Abstract
Butyric acid (BA) induces jugular blood mitochondrial oxidative stress, whereas heme-induced oxidative stress was previously reported to inhibit SIRT1 in vitro. This would imply that BA-induced oxidative stress may similarly affect SIRT1. Here, we elucidated the BA effects on jugular blood cytosolic oxidative stress and SIRT1. Jugular blood cytosol was collected 0, 60, and 180 min after BA injection into rat gingival tissues and used throughout the study. Blood cytosolic oxidative stress induction, heme accumulation, NADPH oxidase (NOX) activation, nicotinamide adenine dinucleotide (NAD+) and NADP pool levels, NAD kinase (NADK), and SIRT1 amounts were determined. We found that BA retention in the gingival tissue induces blood cytosolic oxidative stress and heme accumulation which we correlated to both NOX activation and NADP pool increase. Moreover, we showed that BA-related NADP pool build-up is associated with NADK increase which we suspect decreased NAD+ levels and consequentially lowered SIRT1 amounts in the rat blood cytosol.
Keywords: Butyric acid, NADPH oxidase, Oxidative stress, Rat blood cytosol, Sirtuin-1
Introduction
Mammalian sirtuins consist of seven homologues (SIRT1-7) and belong to a family of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases that can affect stress responses and aging (Satoh et al. 2011). Among the seven sirtuin homologues, SIRT1 has been hypothesized to promote cell survival and longevity (Chen and Guarente 2007; Kloting and Bluher 2005). In addition, SIRT1 is shown to target numerous regulatory factors which can affect stress management and metabolism (Chen and Guarente 2007). It was previously shown that SIRT1 is inhibited by heme-induced oxidative stress in vitro (Puri et al. 2012) which highlights the correlation between oxidative stress and SIRT1.
Butyric acid (BA) is an extracellular metabolite that is commonly found in the mouth, gut, and vagina produced through either the butyrate kinase or butyryl-CoA: acetate CoA-transferase pathways (Louis et al. 2010). Beneficial BA effects include maintaining intestinal colonic health, serving as an energy source for colorectal cells, and positively influencing immune responses (Hu et al. 2011; Maslowski et al. 2009; Wong et al. 2006), whereas non-beneficial BA effects include reactivation of latent viral infection (Imai et al. 2012) and initiation of periodontal pathogenesis (Kurita-Ochiai and Ochiai 2010). In an earlier published report related to our group (Cueno et al. 2013), we showed that BA retention in the gingival tissue induces oxidative stress in the jugular blood mitochondria. It is, however, not clear whether BA retention in the gingival tissue induces blood cytosolic oxidative stress and similarly affects SIRT1.
Here, as a continuation of our earlier work (Cueno et al. 2013), we established that BA retention in the gingival tissue induces blood cytosolic oxidative stress and heme increase. Similarly, we showed that the heme-binding subunit of NADPH oxidase (gp91phox precursor), NADPH, and NADP levels all accumulated which we suspect contributed to BA-induced oxidative stress generation. In addition, we found that NAD kinase activity increased which we hypothesize lead to a decrease in NAD+ levels and consequentially decreased SIRT1 amounts in the rat blood cytosol.
Results and discussion
BA retention in the gingival tissue induces jugular blood cytosolic oxidative stress
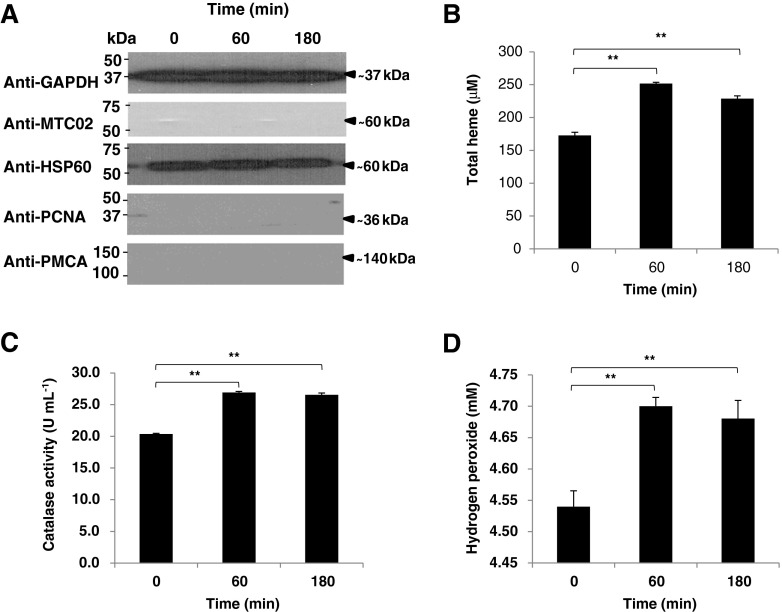
To elucidate whether BA retention in the gingival tissue increases heme amounts and induces oxidative stress in the rat blood cytosol, we isolated jugular blood cytosolic extracts at 0, 60, and 180 min after BA injection similar to our earlier work (Cueno et al. 2013). We found the same pattern of BA retention as we previously observed (data not shown), and subsequently, we measured blood cytosolic heme, catalase (CAT), and hydrogen peroxide (H2O2) levels. Purity of the cytosolic extracts was confirmed using Western blotting (Fig. 1a). We observed that blood cytosolic heme, CAT, and H2O2 amounts were increased after BA injection into the rat gingival tissue (Fig. 1b–d, respectively) which would imply that BA retention in the gingival tissue induces oxidative stress in the blood cytosol and is correlated to heme accumulation. Heme is a biomolecule that interacts with various apo-proteins giving rise to functional heme–proteins which include antioxidant (Chelikani et al. 2005) and pro-oxidant (Espinosa et al. 2009) enzymes. Build-up in cytosolic reactive oxygen species (ROS), like H2O2, triggers mitochondrial oxidative stress (Doughan et al. 2008), whereas elevated mitochondrial ROS activates NADPH oxidase (Wosniak et al. 2009). We suspect that BA-related increase in jugular blood cytosolic H2O2 and heme amounts may have contributed to blood mitochondrial oxidative stress induction (Cueno et al. 2013) which subsequently may have activated NADPH oxidase (NOX).
Fig. 1.
Butyric acid-induced blood cytosolic oxidative stress is correlated to heme accumulation. Jugular blood was collected after BA injection as previously published (Cueno et al. 2013), and animal studies performed were in accordance with the guidelines set by the Kyoto Institute of Nutrition and Pathology Inc. a Jugular blood cytosol purity. Cytosol/Particulate Rapid Separation Kit (BioVision) was used to isolate blood cytosol. Pierce® Detergent Removal Spin Columns (Thermo Scientific) was used to purify samples from traces of detergents. Pierce® Microplate BCA Protein Assay Kit-Reducing Agent Compatible Kit (Thermo Scientific) was used to standardize the protein concentration in all samples used. All kits used were according to manufacturer's recommendation. Western blotting was performed to establish blood cytosol purity. Briefly, blood cytosolic proteins were separated by SDS-PAGE and transferred to Hybond-C nitrocellulose membrane (Amersham Biosciences). Membranes were subsequently blocked with DifcoTM Skim Milk (BD Company) probed with antibodies, and immunoreactive proteins were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Pierce). Anti-GAPDH (GeneTex) was used to detect the glyceraldehydes-3-phosphate in the blood cytosol to serve as control. Anti-HSP60 (StressMarq Biosciences Inc., Canada) was used to determine cytosolic heat-shock protein 60 in the blood cytosol extract to verify the purity of the cytosolic samples. Anti-MTC02 (Novus Biologicals) is a mitochondria-specific antibody used to confirm the absence of mitochondrial components, anti-PCNA (Thermo Scientific) was used to detect the presence of the proliferating cell nuclear antigen, and anti-PMCA (Thermo Scientific) which recognizes the plasma membrane calcium ATPase were used to further establish the purity of the isolated blood cytosol. b Total heme levels in blood cytosolic samples. QuantiChromTM Heme Assay Kit was used to measure blood cytosolic heme levels (free heme and heme–proteins) according to manufacturer's recommendation. c Catalase activity in blood cytosolic samples. EnzyChromTM Catalase Assay Kit (BioAssay Systems) was used to measure cytosolic catalase (CAT) activity according to manufacturer's recommendation. d Hydrogen peroxide amounts in blood cytosolic samples. Red Hydrogen Peroxide Assay Kit (Enzo Life Sciences) was used to measure blood cytosolic hydrogen peroxide (H2O2) amounts according to manufacturer's recommendation. Results shown correspond to n = 5 replicates of six independent samples. Jugular blood cytosol from 0, 60, and 180 min after butyric acid injection are indicated. Statistical analyses were performed using Student's t test (** p < 0.01)
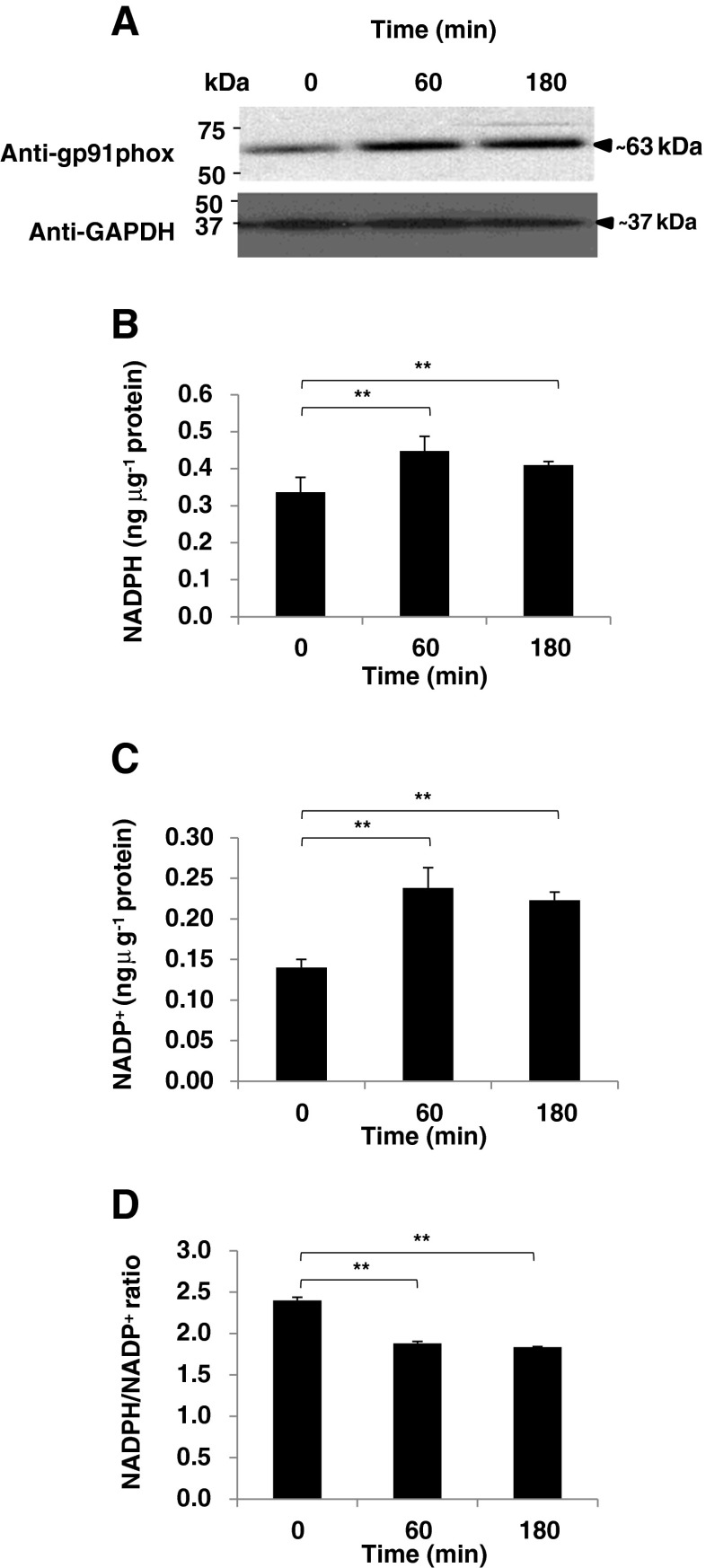
To establish BA-induced NOX activation in the blood cytosol, we detected the gp91phox precursor found in the cytosol and quantified the NADP (NADP+ and NADPH) pool. Heme binding to the gp91phox subunit of the NADPH oxidase enzyme triggers cytosolic ROS production which consequently causes mitochondrial oxidative stress-related ROS increase (Brandes 2005; Yu et al. 1998). We found that the gp91phox precursor (Fig. 2a) and NADP amounts (Fig. 2b, c) were increased in the blood cytosol after BA injection into the rat gingival tissues. In addition, as seen in Fig. 2d, the NADPH/NADP+ ratio in both collection times were decreased which is consistent with oxidative stress (Bernard et al. 2011). We hypothesize that both BA-induced cytosolic oxidative stress and heme increase is associated to both NOX activation and NADP pool increase. Similarly, this would imply that NADK activity may have been affected.
Fig. 2.
Butyric acid causes blood cytosolic NADPH activation and NADP+ pool increase. a Blood cytosolic gp91phox precursor detection. Western blotting was performed using anti-gp91phox (GeneTex) to verify gp91phox precursor amounts in the blood cytosol and anti-GAPDH was used as control. Briefly, blood cytosolic proteins were separated by SDS-PAGE and transferred to Hybond-C nitrocellulose membrane (Amersham Biosciences). Membranes were subsequently blocked with DifcoTM Skim Milk (BD Company) probed with antibodies, and immunoreactive proteins were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Pierce). Blood cytosolic NADP pool [b NADPH and c NADP+] and d NADPH/NADP ratio. EnzyChromTM NADP+/NADPH Assay Kit (BioAssay Systems) was used to determine NADP+ and NADPH levels according to manufacturer's recommendation. Jugular blood cytosol from 0, 60, and 180 min after butyric acid injection are indicated. In all assays performed, results shown are mean±SE, n = 5 replicates of six independent samples. Statistical analyses were performed using Student's t test (** p < 0.01)
BA-induced blood cytosolic oxidative stress is associated with a decrease in SIRT1 amounts
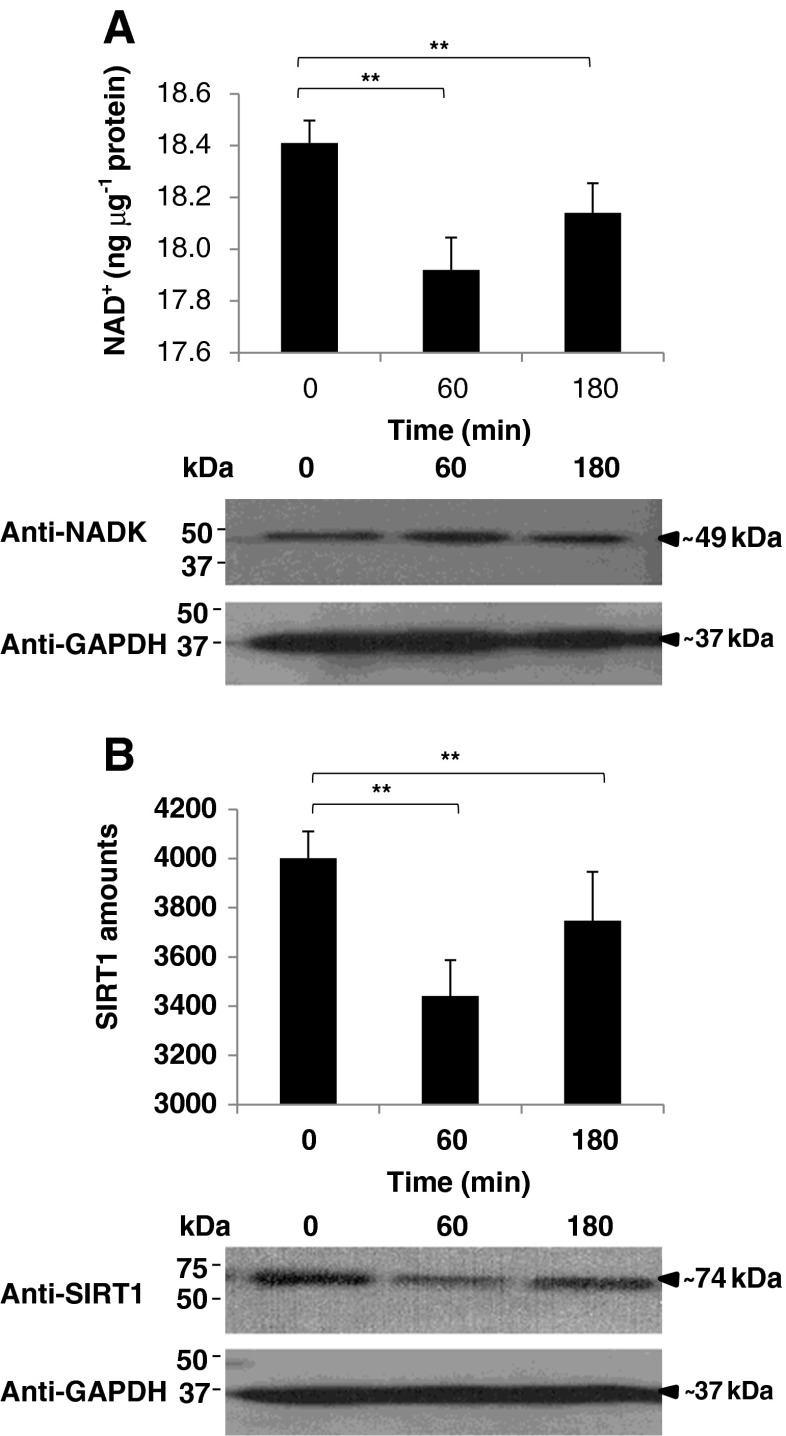
To determine the BA effects on blood cytosolic NADK action, NAD+ levels and NADK protein were detected. We observed that blood cytosolic NAD+ levels were decreased after BA injection (Fig. 3a, upper panel), whereas the detected NADK protein was increased after BA injection (Fig. 3a, lower panel). NADKs play a critical role in determining the NADP pool and one mechanism by which the NADP pool can be increased at the expense of NAD+ is through NADK action (Lerner et al. 2001). This would insinuate that BA could have indirectly contributed to NADK activity increase which we speculate decreased NAD+ levels in the rat blood cytosol. Moreover, as a possible consequence of NAD+ decrease, we suspect that SIRT1 is likewise affected.
Fig. 3.
Butyric acid-related NAD+ pool decrease is associated with lower SIRT1 amounts in the blood cytosol. a Blood cytosolic NAD+ pool. (Upper panel) NAD+ pool levels and (lower panel) NAD kinase (NADK) protein detection are indicated. NAD+/NADH Quantification Kit (BioVision) was used to quantify NAD+ amounts in blood cytosol according to manufacturer's recommendation. Western blotting was performed using anti-NAD kinase (GeneTex) to detect blood cytosolic NADK protein and anti-GAPDH was used as control. Briefly, blood cytosolic proteins were separated by SDS-PAGE and transferred to Hybond-C nitrocellulose membrane (Amersham Biosciences). Membranes were subsequently blocked with DifcoTM Skim Milk (BD Company) probed with antibodies, and immunoreactive proteins were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Pierce). b Blood cytosolic SIRT1 amounts. (Upper panel) Measurement of SIRT1 amounts and (lower panel) SIRT1 protein detection are shown. Fluorogenic SIRT1 (Sir2) Assay Kit (BPS Bioscience) was used to measure blood cytosol SIRT1 amounts. Western blotting was performed using anti-SIRT1 (Aviva Systems Biology) to detect blood cytosolic SIRT1 protein. Jugular blood cytosol from 0, 60, and 180 min after butyric acid injection are indicated. In all assays performed, results shown are mean±SE, n = 5 replicates of six independent samples. Statistical analyses were performed using Student's t test (* p < 0.05; ** p < 0.01)
To expound on the possible consequential effects of blood cytosolic NAD+ decrease on SIRT1, we quantified and detected SIRT1 in the blood cytosol. We found that SIRT1 amounts were similarly decreased after BA injection (Fig. 3b, upper panel). This was concurrently verified using Western blotting (Fig. 3b, lower panel). Both results showed that SIRT1 amounts were decreased after BA injection consistent with NAD+ levels in the blood cytosol (Fig. 3a, upper panel). High SIRT1 amounts promote cell survival, favor longevity, and exhibit better stress response, whereas low SIRT1 amounts have an opposite effect (Chen and Guarente 2007; Kloting and Bluher 2005; Satoh et al. 2011). This highlights the possible detrimental effects of BA-induced SIRT1 decrease in the rat jugular blood. We suspect that BA-induced jugular blood cytosolic oxidative stress and heme accumulation may have indirectly decreased NAD+ levels and consequentially lowered SIRT1 amount which is in agreement with a previous work (Puri et al. 2012). Admittedly, additional experimental work is recommended to further prove the potential relationship between BA and SIRT1.
In conclusion, we established that BA retention in the rat gingival tissue causes jugular blood cytosolic oxidative stress and heme increase. We correlated both BA-induced cytosolic oxidative stress and heme accumulation to both NOX activation and NADP pool increase. Moreover, we showed that BA-related NADK activity increase is associated to a decrease in NAD+ and SIRT1 amounts in the rat blood cytosol.
Acknowledgments
We would like to express our appreciation to T. Tsukahara and N. Matsukawa from Kyoto Institute of Nutrition and Pathology Inc. for helping with the animal experiments. This work was supported by the Dental Research Center–Nihon University School of Dentistry and funded by the Sato Fund–Nihon University School of Dentistry, Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan through the Strategic Research Base Development Program for Private Universities 2010–2014 (S1001024).
Contributor Information
Marni E. Cueno, Phone: +81-3-32198125, FAX: +81-3-32198317, Email: marni.cueno@nihon-u.ac.jp
Kuniyasu Ochiai, Phone: +81-3-32198125, FAX: +81-3-32198317, Email: ochiai.kuniyasu@nihon-u.ac.jp.
References
- Bernard KE, Parkes TL, Merritt TJ. A model of oxidative stress management: moderation of carbohydrate metabolizing enzymes in SOD1-null Drosophila melanogaster. PLoS One. 2011;6:e24518. doi: 10.1371/journal.pone.0024518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension. 2005;45:847–848. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- Chelikani P, Ramana T, Radhakrishnan TM. Catalase: a repertoire of unusual features. Indian J Clin Biochem. 2005;20:131–135. doi: 10.1007/BF02867412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Cueno ME, Imai K, Matsukawa N, Tsukahara T, Kurita-Ochiai T, Ochiai K. Butyric acid retention in gingival tissue induces oxidative stress in jugular blood mitochondria. Cell Stress Chaperones. 2013;18:661–665. doi: 10.1007/s12192-013-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Garcia A, Hartel S, Hidalgo C, Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J Biol Chem. 2009;284:2568–2575. doi: 10.1074/jbc.M804249200. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong TS, Dalal SR, Wu F, Bissonnette M, Kwon JH, Chang EB. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS One. 2011;6:e16221. doi: 10.1371/journal.pone.0016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Inoue H, Tamura M, Cueno ME, Takeichi O, Kusama K, Saito I, Ochiai K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie. 2012;94:839–846. doi: 10.1016/j.biochi.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Kloting N, Bluher M. Extended longevity and insulin signaling in adipose tissue. Exp Gerontol. 2005;40:878–883. doi: 10.1016/j.exger.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T-cells. J Dent Res. 2010;89:689–694. doi: 10.1177/0022034510365456. [DOI] [PubMed] [Google Scholar]
- Lerner F, Niere M, Ludwig A, Ziegler M. Structural and functional characterization of human NAD kinase. Biochem Biophys Res Commun. 2001;288:69–74. doi: 10.1006/bbrc.2001.5735. [DOI] [PubMed] [Google Scholar]
- Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N, Sodhi K, Haarstad M, Kim DH, Bohinc S, Foglio E, Favero G, Abraham NG. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J Cell Biochem. 2012;113:1926–1935. doi: 10.1002/jcb.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Stein L, Imai S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb Exp Pharmacol. 2011;206:125–162. doi: 10.1007/978-3-642-21631-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]