Abstract
Purpose
The aim of the present study was to determine whether supplementation of resveratrol, a stilbenoid antioxidant with therapeutic significance, influences goat (Capra hircus) oocyte maturation and subsequent embryonic development and expression of apoptosis and early embryonic development-related genes.
Methods
Five different concentrations of resveratrol (0.1, 0.25, 0.5, 2.0 and 5.0 μM) were used in in vitro maturation (IVM) medium. Cell tracker blue and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescent stains were used to assay intracellular glutathione and reactive oxygen species levels in mature oocytes. Parthenogenetic activation and hand-made cloning were performed to check the developmental potential following resveratrol treatment. We used quantitative real-time PCR to analyze embryonic gene expression.
Result
Compared to control, no significant improvement was observed in nuclear maturation in resveratrol-treated groups and at 5.0 μM concentration maturation rate decreased significantly (P < 0.05). But resveratrol treatment at the concentrations of 0.25, 0.5 μM significantly reduced intracellular ROS, and increased GSH concentrations. Oocytes treated with 0.25, 0.5 μM resveratrol when subsequently used for PA and HMC, higher extent of blastocyst yields were observed. Expression analysis of proapoptotic (Bax) gene in mature oocytes, cumulus cells, and HMC-derived blastocysts revealed lesser transcript abundances in various resveratrol-treated groups., however no change in the same was observed for antiapoptotic gene (Bcl2). Differential expression of genes associated with developmental competence and nuclear reprogramming was also observed in HMC-derived blastocysts.
Conclusion
Our results show that resveratrol treatment at optimum concentrations (0.25 and 0.5 μM) during IVM produced beneficial microenvironment within oocytes by increasing the intracellular GSH, decreasing ROS level and this in turn, stimulated embryonic development and regulated gene expression.
Keywords: Resveratrol, Goat, Parthenogenesis, Hand-made cloning, Developmental competence
Introduction
The efficiency of reproductive technologies like in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), somatic cell nuclear transfer (SCNT) is dependent on production of viable embryos. Various factors, including oocyte quality, culture conditions, and oocyte activation methods govern the viability of embryos [41]. Complete nuclear and cytoplasmic maturation of in vitro-matured (IVM) oocytes is one of the important factors among them for successful development of embryos in vitro. In vitro maturation (IVM) and developmental potency of oocytes after parthenogenetic activation, in vitro fertilization and somatic cell nuclear transfer are greatly influenced by intracellular glutathione (GSH) level and reactive oxygen species (ROS) [6, 21, 44].
ROS are generated during handling or culturing oocytes or embryos in a high-oxygen atmosphere, and external treatments. Increased level of ROS within cell causes cell membrane damage by lipid peroxidation [31], DNA fragmentation [14], and also influences RNA transcription and protein synthesis [40]. These damaging effects of ROS cease embryonic development and cause early embryonic death. Glutathione, a ubiquitous intracellular free thiol compound, defends the cell from ROS toxicity and regulates the intracellular redox milieu. Apart from that glutathione is also involved in various cellular processes, including the synthesis of DNA and proteins, the metabolism of chemicals, cellular protection, and amino acid transport [19, 29]. Therefore, establishment of an oocyte maturation system that is able to increase the intracellular GSH level of in vitro matured oocytes should improve the viability of IVP embryos and contribute to the development of assisted reproductive technologies in livestock species. Also in the aspect of human fertility preservation and infertility management IVM of oocytes is emerging as promising technique for future use. The recovery of immature oocytes, their subsequent culture and maturation in in vitro condition, eliminates the complications of conventional IVF for which ovarian stimulation is a routine protocol with a concerning risk for ovarian hyperstimulation syndrome [1, 9]. Apart from that IVM also provides a source of invaluable and scarce oocyte [5]. In patients with polycystic ovary syndrome (PCOS) IVM is offered as an important treatment strategy [4, 16]. In spite of so many beneficial sides of IVM over routine IVF technique, this is still confined at experimental level in human. Major reasons behind that is differential quality or competence of oocytes to culminate into developmental term and oxidative stress induced damages that occur during culture of oocytes. Therefore, hunt for suitable antioxidant agent and optimum concentration to be supplemented in media are of utmost importance.
Handful number of chemical substances has been supplemented in the media to enhance the developmental potency of in vitro mature oocytes. Compounds with antioxidative properties such as β-mercatoethanol (β-ME), cysteine [6], cysteamine [30], L-carnitine [44] that augment intracellular glutathione level have been added in IVM as well as in vitro culture (IVC) media. Recently, supplementation of Resveratrol (3,4′,5-trihydroxystilbene), a naturally occurring phytoalexin, has been found to have beneficial impact on porcine IVM and embryonic development [21]. Resveratrol is produced by the interaction of plants with a microorganism present in the roots of Polygonum cuspidatum, Vitis vinifera, red wines, and mulberries and protects these plants against fungal and bacterial infections [23]. Resveratrol exhibits wide variety of pharmacological properties like anti-inflammatory, chemopreventive, antioxidant, antiproliferative, proapoptotic, cardioprotective, and anticancer effects [12, 33]. It also controls the expression of different genes related to DNA synthesis, cell cycle, proliferation, stress response and apoptosis. Resveratrol induces mitochondrial biogenesis and protects against metabolic decline possibly by augmenting activity of sirtuin-1 (SIRT-1), a member of a conserved family of NAD+-dependent deacetylases and ADP-ribosyltransferases. SIRT1 stimulates mitochondrial biogenesis through deacetylation and activation of PGC-1α [10, 36], a key controller of mitochondrial biogenesis that coactivates the nuclear respiratory factors (NRF-1 and NRF-2), which induce the transcription of genes involved in mitochondrial biogenesis [37].
Physiological functions and biological activities of resveratrol for human therapeutic purposes are well-documented. However, our knowledge on effects of resveratrol on livestock oocyte maturation and embryonic developmental competence is inadequate. It has been observed that resveratrol supplementation at lower concentration improved developmental potential of oocytes [21, 24]. Here we investigated the effects of resveratrol on goat IVM and subsequent embryonic development after parthenogenetic activation (PA) and SCNT though hand-made cloning (HMC). We studied the status of oocyte nuclear maturation, intracellular levels of GSH and ROS, embryonic cleavage, morula and blastocyst yield. Also relative transcript abundance of genes associated with apoptosis, early embryonic development and nuclear reprogramming were examined in cumulus cells, oocytes and HMC-derived blastocysts.
Materials and methods
Chemicals
Unless otherwise stated, all chemicals and reagents used in the present study were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Oocyte collection and in vitro maturation
Goat ovaries were obtained from local abattoir and transported to the laboratory at 37 °C in physiological saline solution containing 400 IU/ml Penicillin G and 50 μg/ml streptomycin sulphate. After thorough washing in warm normal saline solution the immature cumulus oocyte complexes (COCs) were punctured from superficial follicles, pooled into 15 ml conical tubes and allowed to precipitate at 37 °C for 5 min. The supernatant was discarded and the precipitate was resuspended in oocyte collection medium (OCM) containing 9.6 g/ml Dulbecco’s Phosphate Buffered Saline (DPBS), 0.1 mg/ml CaCl2, 0.1 mg/ml MgCl2, 3 mg/ml bovine serum albumin (BSA) and 50 μg/ml gentamicin sulphate. COCs with unexpanded, multiple layers of uniform and compact cumulus cells and homogeneous cytoplasmic granulation were chosen and washed thrice in maturation medium [Hepes-buffered TCM-199 + 10 % FBS + 10 μg/ml LH + 5 μg/ml porcine-FSH + 1 μg/ml estradiol-17β + 0.81 mM sodium pyruvate + 2.0 mM L-glutamine + 50 μg/ml gentamicin sulphate]. Groups of 15–20 COCs were transferred into a 100 μl maturation medium drop under mineral oil and subjected to IVM for 24 h in a CO2 incubator (in humidified atmosphere of 5 % CO2 in air) at 38.5 °C. During IVM COCs were treated with different concentrations of resveratrol as mentioned in the experimental design. The resveratrol was dissolved in dimethyl sulfoxide (DMSO) as a 25 mM stock solution and stored at −20 °C before adding to the IVM medium.
Assessment of nuclear status
After 27 h of maturation culture COCs were gently stripped of their cumulus cells with a narrow-bore pipette in DPBS containing 0.1 % hyaluronidase. Completely denuded oocytes were selected and washed in fresh T2 (HEPES-buffered TCM-199 supplemented with 2 % FBS, 2.0 mM L-glutamine, 0.2 mM sodium pyruvate and 50 μg/ml gentamicin sulfate) for removal of cumulus cells. The nuclear status of oocytes was examined by 4′,6′-diamidino-2-phenylindole (DAPI) staining. Oocytes were stained in 2.5 % (wt/vol) DAPI in DPBS for 10 min. Nuclear status was assessed under epifluorescence microscope (Olympus America, Center Valley, PA, USA) and classified as metaphase-I or metaphase-II based on the absence or presence of polar body.
Measurement of intracellular ROS and GSH levels
The oocytes at the MII stage were sampled for measurement of intracellular ROS levels by 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence assay as previously described [31]. In brief, 15–20 oocytes from control and each treatment group were incubated (in the dark) for 30 min in 10 μM H2DCFDA added in HEPES-buffered Tyrode’s medium (TLH) containing 0.05 % (wt/vol) polyvinyl alcohol (TLH-PVA). Following incubation, oocytes were washed with DPBS containing 0.1 % PVA, placed in 50 μl droplets and fluorescence was observed under epifluorescence microscope (Olympus America, Center Valley, PA, USA) equipped with 460 nm filter. Fluorescence images were recorded as TIFF format files and fluorescence intensities of oocytes were analyzed by ImageJ software (Version 1.41; National Institute of Health, Bethesda, MD, USA). The experiment was replicated three times.
Intracellular GSH level of oocytes was determined by 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (Cell tracker blue; CMF2HC; Invitrogen corporation, Carlsbad, CA, USA) method as previously described [44]. 10 μM CMF2HC and 370 nm filter were used for viewing fluorescence.
Parthenogenetic activation of oocytes and in vitro culture of embryos
Oocytes were denuded as described above and those with homogeneous ooplasm and an intact first polar body were transferred in embryo development medium (EDM; Hepe-buffered TCM-199 supplemented with 2.0 mM L-glutamine, 0.2 mM sodium pyruvate, 5 μl/ml non essential amino acid, 10 μl/ml essential amino acid, 10 mg/ml BSA and 50 μg/ml gentamicin). For chemical activation, oocytes were first exposed to 5 μM Ca2+ ionophore A23187 in EDM for 5 min in CO2 incubator (in humidified atmosphere of 5 % CO2 in air). Activated oocytes were washed thrice in EDM for complete removal of Ca2+ ionophore and incubated in EDM containing 2 mM 6-dimethylaminopurine (6-DMAP) at 38.5 °C under 5 % CO2 in humidified air for 4 h. Finally the activated oocytes were washed three times in Research Vitro Cleave (Cook, Queensland, Australia) medium supplemented with 1 % BSA and 50 μg/ml gentamicin sulphate before placing them in 100 μl droplet of the same medium under mineral oil and cultured for 9 days in 5 % CO2 in humidified air. Percentages of embryo cleavage and blastocyst formation were examined at days 2 and 7 respectively considering day of activation as day 0.
Preparation of recipient cytoplasts
The recipient cytoplasts were prepared as described by Jena et al. [18], with minor modifications. Mature oocytes with expanded cumulus cells were transferred into the micro-centrifuge tube containing 0.5 mg ml−1 hyaluronidase in T2 (T denotes HEPES-buffered TCM-199 supplemented with 2.0 mM L-glutamine, 0.2 mM sodium pyruvate, 50 μg ml−1 gentamicin and the number denotes the percentage of FBS) and incubated for 1 min at 38.5 °C, under 5 % CO2 in air. After thorough vortexing for 2–3 min., the contents of the tube were transferred to a 35 mm Petri dish, containing T2. The completely denuded oocytes were chosen and the cumulus cells were removed by washing twice in fresh T2. The denuded oocytes were transferred to another Petri dish, containing 2 mg/ml pronase in T10 and incubated for 2 min at 38.5 °C under 5 % CO2 in air, to digest the zona pellucida. Completely zona-free oocytes were transferred into another Petri dish containing T20 to stop the activity of the pronase enzyme. Zona-free oocytes were washed twice in T20 and incubated in the same medium at 38.5 °C under 5 % CO2 in air, for 20 min., until the protrusion cone, containing nuclear material was visible under the zoom-stereo microscope. Bisection of the zona-free oocytes was performed by the protrusion cone guided method, which eliminates the use of the Hoechst 33342 stain. Protrusion cone bearing oocytes was transferred into the Petri dish containing T20 and 2.5 μg/ml cytochalasin B and manually bisected using an ultra sharp micro blade (Micro Blades, MTB-05; Micromanipulator Microscope Company, Inc., Carson City). The enucleated oocytes were kept in one well of a 4-well dish and transferred to T20 and incubated for 20 min at 38.5 °C, under 5 % CO2 in air.
Activation and the in vitro culture of embryos
Chemical activation of the reprogrammed triplets was performed in T20 medium as described previously in parthenogenetic activation method.
Donor cell preparation
The cells (fetal and adult skin fibroblasts) at the 5th-10th passage were allowed to grow to achieve 100 % confluence, so that majority of the cells reached the G0/G1 stage of the cell cycle. Culture medium of the cells was removed by aspiration and the cells overlaid with Ca2+ and Mg2+-free DPBS, for 5 min. The cells were treated with a 0.25 % trypsin-EDTA solution, after removing the DPBS. The dissociating cells were then harvested in T20 medium and centrifuged at 200 g to produce a loose cell pellet. The pellet was resuspended in T20 and mixed by pipetting, to get single cell suspension in a 1.5 ml tube, kept at room temperature. The cells were then ready for use as nucleus donors.
Pairing and electrofusion of donor cells and cytoplasts
The enucleated demi-oocytes were washed in phytohemagglutinin (0.5 mg/ml in T2) for approximately 4–5 s and then stored in T2 medium containing low-density donor cells. Each demi-oocyte was allowed to attach to a somatic cell by bringing it in the close vicinity to the cell. Then the couplets were put into the fusion medium (0.3 M mannitol, 0.1 mM MgCl2, 0.05 mM CaCl2, and 3 mg/ml BSA). The couplets and the demi-oocytes were picked up, using an unopette (Becton Dickinson, NJ, USA) and kept in the upper and lower parts in the fusion chamber (BTX micro slide 0.5 mm gap, model 450; BTX, San Diego, CA) respectively, containing the fusion medium. Subsequently the couplets were aligned with demi-oocytes 4–5 at a time in between the two electrodes of the chamber with an AC pulse (4 V), using BTX Electro cell Manipulator 200 (BTX, San Diego, CA, USA) and a single DC pulse (2.1 kV/cm, 5 μs pulse width) was applied for fusion. The triplets were maintained in T20 medium and incubated for 4 h at 38.5 °C under 5 % CO2 in air, for reprogramming.
In vitro culture of the cloned embryos
Reconstructed embryos were gently placed (12–15 per well) at a distance on the periphery of the 4-well dish in 400 μl of RVCL (Cook®, Australia with 1 % fatty acid free BSA and 5 % FBS) to avoid central accumulation. The 4-well dish was incubated at 38.5 °C in 5 % CO2 in air, for 7 days.
Gene expression analysis by real-time polymerase chain reaction
Mature COCs with expanded cumulus from different experimental groups (control, 0.25 μM, 0.5 μM, and 5.0 μM resveratrol-treated) were denuded by gently pipetting in 500 μl hyaluronidase (0.5 mg/ml). Completely denuded oocytes with evenly granular cytoplasm, isolated cumulus cells, and HMC-derived blastocysts of corresponding resveratrol groups (Day 7) were separately selected under stereomicroscope for the gene expression study. Total RNA was isolated by using Trizol reagent (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instruction, and quantified by measuring the absorbance at 260 nm. First-strand complementary DNA (cDNA) was synthesized from 500 ng of total RNA by reverse transcription using RevertAid™ First Strand cDNA synthesis kit (Fermentas Life Sciences, EU). The transcript abundances of genes related to developmental competence of embryos were quantified by quantitative real-time PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal reference gene and the expression of each target gene was quantified relative to GAPDH. The sequences of the primer sets used for real-time PCR analysis are shown in Table 1. The primers were amplified on a LightCycler® 480 instrument with software version 1.5 (Roche Diagnostics, Mannheim, Germany). The ‘crossing point’ or Cp values were determined by ‘second-derivative max method’ in the software. All real-time PCR runs were performed in triplicate and each reaction mixture was prepared in a total volume of 10 μL. The reaction mixture consisted of 2 μl of cDNA as template, 5 μl of 2× Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific) containing 0.5 μM of gene specific primer. The following cycling conditions were employed for all the genes: preincubation at 95 °C for 10 min., denaturation at 95 °C for 15 s, annealing at 59 °C for 30 s., 72 °C for 30 s. To circumvent any issue of non-specific amplification melting curve analysis with a temperature gradient of 0.1 °C/s from 70 °C to 95 °C was performed. The relative mRNA level was calculated as 2-ΔΔCp, where, Cp denoted crossing point of target gene amplification, ΔCp denoted (Cptarget gene - Cpinternal reference gene) and ΔΔCp denoted (ΔCpsample – ΔCpcalibrator) [34].
Table 1.
Details of the primers used for gene expression analysis
| Gene | Primer | Sequence (5′-3′) | Product size (bp) | Accession number |
|---|---|---|---|---|
| IGF-1R | Forward | TGATAGTGGGAGGCTTGGTT | 104 | DQ666953.1 |
| Reverse | ATACTCCGGGTTCACAGACG | |||
| Glut1 | Forward | CTGATCCTGGGTCGCTTCA | 81 | JQ343217.1 |
| Reverse | GGACACCTCCCCCACATACA | |||
| Oct4 | Forward | GACTATCTGCCGTTTTGAGG | 164 | FJ970649.1 |
| Reverse | TACTCGTCCGCTTTCTCTTT | |||
| E-cad | Forward | GGACTTTGACTTGAGCCAGTTACAT | 74 | XM_004015093.1 |
| Reverse | TTGGTGCCACGTCATTGC | |||
| Stat3 | Forward | AAAGTACATCCTGGCCCTTTG | 134 | FJ970650.1 |
| Reverse | CTCCTTCTTTGCTGCTTTCACT | |||
| Cx43 | Forward | CTTATTTCAATGGCTGCTCCTC | 135 | AJ293887.1 |
| Reverse | CCCAGTTTTGCTCACTTGCTT | |||
| CHOP-10 | Forward | GGCCACTTCTGACCCTTCCT | 85 | NM_001078163.1 |
| Reverse | CCCCTGCTGAATCGATGGT | |||
| MnSOD | Forward | CGTGACTTTGGTTCCTTTG | 166 | GQ204787.1 |
| Reverse | GGATAAGACCTGTTGTTCCTTG | |||
| DNMT1 | Forward | ATGCGGTGGAAGAGATACAGA | 199 | FJ617538.1 |
| Reverse | GCTGAACCAGAAAAGAGGAG | |||
| Bax | Forward | GCGCATCGGAGATGAATT | 130 | JQ284045.1 |
| Reverse | CCAGTTGAAGTTGCCGTC | |||
| Bcl-2 | Forward | AGTACCTGAACCGGCACCT | 108 | JN036559.1 |
| Reverse | GCCAGGAGAAATCAAACAGG |
Experimental design
In Experiment 1, effect of different resveratrol concentrations (0, 0.1, 0.25, 0.5, 2.0 and 5.0 μM) on goat oocyte maturation was determined. In Experiment 2, effect of different resveratrol concentrations on intracellular ROS and GSH level was observed. In Experiments 3 and 4 in vitro development of parthenogenetically activated (PA) and hand-made cloned (HMC) derived embryos were examined, respectively, following resveratrol supplementation. In Experiments 5 and 6 analysis of gene expression were examined to confirm apoptotic activity in cumulus cells, mature oocytes and HMC-derived blastocyst and early embryonic developmental potential of HMC-derived blastocysts.
Statistical analysis
Statistical analysis was performed using SYSTAT 12 (Systat Software, Inc., Chicago, IL, USA). Percentage data of maturation rate, cleavage and embryonic yield were arcsine-transformed before analysis to maintain homogeneity of variances, compared by one-way ANOVA, followed by Duncan’s multiple range test (DMRT). Comparison of data regarding gene expression among different groups was carried out by one-way ANOVA. Data were presented as mean ± SEM. Differences were considered significant if the P value was less than 0.05. All the experiments were replicated at least for three times.
Results
Experiment-1: Effect of different resveratrol concentrations on goat oocyte maturation
In this set of experiment effect of different concentrations (0, 0.1, 0.25, 0.5, 2.0 and 5.0 μM) of resveratrol was assessed on maturation rate of goat oocytes and results have been presented in Table 2. Nuclear maturation was observed after 27 h of IVM. Compared to control (72.60 %), no significant difference in nuclear maturation was observed among 0.25, 0.5, 2.0 μM resveratrol-treated groups (76.92, 76.5 and 75.34 %). Also, significant (P < 0.05) reduction in nuclear maturation rate was observed in oocytes exposed to 5.0 μM resveratrol group (65.53 %) compared to other groups. No significant effect on degeneration of oocytes was observed following resveratrol supplementation.
Table 2.
Effect of resveratrol treatment during IVM on maturation
| Resveratrol concentration (μM) | Number of oocytes cultured for maturation (N)* | Maturation rate (%) | Degenerate (%) |
|---|---|---|---|
| 0 (Control) | 219 | 159 (72.60 ± 0.32)a | 7.31 ± 0.93 |
| 0.1 | 202 | 149 (73.76 ± 1.58)a | 7.43 ± 1.73 |
| 0.25 | 234 | 180 (76.92 ± 0.86)a | 7.69 ± 1.62 |
| 0.5 | 217 | 166 (76.5 ± 0.76)a | 6.45 ± 1.69 |
| 2.0 | 223 | 168 (75.34 ± 0.80)a | 6.73 ± 0.58 |
| 5.0 | 206 | 135 (65.53 ± 1.08)b | 10.22 ± 0.84 |
Values with different superscript letters within a column differ significantly (P < 0.05)
*3 times replicated
Experiment-2: Effect of different resveratrol concentrations on intracellular ROS and GSH level
Significantly higher (P < 0.05) intracellular GSH content was observed when resveratrol was added in the IVM media at the concentration of 0.25, 0.5 and 2 μM. Intracellular ROS level also decreased (P < 0.05) in 0.1, 0.25, 0.5 and 2 μM resveratrol group compared to control and 5.0 μM groups (Fig. 1).
Fig. 1.
Fluorescent microscope photographs of in vitro mature goat oocytes. A Oocytes were stained with Cell tracker Blue (a-f) and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (g-i) to detect intracellular levels of glutathione (GSH) and reactive oxygen species (ROS), respectively. Mature oocytes derived from the maturation medium treated with 0 μM (a and g), 0.1 μM (b and h), 0.25 μM (c and i), 0.5 μM (d and j), 2.0 μM (e and k), 5.0 μM (f and l). B Effect of resveratrol treatment in IVM medium on intracellular GSH and ROS levels in mature goat oocytes. Within each group (GSH and ROS) different superscript letters are significantly (P < 0.05) different. Experiment was replicated three times
Experiment-3: In vitro development of parthenogenetically activated (PA) embryos following resveratrol supplementation
Different developmental stages of HMC-derived embryos are presented in Fig. 2a–d. As shown in Table 3, significant enhancements (P < 0.05) in morulae and expanded and hatched blastocysts yield were observed in 0.25 and 0.5 μM resveratrol treated groups compared to control. However, no effect of resveratrol-treatment on the cleavage rate was observed and morulae and blastocyst development were significantly reduced (P < 0.05) in 5.0 μM resveratrol-treated group.
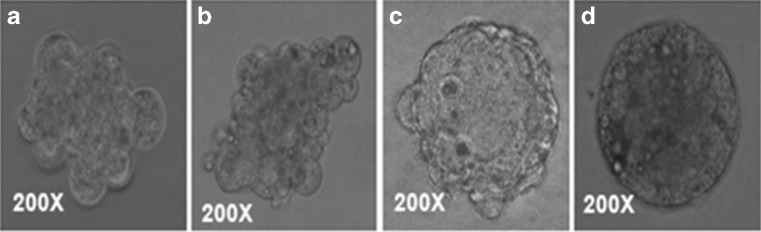
Fig. 2.
Different developmental stages of parthenogenetically activated (PA) (a-d). a 2-cell stage, b morula, c hatching blastocyst, d day 7 blastocyst
Table 3.
Effect of resveratrol treatment during IVM on embryonic development after parthenogenetic activation (PA)
| Resveratrol concentration (μM) | Embryo cultured (n) | % Cleaved | % Morulae | % Expanded blastocyst | % Hatched blastocyst |
|---|---|---|---|---|---|
| 0 (Control) | 204 | 160 (78.43 ± 0.49) | 92 (45.1 ± 0.94)a,b | 42 (20.59 ± 0.6)a | 15 (7.35 ± 0.98)a,b |
| 0.1 | 189 | 153 (80.95 ± 0.56) | 89 (47.1 ± 0.89)b | 38 (20.11 ± 0.98)a | 14 (7.41 ± 0.53)a,b |
| 0.25 | 199 | 162 (81.41 ± 0.97) | 111 (55.78 ± 0.64)c | 57 (28.64 ± 0.54)b | 25 (12.56 ± 0.7)c |
| 0.5 | 188 | 152 (80.85 ± 0.4) | 101 (53.72 ± 0.33)c | 49 (26.06 ± 1.71)b | 19 (10.11 ± 0.98)b,c |
| 2.0 | 195 | 157 (80.51 ± 0.29) | 90 (46.15 ± 1.3)a,b | 41 (21.03 ± 1.47)a | 15 (7.69 ± 1.06)b |
| 5.0 | 201 | 157 (78.11 ± 1.25) | 88 (43.78 ± 1.12)a | 32 (15.92 ± 0.36)c | 8 (3.98 ± 1.78)a |
Values with different superscript letters within a column differ significantly (P < 0.05). The data represent mean ± SEM. The experiment was replicated five times
Experiment-4: In vitro development of hand-made cloned (HMC) embryos following resveratrol supplementation
Different developmental stages of HMC-derived embryos are presented in Fig. 3a–d. It is evident from Table 4 that the HMC embryos in 0.25 and 0.5 μM resveratrol treated groups showed significant increase in development of blastocyst but no improvement in cleavage rate and morula were observed compared to control. Also the cleavage rate, morula and blastocyst formation dropped significantly (P < 0.05) in 5.0 μM resveratrol-treated group.
Fig. 3.
Different developmental stages of hand-made cloned (HMC)-derived embryos (a–d). a 8–16 cells stage, b compact morula and c–d day 8 blastocyst
Table 4.
Effect of resveratrol treatment during IVM on embryonic development after hand-guided cloning
| Resveratrol concentration (μM) | Embryo cultured (n) | % Cleaved | % Morulae | % Blastocyst |
|---|---|---|---|---|
| 0 (Control) | 67 | 48 (71.64 ± 2.02)a | 15 (22.39 ± 1.28)a | 11 (16.42 ± 0.94)a |
| 0.1 | 62 | 45 (72.58 ± 1.36)a | 14 (22.58 ± 1.33)a | 11 (17.74 ± 1.04)a |
| 0.25 | 65 | 48 (73.85 ± 2..72)a | 16 (24.62 ± 1.47)a | 14 (21.54 ± 1.08)b |
| 0.5 | 67 | 49 (73.13 ± 2.19)a | 17 (25.37 ± 1.34)a | 15 (22.39 ± 1.77)b |
| 2.0 | 68 | 50 (73.53 ± 1.12)a | 16 (23.53 ± 1.75)a | 10 (14.71 ± 0.86)a.c |
| 5.0 | 65 | 41 (63.08 ± 2.49)b | 11 (16.92 ± 1.69)b | 6 (9.23 ± 0.87)d |
Values with different superscript letters within a column differ significantly (P < 0.05). The data represent mean ± SEM. The experiment was replicated five times
Experiment-5: Effect of resveratrol treatment on apoptotic gene expression in mature oocytes, cumulus cells and HMC-derived blastocyst
The effect of resveratrol treatment on apoptotic gene expression in mature oocytes, cumulus cells and HMC-derived blastocyst has been shown in Fig. 4. Proapoptotic (Bax) and antiapoptotic (Bcl-2) genes were included in the study. As evident from Fig. 4, relative abundance of Bax gene decreased significantly (P < 0.05) in cumulus cells, mature oocytes exposed to resveratrol as well as HMC-derived blastocysts produced from these oocytes whereas that of Bcl-2 gene expression remain unaltered.
Fig. 4.
Mean ± SEM expression of Bax and Bcl-2 in cumulus cells, mature oocytes and blastocysts treated with different concentrations of Resveratrol during IVM. Within the same mRNA, Mean values without a common superscript letter differed (P < 0.05)
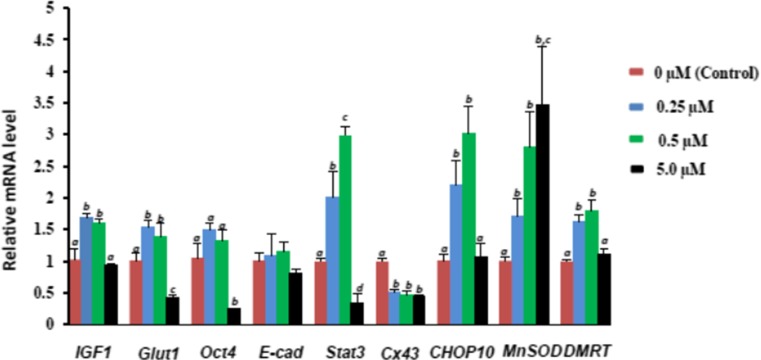
Experiment-6: Effect of resveratrol exposure on expression of developmental competence related HMC-derived blastocyst
The effect of resveratrol treatment during IVM on expression of genes related to early embryonic development and nuclear reprogramming (IGF-1, Glut1, Oct4, E-cad, Stat3, Cx43, CHOP-10, MnSOD, and DNMT) was analyzed and the result was shown in Fig. 5. Except Cx43 and E-cad all the genes showed significant surge in their transcript abundance after resveratrol treatment at 0.25 and 0.5 μM resveratrol exposed groups whereas expression of IGF-1, Glut1, Oct4, E-cad, Stat3, CHOP-10, and DNMT decreased significantly (P < 0.05) in 5.0 μM resveratrol exposed group. The relative abundance of Cx43 decreased significantly (P < 0.05) in all the groups and no alteration in expression pattern of E-cad gene was noted in any of the groups.
Fig. 5.
Mean ± SEM expression of IGF1, Glut1, Oct4, E-cad, Stat3, Cx43, CHOP-10, MnSOD and DMRT in blastocysts treated with different concentrations of resveratrol during IVM. Within the same mRNA, mean values without a common superscript letter differed (P < 0.05)
Discussion
In the present study the effects of supplementation of resveratrol, an antioxidant, on oocyte maturation, intracellular levels of GSH and ROS in mature oocytes and embryonic development after PA and HMC were evaluated through a series of experiments. Also, expression levels of several genes having decisive role in development and apoptosis were analyzed in HMC-derived embryos that were obtained from resveratrol-treated oocytes. Our findings revealed that resveratrol administration during the maturation period resulted in concentration-dependent effects. Treatment of goat oocytes with 0.25 and 0.5 μM resveratrol during IVM effectively scavenged intracellular ROS, increased GSH concentration in mature oocytes, increased embryonic development and were found to have impact on expression of apoptosis-related and developmental competence-associated genes in cumulus cells, mature oocytes and blastocysts.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural polyphenol compound, and a phytoalexin produced naturally by several plants especially the roots of the Japanese Knotweed when challenged from bacterial or fungal attack. Diverse effects of resveratrol as cardioprotective, anticarcinogenic, immunomodulatory, antioxidant, and antiapoptotic agent have been reported previously [12, 17, 33]. One of the most robust and reproducible effects of resveratrol is to increase the mitochondrial mass [3, 22]. The fuelling effect of resveratrol on mitochondrial biogenesis is executed probably by activating Sirtuin1 (SIRT1), a member of a conserved family of NAD+-dependent deacetylases and ADP-ribosyltransferases. SIRT1 stimulates mitochondrial biogenesis through deacetylation and activation of PGC-1α [10, 36], a key controller of mitochondrial biogenesis that coactivates the nuclear respiratory factors (NRF-1 and NRF-2), which induce the transcription of genes involved in mitochondrial biogenesis [37]. The beneficial effect of resveratrol on porcine oocyte maturation and embryonic development has been reported recently [21, 24]. However, no report is available on the effect of resveratrol treatment on embryonic development in goat. Therefore, current study investigated the effect of five different concentrations on oocyte maturation and embryonic development following PA and HMC. Although IVF or ICSI is frequently used to generate embryos PA and HMC were used in the present study as our aim was to find out the effect of the resveratrol on oocyte-related factors only and in the former cases the effect of resveratrol might be overshadowed by the sperm intracellular ROS level. Based on our observation it was revealed that although resveratrol treatment at the concentrations of 0.25 and 0.5 μM produce optimum effect the higher concentration of this (5 μM) has detrimental impact. This is in line with the earlier reports of dose-dependent effect exerted by resveratrol in cell proliferation [20, 39] as well as mammalian embryonic development [21, 24].
Supplementation of antioxidants in culture medium influences oocyte maturation and embryonic development. In vitro studies in pigs and cattle have indicated that antioxidants like cysteine and β-mercatoethanol improve embryonic development by increasing intracellular glutathione (GSH) levels. GSH is the major nonprotein sulphydryl compound found in mammalian cells with strong basal ROS scavenging activity [8]. Although plethora of factors contributes to the events of oocyte maturation, fertilization and subsequent embryonic development maintenance of adequate GSH levels is of prime importance [11]. In the present study, IVM medium supplemented with resveratrol did not show any significant boost in nuclear maturation and maturation rate decreased when higher concentration (5.0 μM) was used. However, resveratrol treatment at different concentrations effectively decreased ROS level in mature oocytes. This may be due to augmenting intracellular GSH level following resveratrol supplementation. The most striking effect was observed at 0.25 and 0.5 μM resveratrol-treated groups which show subsequent uplift in morulae and blastocysts yield following PA and HMC. So it may be inferred that supplementation of resveratrol in the IVM medium promotes cytoplasmic maturation of oocytes and increased intracellular GSH level contributed to the higher embryonic development following PA and HMC. Previously, two separate studies to find out the effect of resveratrol treatment on porcine oocyte development reported that resveratrol at the concentration of 0.5 μM [24] and 2.0 μM [21] increased the blastocyst yield following PA and IVF. The later also reported increased intracellular GSH concentration at 0.5 and 2.0 μM resveratrol-treated groups. Our study demonstrated that 0.25 and 0.5 μM resveratrol concentrations are optimum for goat oocytes maturation and embryonic development. So, there exist species-specific differences in the oocyte maturation and embryonic development with different resveratrol concentrations. This may be due to the species-to-species variation in sensitivity of oocytes to resveratrol and molecular interplay during these procedures.
Mitochondria are the major source of ROS and, therefore, cells always try to protect it from oxidative stress. Oocytes and embryos have multiple enzymatic and nonenzymatic resistance mechanisms against ROS [7, 11]. But excessive amount of ROS that is beyond the capability of this defense system to detoxify, especially when the GSH levels of oocytes drop after zygotic genome activation [26], may destroy mitochondrial integrity and decrease embryonic development. From this study, it was not revealed whether high level of GSH defends the mitochondrial integrity and functionality from oxidative stress. However, our result of decreased ROS levels in resveratrol-treated oocytes indicated that the increased GSH level due to the resveratrol treatment improved embryonic development. This effect was probably due to ROS scavenging, which led to the protection of micro-organelles, including mitochondria. Improved success rate of assisted reproductive techniques (ART) has been reported in different species when embryos are cultured under reduced oxygen concentration (5 %), due to overall reduction of the cumulative detrimental effects of reactive oxygen species. A recent meta-analysis on long-term data of IVF/ICSI outcomes in human pointed out the dubious role of low oxygen concentration on better fertilization, implantation and pregnancy rate [38]. One of the reasons behind this may be the intracellular source of ROS generated by enzymatic activity and cause oxidative damage to embryos even if at lower level of extracellular O2 concentration. The imbalance between total antioxidant capacity (TAC) and increased ROS levels in embryonic surrounding may lead to oxidative stress [11]. Therefore, supplementation of antioxidants is required when the embryos are cultured at lower O2 concentrtion. Hence resveratrol in the media may produce beneficial effect on developmental competence by combating the intracellular ROS even when the oocytes and embryos are cultured at lower O2 concentration.
Apoptosis is a normal cellular procedure by which cells with DNA or chromosomal abnormalities are eliminated [28]. Growing body of evidences suggests that environmental stress factor such as oxidative stress derived from ROS generation when oocytes or embryos are cultured in vitro induce apoptosis and resveratrol acts as an antioxidant in animal cells or embryos and reduces apoptosis induced by oxidative stress by increasing intracellular GSH level (Jang et al.2000; [27]; Lee et al.2010; [21]). So, we examined the mRNA expression of proapoptotic (Bax) and antiapoptotic (Bcl-2) genes in mature oocytes, cumulus cells, and HMC-derived blastocysts. Treatment with 0.25 and 0.5 μM resveratrol resulted in significantly lower mRNA expression of proapoptotic gene, Bax compared with the control group. But, transcript abundance of antiapoptotic gene, Bcl-2 did not differ significantly in either of the groups. Presence of resveratrol in the medium may be advantageous for cumulus cells to maintain their viability and expansion through proproliferative and antiapoptotic effects. Appropriate oocyte maturation largely depends on the presence of surrounding cumulus cells. Cumulus cells augment oocyte development during IVM, either by releasing soluble factors, which stimulate developmental competence, or by eliminating inhibitory components from maturation environment [15]. Another possible influence of cumulus cells during IVM of bovine oocytes might be that they decrease oxygen tension in the close proximity of the oocyte as a result of active cumulus cell metabolism [2]. Therefore, it is likely that overall positive impact on maturation and embryonic development exerted by resveratrol is due to modulation of apoptosis related genes.
Modifications in culture environment can modulate gene expression in mammalian cells and embryos [32, 45]. In case of HMC-derived blastocysts reconstructed from mature oocytes of different resveratrol treatment groups, expression of a set of genes which are having relevance in early embryonic development [13, 25, 35, 42, 43] were checked. The genes included in the present study are involved in pre- and post-natal growth (IGF-1), metabolism (Glut1), pluripotency (Oct4), morphogenesis and biogenesis of the trophoectoderm (E-cad), lineage determination and cavitation (Stat3), gap junction, cavitation and cryosurvival (Cx43), growth arrest (CHOP-10), oxidative stress (MnSOD), and DNA methylation (DNMT). The expression profiling of these genes revealed that at lower concentrations of resveratrol major surge in the transcript levels of these genes occurred except E-cad and Cx43. The exact relation between the surge in mRNA expression and resveratrol treatment is not clear but it can be speculated that resveratrol treatment at the earlier stage of oocyte maturation offered beneficial microenvironment by increased intracellular GSH level and reduced ROS level. The better cytoplasmic milieu in turn, induced effective alteration in the expression of genes of developmental relevance, which is reflected by the increased yield of embryos. We did not study mRNA expression of these genes in PA-derived blastocyst because the phenomenon of nuclear reprogramming is different from that of HMC-derived blastocyst and therefore, cannot be compared simultaneously.
Conclusion
In summary supplementation of resveratrol at optimum concentration (0.25 and 0.5 μM) during IVM of oocytes improved developmental potential of parthenogenetic and hand-guided cloned embryos. This improvement is due to production of beneficial microenvironment within oocytes by increasing the intracellular GSH, decreasing ROS level. The addition of resveratrol during oocyte maturation also decreased abundance of transcripts of proapoptotic genes in cumulus cells, mature oocytes as well as blastocysts. Also the occurrence of nuclear reprogramming and chronicle of development were more apposite as observed from modulated expression of relevant genes in blastocysts derived from resveratrol-treated oocytes.
Footnotes
Capsule Optimum concentration of Resveratrol supplementation during goat oocyte maturation has beneficial effect on embryonic development and modulation of developmental competence related gene expression.
References
- 1.Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update. 2003;9(3):275–89. doi: 10.1093/humupd/dmg018. [DOI] [PubMed] [Google Scholar]
- 2.Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59:939–49. doi: 10.1016/S0093-691X(02)01125-1. [DOI] [PubMed] [Google Scholar]
- 3.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in-vitro maturation and in-vitro fertilization-embryo transfer. Fertil Steril. 2005;83:1461–5. doi: 10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18(6):864–80. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of β-mercaptoethanol, cysteine, and cystine. Theriogenology. 2000;53:761–71. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- 7.Donnay I, Knoops B. Peroxiredoxins in gametogenesis and embryo development. Subcell Biochem. 2007;44:345–55. doi: 10.1007/978-1-4020-6051-9_16. [DOI] [PubMed] [Google Scholar]
- 8.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14(5):236–42. doi: 10.1016/S1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 10.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 12.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–7. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez-Adán A, Rizos D, Fair T, Moreira PN, Pintado B, de la Fuente J, et al. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol Reprod Dev. 2004;68(4):441–8. doi: 10.1002/mrd.20113. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology. 1998;49:1451–63. doi: 10.1016/S0093-691X(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 16.Holzer H, Scharf E, Chian RC, Demirtas E, Buckett W, Tan SL. In-vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertil Steril. 2007;88:62–7. doi: 10.1016/j.fertnstert.2006.11.087. [DOI] [PubMed] [Google Scholar]
- 17.Jang JH, Surh YJ. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res. 2001;496:181–90. doi: 10.1016/S1383-5718(01)00233-9. [DOI] [PubMed] [Google Scholar]
- 18.Jena MK, Malakar D, De AK, Garg S, Akshey YS, Dutta R, et al. Handmade cloned and parthenogenetic goat embryos—a comparison of different culture media and donor cells. Small Ruminant Res. 2012;105:255–62. doi: 10.1016/j.smallrumres.2012.03.001. [DOI] [Google Scholar]
- 19.Kosower NS, Kosower EM. The glutathione status of cells. Int Rev Cytol. 1978;54:109–60. doi: 10.1016/S0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 20.Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy PVG. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res. 2002;62(9):2488–92. [PubMed] [Google Scholar]
- 21.Kwak SS, Cheong SA, Jeon Y, Lee E, Choi KC, Jeung EB, et al. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology. 2012;78(1):86–101. doi: 10.1016/j.theriogenology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Langcake P, Cornford C, Pryce R. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry. 1979;18:1025–7. doi: 10.1016/S0031-9422(00)91470-5. [DOI] [Google Scholar]
- 24.Lee K, Wang C, Chaille JM, Machaty Z. Effect of resveratrol on the development of porcine embryos produced in vitro. J Reprod Dev. 2010;56:330–5. doi: 10.1262/jrd.09-174K. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Amarnath D, Kato Y, Tsunoda Y. Analysis of development-related gene expression in cloned bovine blastocysts with different developmental potential. J Reprod Dev. 2007;53(6):1247–63. doi: 10.1262/jrd.19096. [DOI] [PubMed] [Google Scholar]
- 26.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- 27.Mahal H, Mukherjee T. Scavenging of reactive oxygen radicals by resveratrol: antioxidant effect. Res Chem Intermedi. 2006;32:59–71. doi: 10.1163/156856706775012941. [DOI] [Google Scholar]
- 28.Matwee C, Betts DH, King WA. Apoptosis in the early bovine embryo. Zygote. 2000;8:57–68. doi: 10.1017/S0967199400000836. [DOI] [PubMed] [Google Scholar]
- 29.Meister A, Anderson ME. Glutathione. Annu Review Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee A, Kumar D, Singh KP, Chauhan MS, Singla SK, Palta P, et al. Assessment of DNA damage during in vitro development of buffalo (Bubalus bubalis) embryos: effect of cysteamine. Reprod Domest Anim. 2010;45(6):1118–21. doi: 10.1111/j.1439-0531.2009.01484.x. [DOI] [PubMed] [Google Scholar]
- 31.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–7. doi: 10.1242/dev.109.2.501. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Park SB, Kim NH. Expression of early development related genes in bovine nuclear transferred and fertilized embryos. Zygote. 2003;11:355–60. doi: 10.1017/S0967199403002454. [DOI] [PubMed] [Google Scholar]
- 33.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–97. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizos D, Lonergan P, Boland MP, Arroyo-García R, Pintado B, de la Fuente J, et al. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod. 2002;66(3):589–95. doi: 10.1095/biolreprod66.3.589. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature. 2005;434:113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 37.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–78. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobrinho DBG, Oliveira JBA, Petersen CG, Al M, Silva LFI, Massaro FC, Baruffi RLR, Cavagna M, Franco JG, et al. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrin. 2011;9:143–154. doi: 10.1186/1477-7827-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szende B, Tyihák E, Király-Véghely Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med. 2000;32(2):88–92. doi: 10.1038/emm.2000.16. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology. 2000;54:137–45. doi: 10.1016/S0093-691X(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 41.Whitworth KM, Li R, Spate LD, et al. Method of oocyte activation affects cloning efficiency in pigs. Mol Reprod Dev. 2009;76:490–500. doi: 10.1002/mrd.20987. [DOI] [PubMed] [Google Scholar]
- 42.Wrenzycki C, Herrmann D, Lucas-Hahn A, Lemme E, Korsawe K, Niemann H. Gene expression patterns in in vitro-produced and somatic nuclear transfer-derived preimplantation bovine embryos: relationship to the large offspring syndrome? Anim Reprod Sci. 2004;82:593–603. doi: 10.1016/j.anireprosci.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Yaseen MA, Wrenzycki C, Herrmann D, Carnwath JW, Niemann H. Changes in the relative abundance of mRNA transcripts for insulin-like growth factor (IGF-I and IGF-II) ligands and their receptors (IGF-IR/IGF-IIR) in preimplantation bovine embryos derived from different in vitro systems. Reproduction. 2001;122(4):601–10. doi: 10.1530/rep.0.1220601. [DOI] [PubMed] [Google Scholar]
- 44.You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology. 2010;74:777–85. doi: 10.1016/j.theriogenology.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W, Xiang T, Walker S, et al. Global gene expression analysis of bovine blastocysts produced by multiple methods. Mol Reprod Dev. 2008;75:744–58. doi: 10.1002/mrd.20797. [DOI] [PubMed] [Google Scholar]