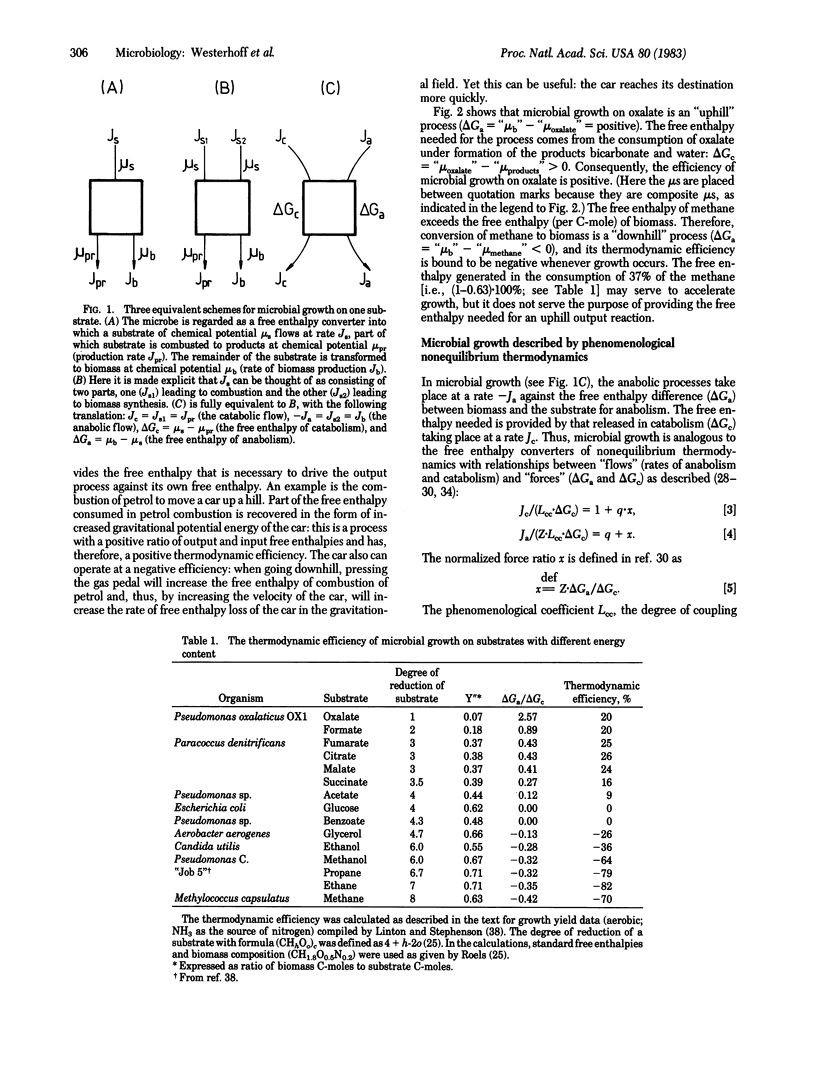
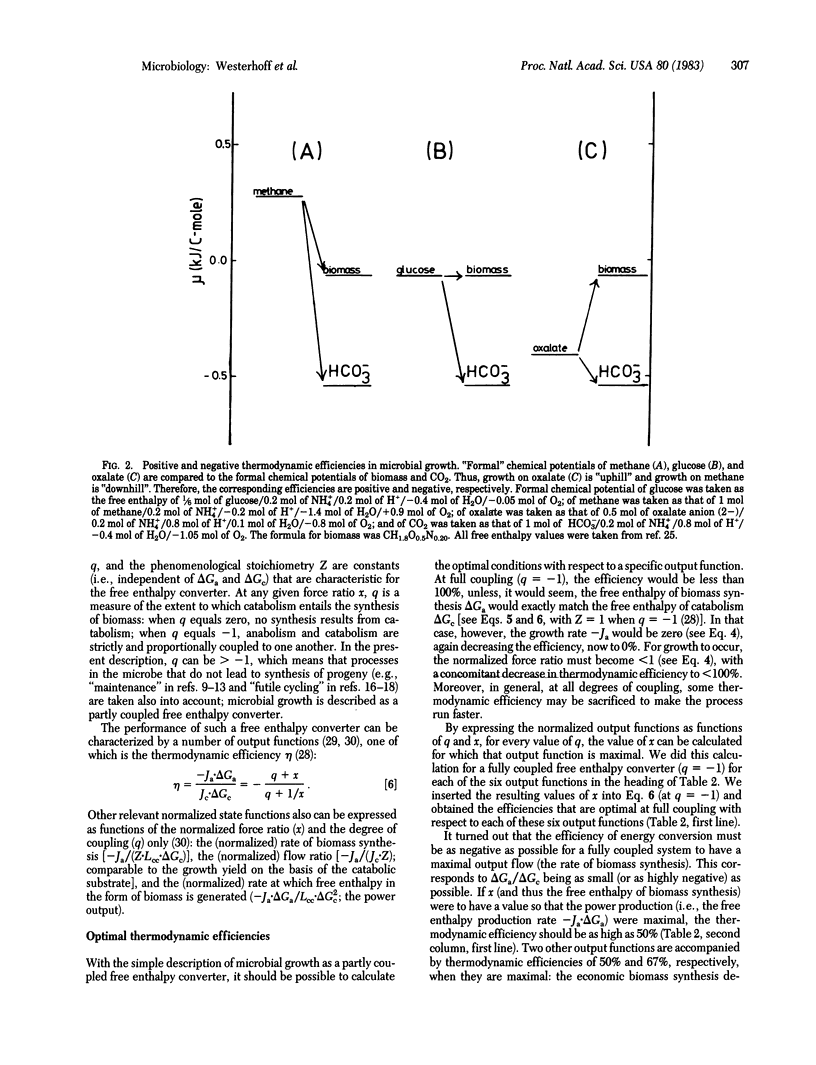
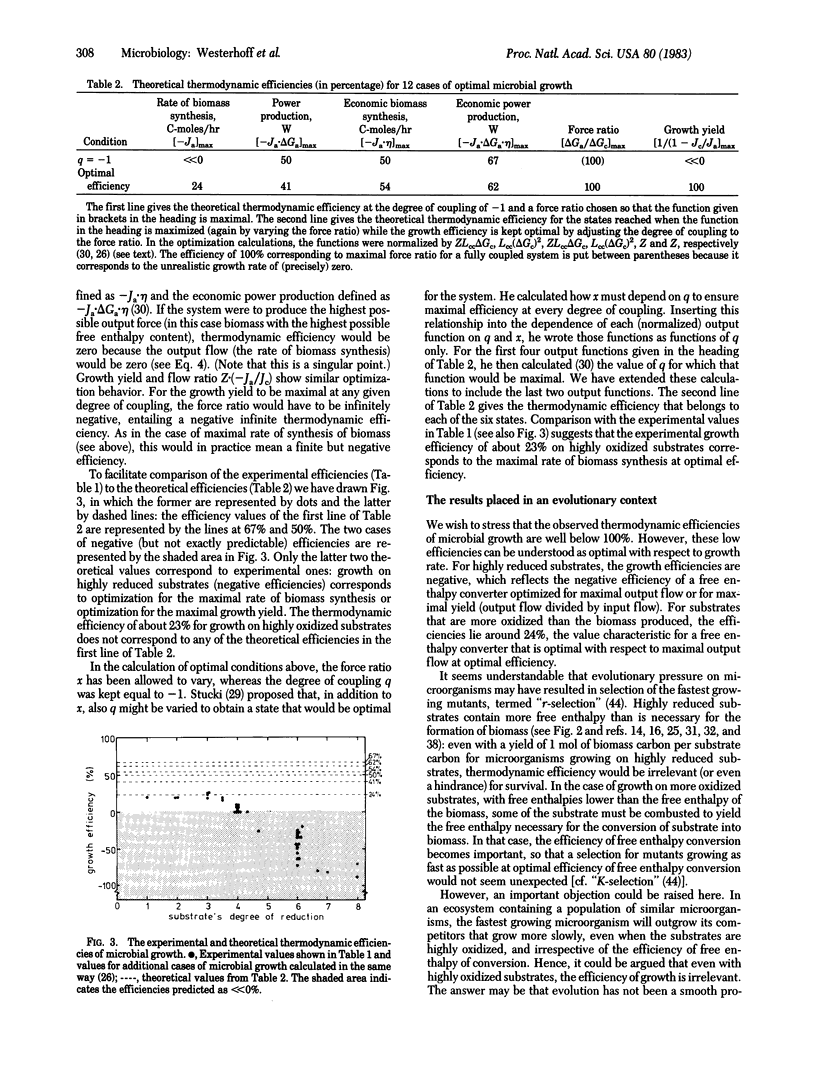
Abstract
Thermodynamic efficiency of microbial growth on substrates that are more oxidized than biomass approaches 24%. This is the theoretical value for a linear energy converter optimized for maximal output flow at optimal efficiency. For growth on substrates more reduced than biomass, thermodynamic efficiencies correspond to those predicted for optimization to maximal growth rate (or yield) only.
Keywords: nonequilibrium thermodynamics, growth yield, r- and K-selection, uncoupling, energy wastage
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- Light P. A., Garland P. B. A comparison of mitochondria from Torulopsis utilis grown in continuous culture with glycerol, iron, ammonium, magnesium or phosphate as the growth-limiting nutrient. Biochem J. 1971 Aug;124(1):123–134. doi: 10.1042/bj1240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkevich I. G., Eroshin V. K. Productivity and heat generation of fermentation under oxygen limitation. Folia Microbiol (Praha) 1973;18(5):376–385. doi: 10.1007/BF02875932. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Arch Microbiol. 1975 Dec 31;106(3):251–258. doi: 10.1007/BF00446531. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Stucki J. W. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem. 1980 Aug;109(1):269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Lolkema J. S., Otto R., Hellingwerf K. J. Thermodynamics of growth. Non-equilibrium thermodynamics of bacterial growth. The phenomenological and the mosaic approach. Biochim Biophys Acta. 1982 Dec 31;683(3-4):181–220. doi: 10.1016/0304-4173(82)90001-5. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Stouthamer A. H. Growth yields and the efficiency of oxidative phosphorylation during autotrophic growth of Paracoccus denitrificans on methanol and formate. Arch Microbiol. 1978 Jul;118(1):21–26. doi: 10.1007/BF00406069. [DOI] [PubMed] [Google Scholar]



