Abstract
Intestinal inflammatory diseases are the result of multiple processes, including mucosal oxidative stress and perturbed homeostasis between commensal bacteria and mucosal immunity. Toll-like receptors (TLRs) recognize molecular-associated microorganisms' patterns and trigger innate immunity responses contributing to intestinal homeostasis and inflammatory responses. However, TLRs effects on redox balance in intestinal mucosa remain unknown. Therefore, the present study analyzes the effect of TLR2, TLR3, and TLR4 on both oxidative damage of lipids and proteins, and the activity of antioxidant enzymes in enterocyte-like Caco-2 cells. The results show that the activation of these TLRs increased lipid and protein oxidation levels; however, the effect on the antioxidant enzymes activity is different depending on the TLR activated. These results suggest that the activation of TLR2, TLR3, and TLR4 might affect intestinal inflammation by not only their inherent innate immunity responses, but also their pro-oxidative effects on intestinal epithelial cells.
Keywords: TLR2, TLR3, TLR4, Oxidative stress, Antioxidant enzymes, Caco-2 cells
Introduction
The intestinal epithelium is a critical anatomical and immunological barrier between the body and a large variety of luminal microorganisms (microbiota) activating intestinal innate immunity responses to maintain both the integrity of the mucosal barrier and the intestinal physiology (Cario 2008; Mendoza et al. 2009, 2012). One of the mechanisms triggered by innate immunity is mediated by toll-like receptors (TLRs), which recognize molecular-associated microorganisms' patterns. TLR2 and TLR4 recognize cell components of gram-positive (lipoteichoic acid, lipoprotein, lipopeptide, and peptidoglycan) and gram-negative (lipopolysaccharide, LPS) bacteria, respectively; whereas TLR3 reacts with viral-derived double-strand RNA. Intestinal epithelial cells express TLRs, which after activation lead to the production of anti- or proinflammatory cytokines contributing to inflammatory responses (Fukata et al. 2009).
The intestinal epithelium is a major target for oxidative damage due to constant exposure of reactive oxygen species (ROS) generated by luminal contents (Ames 1983). This oxidative stress may lead to intestinal inflammation (Finkel and Holbrook 2000; Klaunig and Kamendulis 2004) and inflammatory bowel diseases (McKenzie et al. 1996; Zhu and Li 2012; Kruidenier et al. 2003). Thus, chronic gut inflammation is associated with enhanced production of ROS (Grisham 1994), and disturbances in redox equilibrium can lead to a proinflammatory state (Gill et al. 2010).
The effect of TLRs on oxidative stress appears controversial. In fact, TLRs may induce oxidative damage (Kadl et al. 2011; Ko et al. 2011) or protect from it (Frantz et al. 2001; Patel and Hackam 2013), depending on analyzed tissue. The oxidative response in intestinal epithelial cells yielded by the activation of TLRs remains unknown. Therefore, the aim of the present work has been to determine the contribution of the activation of TLR2, TLR3, and TLR4 to the oxidative stress in the human enterocyte-like Caco-2 cells by measuring the levels of both lipid peroxidation (MDA + 4-HDA) and protein carbonyls. Moreover, the activity of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) was also analyzed.
Experimental
Cell culture and cell homogenate preparation
Caco-2/TC7 cells were cultured at 37 °C in an atmosphere of 5 % CO2 and maintained in high-glucose DMEM, supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 % nonessential amino acids, and 20 % fetal bovine serum (FBS) (from Life Technologies, Carlsbad, CA, USA). The experiments were carried out in the cells 14 days after seeding (9 days after confluence), and the cell medium was FBS-free 24 h before using the cells. This condition did not affect the functional differentiation status of the Caco-2 cells (Mendoza et al. 2009). Specific TLR ligands were added to cell medium 1 day before the measurement of oxidative parameters. The concentrations assayed have been shown to affect the activity of epithelial cells (Mendoza et al. 2009, 2012; Mintz et al. 2013). TNFα treatment at 5 ng/ml was used as a reference of prooxidant condition (Al-Shudiefat et al. 2013).
For cell homogenate preparation, the cells were resuspended and homogenized with a cold Tris-mannitol buffer (Tris 2 mM, mannitol 50 mM, pH 7.1, protease inhibitors, and 0.02 % sodium azide). Then, the homogenate was disrupted by sonication (15 1-s bursts, 60 W). Antioxidant enzymes' activity was measured in the cellular homogenate. For lipid peroxidation and protein carbonyl analysis, the homogenate was additionally centrifuged for 10 min at 3,000 g, and the supernatant was taken for the study. Protein content was measured by following the Bradford method (Bio-Rad, Hercules, CA, USA).
Analysis of lipid and protein oxidation and antioxidant enzyme activity
The level of lipid peroxidation was determined by measuring the concentration of malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA) following the method of Esterbauer and Cheeseman (1990). Protein oxidation was analyzed by determining the protein carbonyl level in the cells following the method described by Wehr and Levine (2013).
Adding enzyme-specific substrate to the cell homogenate and measuring the rates of disappearance of the substrate by spectrophotometry determined the activities of the antioxidant enzymes CAT, SOD, and GPx. CAT activity (Aebi 1984) was calculated as the reduction of H2O2 in nanomole per milligram protein × minute. For SOD activity (McCord and Fridovich 1969), the production of a superoxide radical was determined by using a xanthine/xanthine-oxidase system to reduce cytochrome C, and it was calculated as units per milligram protein × minute. For GPx activity (Flohé and Günzler 1984), the decrease in NADPH during oxidation to NADP was measured, and it was calculated as NADPH nanomole per milligram protein × minute.
Statistical analysis
All results are expressed as means ± standard error of the mean. Statistical comparisons were performed using one-way ANOVA followed by the Bonferroni posttest with a confidence interval of 95 % (p < 0.05). Normal distribution was previously confirmed with the D'Agostino–Pearson test. Statistical analysis was carried out by the computer-assisted Prism GraphPad Program (Prism version 4.0, GraphPad Software, San Diego, CA, USA).
Results and discussion
The present study analyzes the effect of the TLR2, TLR3, and TL4 activation on the oxidative damage of lipids and proteins in the intestinal epithelial cell line Caco-2. Since oxidative damage may appear in the cells resulting from an unbalance between pro- and antioxidant activities, and the main cellular defense against free radicals is the activity of antioxidant enzymes, we have also analyzed the effect of the activation of TLR2, TLR3, and TLR4 on the activity of antioxidant enzymes CAT, SOD, and GPx. Previous studies in our laboratory have shown that Caco-2 cells expressed TLR3 and TLR4 (Mendoza et al. 2009, 2012). The expression of TLR2 was also demonstrated before carrying out the study (data not shown).
The figures' results were expressed as a percentage of the control value (untreated cells; control 100 %), being the absolute values obtained under control conditions as follows: 0.30 ± 0.03 nmol MDA + 4-HDA/mg protein (lipid peroxidation), 21.82 ± 2.30 nmol carbonyl groups/mg protein (protein oxidation), 4.75 ± 0.19 H2O2 nmol/mg protein × minute (CAT activity), 71.40 ± 5.90 U/mg protein × minute (SOD activity), and 1650.37 ± 123.17 NADPH nmol/mg protein × minute (GPx activity).
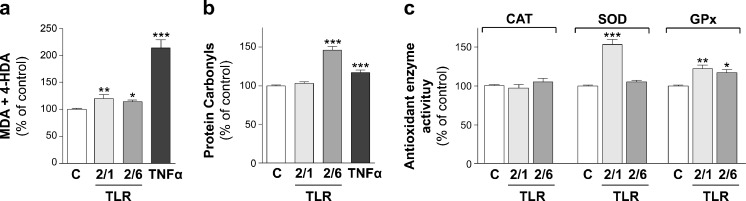
In order to analyze the effects of TLR2 activation, TLR2/1 and TLR2/6 heterodimers were assessed. Thus, Caco-2 cells were treated during 24 h with Pam3CSK4 (TLR2/1 ligand) or Pam2CSK4 (TLR2/6 ligand) at 5 μg/ml or 50 ng/ml, respectively. The results show that the activation of TLR2/6 induced a significant increase in the oxidation of both lipids and proteins compared with control. However, the activation of TLR2/1 yielded a significant increase in the level of lipid peroxidation (Fig. 1a, b). Concerning antioxidant enzymes' activity, TLR2/1 and TLR2/6 activation did not modify CAT activity; however, TLR2/1 activation seemed to significantly stimulate SOD and GPx activities, whereas TLR2/6 activation significantly increased only GPx activity (Fig. 1c). These results infer that stimulation of the antioxidant enzymes' activity SOD and GPx induced by TLR2/1 might avoid an oxidative effect, mainly on proteins. This approach agrees with results obtained in retinitis pigmentosa, which have shown that SOD (Usui et al. 2009) and GPx (Lu et al. 2009) reduced protein carbonyl levels. Previous studies have described TLR2 as a sensor of oxidation, providing a key link connecting inflammation and oxidative stress in inflammatory cells (Kadl et al. 2011; Paul-Clark et al. 2009). TLR2 has also been shown to mediate neural oxidative damage yielded by virus (Schachtele et al. 2010). According to these studies, the results of the present study suggest a prooxidant effect of TLR2 in intestinal epithelial cells that seems to be different depending on the activation of TLR2/1 or TLR2/6 heterodimers.
Fig. 1.
The effect of the activation of TLR2 on lipid peroxidation (a), protein oxidation (b), and CAT, SOD, and GPx antioxidant enzyme activity (c) is as follows. Caco-2 cells were treated during 1 day with either Pam3CSK4 (TLR2/1 ligand) or Pam2CSK4 (TLR2/6 ligand) at 5 μg/ml, or 50 ng/ml, respectively. Treatment with TNFα 5 ng/ml was used as a reference of oxidative level. Results were expressed as the percentage of the control value (100 %) and were indicated as the mean ± SE of five independent experiments in lipid and protein oxidation and four independent experiments in antioxidant activity enzymes. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with control
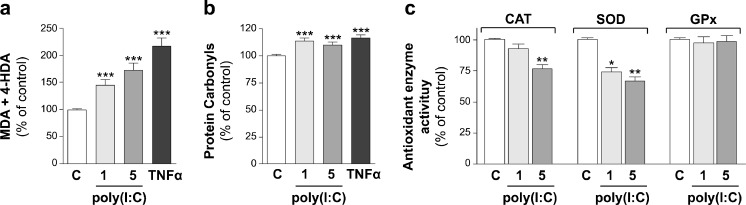
Regarding the analysis of TLR3 effects, Caco-2 cells were treated during 24 h with poly(I:C) (TLR3 agonist) at two concentrations, 1 or 5 μg/ml. The results showed that TLR3 activation by poly(I:C) yielded a significant increase of oxidative damage of lipids (Fig. 2a) and proteins (Fig. 2b) at the two concentrations assayed. Moreover, poly(I:C) significantly reduced CAT activity at high concentration and induced a reduction of SOD activity at the two concentrations assayed. However, GPx activity did not seem to be affected by TLR3 activation (Fig. 2c). The decrease observed on antioxidant enzyme activity after poly(I:C) treatment may explain the prooxidant effect of TLR3 observed on lipids and proteins. Previous results obtained in airway epithelial cells conclude that TLR3 stimulates inflammatory and oxidant responses (Koarai et al. 2010). In contrast, recent studies have demonstrated neuroprotective and antioxidant effects of TLR3; however, these effects were obtained after activation with a poly(I:C) concentration 10 to 20 times higher than in present study (Patel and Hackam 2013; Borysiewicz et al. 2013).
Fig. 2.
The effect of the activation of TLR3 on lipid peroxidation (a), protein oxidation (b), and CAT, SOD, and GPx antioxidant enzyme activity (c) is as follows. Caco-2 cells were treated during 1 day with poly(I:C) (TLR3 ligand) at 1 or 5 μg/ml. Treatment with TNFα 5 ng/ml was used as a reference of oxidative level. Results were expressed as the percentage of the control value (100 %) and were indicated as the mean ± SE of five independent experiments in lipid and protein oxidation and four independent experiments in antioxidant activity enzymes. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with control
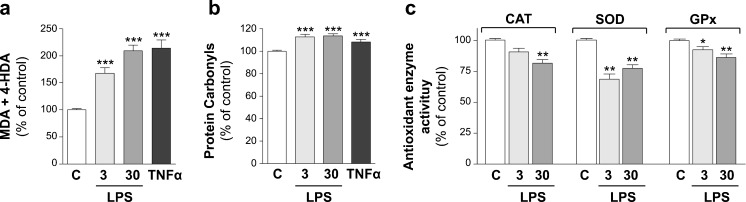
To study TLR4 effects on oxidative damage, Caco-2 cells were treated during 24 h with LPS (TLR4 ligand) at either 3 or 30 μg/ml. The results showed that LPS induced a significant increase of the oxidative level of both lipids (Fig. 3a) and proteins (Fig. 3b) at the two concentrations assayed. Concerning the activity of antioxidant enzymes, the activation of TLR4 decreased the enzymes activity, depending on the concentration used. Thus, LPS at high concentration has shown to reduce CAT, SOD, and GPx activities, whereas LPS at low concentration reduced SOD and GPx activities (Fig. 3c). These results may explain the high and significant oxidative damage obtained in lipids and proteins in cells treated with LPS. Our results agree with recent studies carried out in different tissues that demonstrate TLR4 activation induced oxidative stress (Ko et al. 2011; Lee et al. 2012; Gárate et al. 2013).
Fig. 3.
The effect of the activation of TLR4 on lipids peroxidation (a), protein oxidation (b), and CAT, SOD, and GPx antioxidant enzyme activity (c) is as follows. Caco-2 cells were treated during 1 day with LPS (TLR4 ligand) at 3 or 30 μg/ml. Treatment with TNFα 5 ng/ml was used as a reference of oxidative level. Results were expressed as the percentage of the control value (100 %) and were indicated as the mean ± SE of five independent experiments in lipid and protein oxidation and four independent experiments in antioxidant activity enzymes. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with control
To summarize, the results of the present work demonstrate that the activation of TLR2, TLR3, and TLR4 by specific ligands increased the oxidative status of intestinal epithelial cells. However, the level of prooxidant effect on lipids and proteins and the effect on antioxidant enzymes activity seemed to differ depending on the TLR activated. Thus, TLR3 and TLR4 activation has shown a similar pattern on lipid and protein oxidation, but they show slight differences in the antioxidant enzymes effects. Conversely, activation of TLR2 has shown a different effect on oxidative damage in Caco-2 cells. Lipid peroxidation levels were lower than observed in TLR3 and TLR4 activation. Interestingly, GPx activity, which has been described to reduce oxidized lipids to their nontoxic metabolites (Buijsse et al. 2012), was significantly increased by TLR2, whereas it was significantly decreased by TLR3 or TLR4 activation. These effects on GPx may explain why TLR2 induces less lipid oxidation level than TLR3 and TLR4. Finally and unexpectedly, protein oxidation yielded by TLR2/6 ligand was higher than obtained by activation of TLR3 and TLR4.
TLRs activity, triggered by intestinal microbiota, has been described to contribute to intestinal physiology and pathology. Thus, in relation to the intestinal physiology, TLRs have been described to be involved in immune responses by acting on immunological cells. In this context, the pro-oxidative effect of TLR2, TLR3, and TLR4 in intestinal epithelial cells might also contribute to the intestinal physiology by inducing epithelial turnover. In relation to intestinal pathology, TLRs deregulation has been described to be involved in intestinal inflammatory processes by TLRs inherent innate immune responses. Since oxidative stress has been described as contributing to inflammatory processes, pro-oxidative activity of TLRs might deteriorate the intestinal inflammatory injury.
The results obtained in the study of TLRs effects on intestinal oxidative stress and the analysis of the different antioxidant mechanisms involved might contribute to the design of specific therapies in the treatment of intestinal inflammatory processes. These results also provide a deeper knowledge of mechanisms triggered by TLRs in intestinal physiology and pathology.
Acknowledgments
This work was funded by grants from the Spanish Ministry of Science and Innovation and the European Regional Development Fund (ERDF/FEDER) (BFU2010-18971), European Social Found (ESF) and the Aragon Regional Government (B61) and the Foundation for the Study of Inflammatory Bowel Diseases in Aragón (ARAINF 012/2008). The authors would like to thank Dr. Brot-Laroche (INSERM, UMR S 872, Centre de Recherche des Cordeliers, Paris) for providing Caco-2/TC7 cells. E. Latorre and E. Layunta are PhD student fellows from Aragon Regional Government (B105/11 and B022/13, respectively).
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Al-Shudiefat AA, Sharma AK, Bagchi AK, Dhingra S, Singal PK. Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Mol Cell Biochem. 2013;372:75–82. doi: 10.1007/s11010-012-1447-z. [DOI] [PubMed] [Google Scholar]
- Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals, and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Borysiewicz E, Doppalapudi S, Kirschman LT, Konat GW. TLR3 ligation protects human astrocytes against oxidative stress. J Neuroimmunol. 2013;255:54–59. doi: 10.1016/j.jneuroim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Buijsse B, Lee DH, Steffen L, Erickson RR, Luepker RV, Jacobs DR, Jr, Holtzman JL. Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc's. PLoS One. 2012;7(6):e38901. doi: 10.1371/journal.pone.0038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E. Barrier-protective function of intestinal epithelial toll-like receptor 2. Mucosal Immunol. 2008;1:S62–S66. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress, and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/S0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic Biol Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Ko MK, Saraswathy S, Parikh JG, Rao NA. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci. 2011;52:5824–5835. doi: 10.1167/iovs.10-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarai A, Sugiura H, Yanagisawa S, Ichikawa T, Minakata Y, Matsunaga K, Hirano T, Akamatsu K, Ichinose M. Oxidative stress enhances toll-like receptor 3 response to double-stranded RNA in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:651–660. doi: 10.1165/rcmb.2008-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Sig. 2012;10:33. doi: 10.1186/1478-811X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo YJ, Lauer TW, Usui S, Komeima K, Xie B, Campochiaro PA. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid Redox Signal. 2009;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–6063. [PubMed] [Google Scholar]
- McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun. 2009;15:243–250. doi: 10.1177/1753425909104781. [DOI] [PubMed] [Google Scholar]
- Mendoza C, Matheus N, Latorre E, Castro M, Mesonero JE, Alcalde AI. Toll-like receptor 3 activation affects serotonin transporter activity and expression in human enterocyte-like Caco-2 cells. Cell Physiol Biochem. 2012;30:187–198. doi: 10.1159/000339057. [DOI] [PubMed] [Google Scholar]
- Mintz M, Dvir D, Ilia-Ezra R, Shpigel NY. Pam3CSK4/TLR2 signaling elicits neutrophil recruitment and restricts invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine mastitis model. Vet Immunol Immunopathol. 2013;152:168–175. doi: 10.1016/j.vetimm.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Patel AK, Hackam AS. Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol. 2013;54:122–131. doi: 10.1016/j.molimm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am J Respir Crit Care Med. 2009;179:299–306. doi: 10.1164/rccm.200707-1019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele SJ, Hu S, Little MR, Lokensgard JR. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J Neuroinflammation. 2010;7:35. doi: 10.1186/1742-2094-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, Campochiaro PA. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther. 2009;17:778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr NB, Levine RL. Quantification of protein carbonylation. Methods Mol Biol. 2013;965:265–281. doi: 10.1007/978-1-62703-239-1_18. [DOI] [PubMed] [Google Scholar]
- Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med. 2012;237:474–480. doi: 10.1258/ebm.2011.011358. [DOI] [PubMed] [Google Scholar]