PCOS may be caused by a disruption in folliculogenesis, leading to excessive accumulation of pre-antral and small antral follicles.
Some investigators have emphasized that AMH concentration is related to the intensification of hormonal and morphological changes in PCOS [39]. AMH concentrations are strongly associated with the main phenotypic features of PCOS, including ovulatory dysfunction and HA. Amenorrheic women with PCOS display higher serum AMH levels than do oligomenorrheic women with PCOS [40].
Whether there is an association between serum AMH levels and different anthropometric, metabolic, and endocrine parameters in patients with PCOS is controversial; age has been reported to be both negatively related [38,40] and not correlated [41] with AMH. We found a negative correlation between age and AMH.
Eilertsen et al. [42] recently concluded that AMH could successfully replace PCOM for the diagnosis of PCOS, and that substitution of AMH with PCOM was equally effective for diagnosis compared to both the AES and ROT criteria. Our results (from the fourth group) clearly support the substitution of AMH with PCOM, which had similar sensitivity for diagnosing PCOS compared to both the ROT and AES criteria. However, the specificity was relatively lower than with the AES criteria. Although PCOM is not even a diagnostic parameter in the NIH criteria, our fourth group reached acceptable levels of sensitivity and specificity. Thus, our results show that AMH can reliably replace PCOM in both the ROT and AES criteria and can even be implemented in the NIH criteria for diagnosing PCOS.
There are some limitations to our study. First, it was not an epidemiologic study and could not predict prevalence, although we including more patients and controls than many previous studies. There may be selection bias in our study sample because our participants were admitted to our clinic with complaints such as menstrual irregularity or HA, and therefore had a high probability of being diagnosed with PCOS.
In conclusion, the serum AMH level alone is a useful marker for diagnosing PCOS and is correlated with conventional diagnostic criteria. However, the combination of the serum AMH level with HA and/or OA markedly increases the diagnostic capability for PCOS and can be introduced as an objective and well-structured criterion.
Acknowledgments
We would like to thank Scott Mathyk, Begum Aydogan MD, Metehan Imamoglu MD, Yasemin Kurban, Kiymet Guler for their sincere support and technical assistance.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Capsule Serum AMH levels alone is a useful marker for diagnosis of PCOS. The combination of the serum AMH level with HA and/or OA markedly increases the diagnostic capability for PCOS and can be introduced as an objective and well structured criterion.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, et al. Müllerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 4.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. AntiMüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RA. What does anti-Müllerian hormone tell you about ovarian function? Clin Endocrinol (Oxf) 2012;77:652–655. doi: 10.1111/j.1365-2265.2012.04451.x. [DOI] [PubMed] [Google Scholar]
- 6.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Müllerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 7.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 8.Sahmay S, Cetin M, Ocal P, Kaleli S, Senol H, Birol F, et al. Serum anti-Müllerian hormone level as a predictor of poor ovarian response in in vitro fertilization patients. Reprod Biol Endocrinol. 2011;10:9–14. doi: 10.1007/s12522-010-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum anti-Müllerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet. 2011;28:1197–1203. doi: 10.1007/s10815-011-9627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahmay S, Demirayak G, Guralp O, Ocal P, Senturk LM, Oral E, et al. Serum anti-Müllerian hormone, follicle stimulating hormone and antral follicle count measurement cannot predict pregnancy rates in IVF/ICSI cycles. J Assist Reprod Genet. 2012;29:589–595. doi: 10.1007/s10815-012-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahmay S, Guralp O, Aydogan B, Cepni I, Oral E, Irez T. Anti-Müllerian hormone and polycystic ovary syndrome: assessment of the clinical pregnancy rates in in vitro fertilization patients. Gynecol Endocrinol. 2013;29:440–443. doi: 10.3109/09513590.2013.769519. [DOI] [PubMed] [Google Scholar]
- 12.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 13.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 14.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 15.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 16.Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10:107–117. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 17.Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E, et al. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:4399–4405. doi: 10.1210/jc.2010-0334. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfield RL, Wroblewski K, Padmanabhan V, Littlejohn E, Mortensen M, Ehrmann DA. AntiMüllerian hormone levels are independently related to ovarian hyperandrogenism and polycystic ovaries. Fertil Steril. 2012;98:242–249. doi: 10.1016/j.fertnstert.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JG, Nakhuda GS, Guarnaccia MM, Sauer MV, Lobo RA. Müllerian inhibiting substance and disrupted folliculogenesis in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:77 e1–5. [DOI] [PubMed]
- 20.Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Müllerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod. 2008;23:2122–2126. doi: 10.1093/humrep/den185. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab. 2005;90:2571–2579. doi: 10.1210/jc.2004-0219. [DOI] [PubMed] [Google Scholar]
- 23.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25 [DOI] [PubMed]
- 24.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. p. 377. [Google Scholar]
- 26.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–1379. doi: 10.1016/S0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 27.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 28.Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94:1044–1051. doi: 10.1016/j.fertnstert.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Sahmay S, Atakul N, Ocul M, Tuten A, Aydogan B, Seyisoglu H. Serum anti-Müllerian hormone levels in the main phenotypes of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):157–161. doi: 10.1016/j.ejogrb.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Weerakiet S, Lertvikool S, Tingthanatikul Y, Wansumrith S, Leelaphiwat S, Jultanmas R. Ovarian reserve in women with polycystic ovary syndrome who underwent laparoscopic ovarian drilling. Gynecol Endocrinol. 2007;23:455–460. doi: 10.1080/09513590701485212. [DOI] [PubMed] [Google Scholar]
- 31.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum Müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–146. doi: 10.1016/S0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 32.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–1826. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 33.Sahmay S, Guralp O, Senturk L, Imamoglu M, Kucuk M, Irez T. Serum anti-Müllerian hormone concentrations in reproductive age women with and without polycystic ovary syndrome: the influence of body mass index. Reprod Med Biol. 2011;10:113–120. doi: 10.1007/s12522-011-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ, et al. The effect of weight loss on anti-Müllerian hormone levels in overweight and obese women with polycystic ovary syndrome and reproductive impairment. Hum Reprod. 2009;24:1976–1981. doi: 10.1093/humrep/dep101. [DOI] [PubMed] [Google Scholar]
- 35.Bungum L, Jacobsson AK, Rosen F, Becker C, Yding Andersen C, Guner N, et al. Circadian variation in concentration of anti-Müllerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011;26:678–684. doi: 10.1093/humrep/deq380. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Ma Y, Chen X, Wang W, Zhang Q, Yang D. Different diagnostic power of anti-Müllerian hormone in evaluating women with polycystic ovaries with and without hyperandrogenism. J Assist Reprod Genet. 2012;29:1147–1151. doi: 10.1007/s10815-012-9839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E, et al. Serum anti-Müllerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- 38.Fleming R, Harborne L, MacLaughlin DT, Ling D, Norman J, Sattar N, et al. Metformin reduces serum Müllerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil Steril. 2005;83:130–136. doi: 10.1016/j.fertnstert.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 39.La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–972. doi: 10.1016/j.fertnstert.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–323. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 41.Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Müllerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- 42.Eilertsen TB, Vanky E, Carlsen SM. Anti-Müllerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod. 2012;27:2494–2502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]