Abstract
Peroxiredoxins, a group of antioxidant protein enzymes (PRDX1 to 6), are reported as antiatherogenic factors in animals; however, human studies are lacking. The present work aims to provide baseline data regarding the phenotype of PRDX1, 2, 4, and 6 in diabetic patients with peripheral atherosclerosis disease (PAD) and their relation to endothelial dysfunction (ED) and disease severity. Plasma levels of PRDX1, 2, 4, and 6 and markers of endothelial dysfunction (ICAM-1 and VCAM-1) were measured using ELISA in 55 type 2 diabetic patients having PAD and 25 healthy subjects. Ankle–brachial index (ABI), body mass index (BMI), triglycerides (TG), total cholesterol, HbA1c, and insulin resistance (HOMA IR) were measured. PRDX1, 2, 4, and 6 levels were significantly higher in patients compared to controls (PRDX1 21.9 ± 5.71 vs 16.8 ± 3.9 ng/ml, P < 0.001, PRDX2 36.5 ± 14.83 vs 20.4 ± 8.61 ng/ml, P < 0.001, PRDX4 3,840 ± 1,440 vs 2,696 ± 1,972 pg/ml, P < 0.005, PRDX6 311 ± 110 vs 287.9 ± 114 pg/ml, P < 0.05). PRDX1 and PRDX4 correlated negatively with ABI (r = −0.273, P < 0.05 and r = −0.28, P < 0.05, respectively), while PRDX1 and PRDX2 correlated positively with HOMA/IR and TG (r = 0.276, P < 0.01 and r = 0.295, P < 0.01, respectively). ICAM-1 was associated with PRDX2 and log PRDX6 (r = 0.345, P = 0.0037 and r = 0.344, P = 0.0038). Our results provide strong links among PRDXs, ED, and severity of PAD in diabetic patients which warrants further evaluation to clarify whether high circulating levels of PRDXs are a consequence of chronic atherosclerotic disease or a predisposing factor for later cardiovascular events.
Keywords: Peroxiredoxins, Oxidative stress, Atherosclerosis, Diabetes, Endothelial dysfunction, ABI
Introduction
Peripheral arterial disease (PAD) is a relatively common manifestation of atherosclerotic vascular disease that affects large- and medium-sized arteries of most circulatory beds, especially lower extremity, and is the leading cause of death and disability in developed countries (Khawaja and Kullo 2009). Although conventional risk factors including diabetes are known to contribute to the development of PAD, the role of biomarkers in pathways such as oxidative stress in determining susceptibility to and severity of PAD is not fully elucidated (Khawaja and Kullo 2009). Therefore, exploring circulating levels of novel proteins in PAD may allow earlier detection, an improved understanding of disease etiology and progression, and the development of new therapies.
Reactive oxygen species (ROS) contribute to the pathogenesis of cardiovascular diseases including atherosclerosis, by inducing oxidative damage to lipids and proteins (Madamanchi et al. 2005). To protect against the toxic effects of ROS, cells and tissues have developed several enzymatic antioxidant defense systems that regulate the concentration of these species inside and outside of the cells (Bergamini et al. 2004). Local imbalance between ROS and scavenging activity of anti-oxidant enzymes due to overproduction of ROS results in various diseases including atherosclerosis (Park and Oh 2011). Fortunately, naturally occurring antioxidants are proposed to be atheroprotective. One such antioxidant family is the peroxiredoxins (PRDXS; Rhee et al. 2005).
PRDXs are antioxidant protein enzymes containing essential catalytic cysteine residues that use thioredoxin to scavenge hydrogen peroxide, lipid hydroperoxides, and peroxynitrite. PRDXs make up a potent defense mechanism for maintaining redox balance under normal conditions and oxidative stress. The six mammalian PRDXs (PRDX1 to 6) are distributed at sites of ROS production, including the cytosol, mitochondria, and peroxisomes (Rhee et al. 2005).
Although the six members of PRDX family share an antioxidant scavenging function, they have other differential functions specified for each member. For example, PRDX1 is induced in macrophages by oxidized lipoprotein lipase exposure (Conway and Kinter 2006) and in endothelial cells by laminar shear stress (Mowbray et al. 2008). In addition, PRDX1 deficiency results in the excessive release of P-selectin and von Willebrand factor (Kisucka et al. 2008). PRDX2 induces many functions including, removal of H2O2 transiently produced by activation of various cell surface receptors (Rhee et al. 2005), inhibition of general immune cell responsiveness (Moon et al. 2006), inhibition of low-density lipoprotein (LDL) oxidation, and reduction of plasma lipid peroxide levels (Park et al. 2011).
A recent study by Martinez-Pinna and colleagues (2011) identified peroxiredoxin-1 as a marker of abdominal aortic aneurysm. On the other hand, animal studies provided some evidences implicating peroxiredoxins in the protection against the atherosclerotic process. For example, PRDX1 deficiency increased vulnerability to the development of excessive endothelial cell activation and early atherosclerosis in mouse (Kisucka et al. 2008). Deletion of PRDX2 led to increased expression of vascular cell adhesion molecule-1(VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and monocyte chemo-attractant protein-1(MCP-1; Park et al. 2011); all are markers of endothelial dysfunction (ED) and inducers of atherosclerotic plaques (Brevetti et al. 2006). On the other hand, PRDX2 was demonstrated to ameliorate atherosclerosis in ApoE−/− mice and the strong expression of PRXD2 in endothelial and immune cells in the atherosclerotic lesion blocked the up regulation of endogenous H2O2 induced by pro-inflammatory cytokines (Park et al. 2011). Finally, PRDX6-deficient mice showed significantly larger aortic root lesions than the wild-type. However, this protein does not seem to play a major anti-atherogenic role in mice (Wang et al. 2003), contrary to PRDX1 and 2.
Despite the extensive animal work on the role of peroxiredoxins in atherosclerosis, data pertaining to any possible association between PAD and peroxiredoxins in human studies are insufficient. Therefore, we aimed to measure levels of circulating peroxiredoxins 1, 2, 4, and 6 and investigate their relation to the severity of atherosclerotic disease, markers of endothelial dysfunction and comorbidity factors as hyperlipedemia and insulin resistance, in type II diabetic patients (T2DM) suffering from peripheral atherosclerotic disease (PAD).
Methods and subjects
Study population
This is a case–control study, where 55 patients suffering from type II diabetes mellitus (T2DM) were recruited at the outpatient clinic of the Department of Vascular Surgery of King Khalid University Hospital between June and December 2009. Consecutive patients with present symptoms of atherosclerotic chronic lower limb ischemia or carotid artery stenosis were considered for inclusion in the study. Patients who had acute onset of lower limb ischemia, clinical or laboratory signs of acute infection, myocardial infarction, stroke, trauma, or surgical procedure in the last 6 months were excluded. Patients with coexisting malignant tumor, hepatic disease, end stage renal disease (dialysis) or immune suppression were also excluded. The full medical record of the patients was registered with detailed physical status and routine clinical laboratory tests. Twenty-five healthy subjects were recruited as the control group. The study was carried out in accordance with the institutional review board regulations of Medical College at King Saud University. All patients and healthy subjects signed a written consent.
Clinical and demographic data
All patients had a medical history interview and physical examination. A questionnaire was used for recording the relevant demographic and clinical data (age, weight, height, smoking habit, medications, and associated disease). The index of basal insulin resistance was assessed using the homeostasis model (HOMA/IR), originally described by Matthews et al. (1985), in which HOMA/IR (mmol/l × μIU/ml) = fasting glucose (mmol/l) × fasting insulin (μIU/ml)/22.5. (HOMA/IR of a value of more than 2.5 was considered to represent insulin resistance).
Body weight was measured to the nearest 0.1 kg using a digital scale. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (kg/m2).
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded in supine position in the left arm using an automated digital blood pressure device. All variables were measured in duplicate and the average of two measurements was used.
Ankle–brachial index (ABI)
Ankle–brachial index (ABI) is an objective, reproducible, non-invasive measure that correlates with PAD severity. PAD was defined as ABI ≤ 0.90 which is 95 % sensitive and 90 % specific for the presence of a ≥50 % narrowing of a lower extremity artery and is used in the clinical setting to establish a diagnosis of PAD (Doobay and Anand 2005). Ankle–brachial index was measured with Doppler ultrasound (Vaso-guard,) and carried out by an experienced technician at Vascular Surgery Lab. The patient lied down in a supine position after resting at normal room temperature; measurements were taken at each ankle over the posterior tibial and dorsal pedal arteries.
Detection of carotid stenosis
Carotid stenosis was determined using Duplex ultrasound. Stenosis of 70 % or above was determined as significant (Grant et al. 2003). Carotid artery ultrasound was performed by an experienced sonographer blinded to laboratory results at the vascular lab. Patients were placed in a supine position and images were taken from carotid artery in a standardized fashion. After having measured both carotid arteries, the more stenotic artery was determined as the maximal carotid stenosis.
Blood samples and determination of PRDXs and endothelial biomarkers
Blood samples were taken after 10–12 h of overnight fasting via antecubital vein into native ethylenediaminetetraacetic acid (EDTA) tubes. The samples were centrifuged and aliquots of plasma were stored at −70 °C until assayed.
PRDX 2, 4, and 6 were measured in the plasma of patients and control subjects using commercially available ELISA kits (Wuhan EIAab Science Co., Ltd., China) and PRDX1 (Abfrontier, China) following manufacturer’s instructions. ICAM-1 and VCAM-1 and insulin levels were determined in plasma of patients and controls using ELISA kits according to the manufacturer’s instructions (R&D Systems, USA).
Determination of other laboratory parameters
Fasting serum samples were also used for measuring standard clinical laboratory measurements. Serum cholesterol and triglycerides, high- and low-density lipoproteins (HDL and LDL) levels, and fasting blood sugar were analyzed at King Khalid University Hospital biochemistry central lab (Konelab Intelligent Diagnostics Systems (Konelab Corporation, Finland). HbA1c was measured by using Helena Glyco-Tek Affinity Colum method (Helena Biosciences, Colima Avenue, Sunderland Enterprise Park, UK).
Statistical analysis
Normally distributed values are presented as means ± SD, whereas non-normally distributed values are presented as median (average). Statistical significance was set at the level of P < 0.05. Comparisons between two groups were assessed for continuous variables with the Student’s unpaired t test, Mann–Whitney test or X2 test, as appropriate. Spearman’s rank correlation was used to determine correlations of PRDXs and other variables. Logarithmic transformation was made for PRDX6 to obtain a normal distribution and a linear relationship with independent variables. All statistical analyses were performed with SAS statistical software (Version 9.1; SAS Institute, Inc., Cary, NC, USA).
Results
Clinical characteristics of the study subjects
This study included 55 patients (58.18 % males and 41.82 % females) with an average age of 61 ± 8.97 years, and 25 control subjects with an average age of 54 ± 15.39 years of which 76 % was male and 24 % was female. The baseline clinical and laboratory characteristics of the study population are represented in Table 1. Nine patients (16.2 %) had severe carotid artery stenosis (above 70 %, according to Grant et al. 2003), 34 patients with severe PAD (61 %), whereas 12 patients suffered from both diseases (21.8 %). The mean values of ankle–brachial index showed significant reduction compared to control subjects by more than 30 %. Clinical and laboratory features of the studied groups are presented in Table 2. Lipid profile: triglycerides, LDL, and total cholesterol were higher in patients compared with controls. Also markers of endothelial dysfunction (ICMA-1 and VCAM-1) reported higher levels in patients compared with controls (Table 2).
Table 1.
Age, gender, body mass index, systolic and diastolic blood pressure, ankle–brachial index, and carotid artery stenosis percentage in control and patient groups
| Parameter | Control subjects (n = 25) | Patients (n = 55) | P value |
|---|---|---|---|
| Age (year) | 54 ± 15.39 | 61 ± 8.97 | <0.05 |
| Gender (M/F) | 7/18 | 24/31 | |
| BMI (kg/m2) | 25.3 ± 4.06 | 27.1 ± 4.4 | NS |
| SBP (mmHg) | 117 ± 3.91 | 140.2 ± 23.22 | <0.001 |
| DBP (mmHg) | 75.9 ± 6.28 | 78.1 ± 9.5 | NS |
| ABI | 1.1 ± 0.05 | 0.7 ± 0.35 | <0.001 |
| Carotid artery stenosis | – | 40.8 % ± 28.42 | |
| (Rt)% | |||
| (Lt)% | – | 43.6 ± 34.55 |
Comparisons were made using Mann–Whitney test. P < 0.05 is statistically significant
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, ABI ankle–brachial index, NS non-significant
Table 2.
Clinical and laboratory features of control subjects and patients: serum cholesterol, triglycerides, high- and low-density lipoproteins, glycosylated hemoglobin, plasma insulin, fasting blood sugar level, insulin resistance index, serum creatinine, erythrocyte sedimentation rate, and plasma level of ICAM-1 and VCAM-1
| Parameter | Control subjects (n = 25) | Patients (n = 55) | P value |
|---|---|---|---|
| Total cholesterol (mmol/l) | 4.5 ± 0.62 | 5.1 ± 1.46 | <0.05 |
| Triglycerides (mmol/l) | 0.9 ± 0.39 | 2.0 ± 1.01 | <0.001 |
| HDL cholesterol (mmol/l) | 0.9 ± 0.29 | 1.3 ± 1.2 | NS |
| LDL (mmol/l) | 2.9 ± 0.76 | 3.9 ± 1.38 | <0.001 |
| HbA1c (%) | ND | 8.1 ± 2.16 | |
| Plasma Insulin (IU/ml) | 13.2 ± 5.86 | 24 ± 22.1 | <0.05 |
| Fasting blood sugar (mmol/l) | 4.7 ± 0.54 | 8.9 ± 3.88 | <0.001 |
| HOMA-IR | 2.8 ± 1.44 | 8.8 ± 1.5 | <0.01 |
| Creatinine (μmol/l) | 87.1 ± 14.1 | 123.3 ± 10.6 | NS |
| ESR (mm/h) | 13.2 ± 12.79 | 59.6 ± 38.37 | <0.001 |
| ICAM-1 (ng/ml) | 13.468 ± 5.89 | 17.5 ± 7.77 | 0.02 |
| VCAM-1 (ng/ml) | 29.48 ± 5.799 | 48.28 ± 25.4 | <0.001 |
Comparisons were made using Mann–Whitney test. P < 0.05 is statistically significant
HbA 1c glycosylated hemoglobin, HOMA IR insulin resistance index, ESR erythrocyte sedimentation rate, ICAM-1 cellular adhesion molecule-1, VCAM-1 vascular cellular adhesion molecule-1, ND non-detectable, NS non-significant
Plasma levels of peroxiredoxins
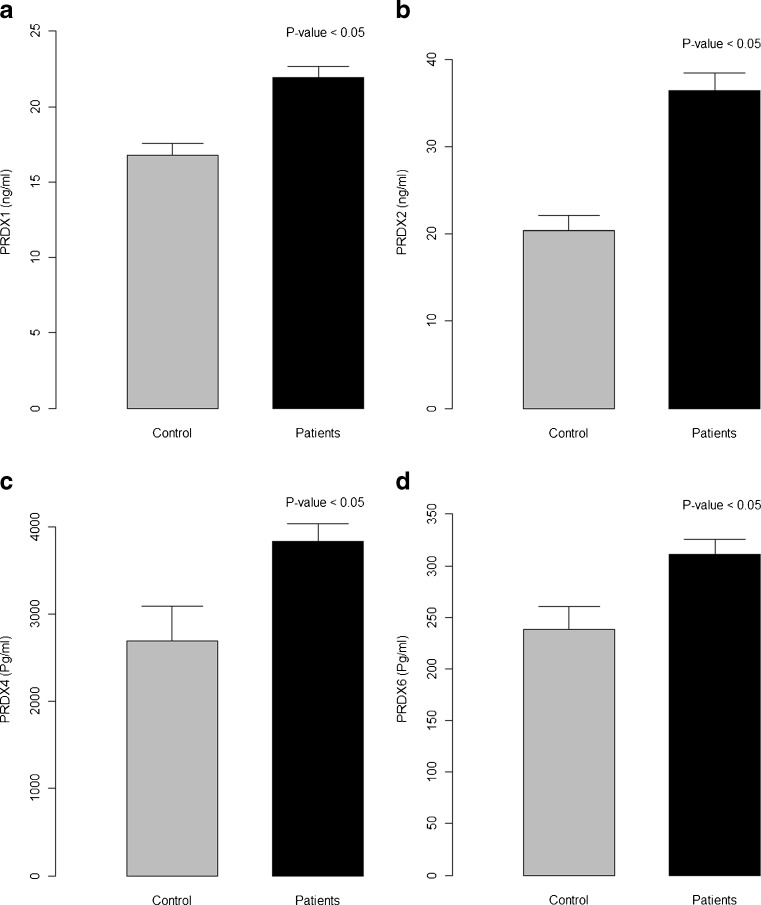
In the current study, plasma peroxiredoxins reported significantly higher levels in diabetic patients suffering from PAD, compared to normal healthy controls: PRDX1 21.9 ± 5.7 ng/ml vs 16.8 ± 3.9 ng/ml, P < 0.001 (Fig. 1a); PRDX2 36.5 ± 14.8 ng/ml vs 20.4 ± 8.6 ng/ml, P < 0.001 (Fig. 1b); PRDX4 3,840 ± 1,440.7 pg/ml vs 2,696.9 ± 1,972.6 pg/ml, P = 0.005 (Fig. 1c); and PRDX6 311 ± 110.1 pg/ml vs 238.2 ± 111.5 pg/ml, P = 0.008 (Fig. 1d; respectively, in patients vs control).
Fig. 1.
Plasma levels of peroxiredoxins in healthy control subjects and diabetic patients with peripheral atherosclerotic disease: a PRDX1, b PRDX2, c PRDX4, and d PRDX6. Results are expressed as mean ± SD; P < 0.05 is statistically significant
Biomarkers of endothelial dysfunction
Results for biomarkers of endothelial dysfunction in patients and control individuals are shown in Table 2. VCAM-1 and ICAM-1 attained significantly higher levels in patients compared to control subjects (29.48 ± 5.799 vs 48.28 ± 25.4 ng/ml, P < 0.001 and 13.468 ± 5.89 vs 17.5 ± 7.77 ng/ml, P = 0.02, respectively in control subjects vs patients).
Association of peroxiredoxins with laboratory parameters and clinical characteristics
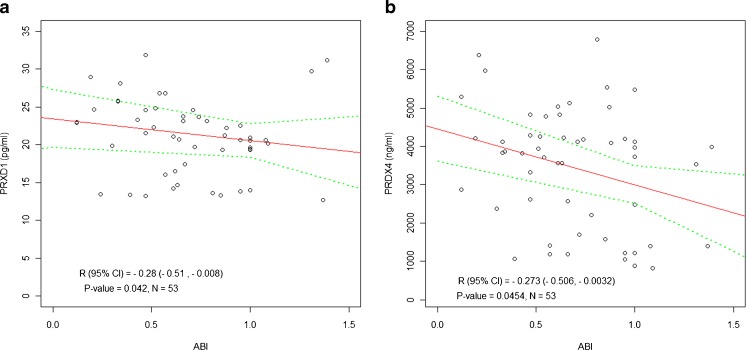
Analysis of laboratory markers revealed significant positive correlation between PRDX1 and triglycerides (TG; P = 0.009; Fig. 2a). Also PRDX2 plasma levels showed a strong association with HOMA-IR (P = 0.018; Fig. 2b). Only PRDX1 and PRDX4 plasma concentrations were negatively correlated with ABI (P = 0.042 and P = 0.045, respectively; Fig. 3a, b).
Fig. 2.
Ankle–brachial index (ABI), an indicator of peripheral artery disease severity, correlates with circulating levels of a PRDX1 and b PRDX4 in diabetic patients with peripheral atherosclerotic disease
Fig. 3.
The association of peroxiredoxins with comorbidity factors. a PRDX1 correlates with triglycerides (TG) and b PRDX2 correlates with HOMA-IR in whole population
Association of peroxiredoxins with biomarkers of endothelial dysfunction
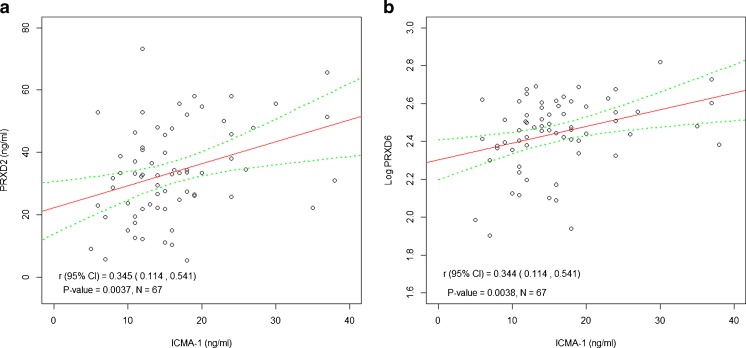
We investigated the association between peroxiredoxins plasma levels and endothelial biomarkers. A strong positive association was reported between ICAM-1 and PRDX1 plasma concentration (P = 0.0037; Fig. 4a). Also, ICAM-1 was associated with log PRDX6 plasma levels (P = 0.0038; Fig. 4b).
Fig. 4.
The association of circulating levels of peroxiredoxins: a PRDX2 and b Log PRDX6, with ICAM-1, a marker of endothelial dysfunction
Discussion
In the present study, the antioxidant status was investigated by measuring the levels of peroxiredoxins in type II diabetic patients with peripheral atherosclerotic disease (PAD). The novel finding of this study is the high circulating levels of PRDX1, 2, 4, and 6 reported in diabetic patients with PAD. This increase in peroxiredoxins levels was associated with endothelial dysfunction, severity of peripheral arterial disease, and comorbidities as triglycerides and insulin resistance. Because of the study design, only correlative associations have been presented and thus, the current study cannot provide mechanistic explanations. Although oxidative damage to lipids and proteins is implicated in the development of atherosclerotic vascular disease (Madamanchi et al. 2005), data relating any possible association between PAD and antioxidant status are insufficient. Up to the best of our knowledge, this is the first human study, investigating the circulating levels of peroxiredoxins with the extent of atherosclerosis in diabetic patients.
Oxidative stress, a situation with increased reactive oxygen species (ROS) production and/or decreased antioxidant defense mechanisms, is evident in the pathogenesis of atherosclerosis (Madamanchi et al. 2005). The present study investigated four members of the peroxiredoxin family and demonstrated that circulating levels of PRDX1, 2, 4, and 6 are markedly raised in diabetic patients with PAD. Although little is known about the pathophysiological role of peroxiredoxins, there is an evidence that they are upregulated in conditions of increased ROS generation (Bast et al. 2002; Schulte et al. 2011), which supports our observation. Previous reports showed that exposure of cells to oxidative stress and heat shock induced chaperone function of PRDXs, in order to enhance cellular resistance to stressful stimuli (Jang et al. 2004). Therefore, the high levels of PRDXs reported here possibly reflect the chaperone function of these proteins under such pathological conditions of hyperlipedemia and hyperglycemia.
PRDX1, the first member in peroxiredoxin family investigated in the present study, has been shown to be expressed and induced in endothelium in vitro by laminar shear stress (Mowbray et al. 2008) and to protect red blood cells from ROS (Neumann et al. 2003). Among the stimuli that can lead to an increase in the levels of PRDX1 is the oxidized LDL (Conway and Kinter 2006), which has been proposed to participate in atherogenesis (Grundy et al. 1998). Although we demonstrated a higher level of LDL in the diabetic atherosclerotic patients parallel to an elevation in PRDX1, no correlation was detected. However, we reported a positive association of PRDX1 plasma concentration with the level of triglycerides (TG). Many prospective studies have reported a positive relationship between levels of TG and cardiovascular risk (Austin et al. 1998). In addition, lipoprotein metabolism is integrally linked with TG, and elevations of plasma TG can be confounded by significant correlations with LDL and HDL-C levels (Grundy et al. 1998). Therefore, many persons with elevated TG are at increased risk of cardiovascular disease, even when this greater risk cannot be independently explained by TG (Grundy et al. 1998). While animal studies demonstrated the anti-atherogenic and anti-oxidant effects of PRDX1 in Prdx1−/− mice (Kisucka et al. 2008), future human studies are encouraged to determine whether anti-atherosclerosis therapeutic measures have impact on PRXD1 and reactive oxygen species. Given that PRDX1 correlated with TG as reported from the current study, it can be speculated that PRDX1 could be a marker of increased cardiovascular risk.
Peroxiredoxin 2 also reported higher levels in patients compared to control subjects. In this regard, PRDX2 possibly induces many protective effects including scavenging activity (Moon et al. 2006), inhibition of LDL oxidation, and reduction of plasma lipid peroxide levels (Park et al. 2011). Therefore, strategies increasing PRDX2 may be beneficial in PAD.
Peroxiredoxin 4 (PRDX4), the third member investigated in the present study, showed an increment in patients with PAD. It is a hydrogen peroxide degrading peroxidase recently found circulating in blood of septic patients and potentially reflecting an antioxidant system in imbalance (Schulte et al. 2011), similar to atherosclerosis (Madamanchi et al. 2005). The amount of secreted PRDX4 is most likely proportional to the initial intracellular expression levels (Okado-Matsumoto et al. 2000). Consequently, the tissue with high basal or upregulated cellular expression of PRDX4 likely represents a source for circulating PRDX4. Okado-Matsumoto and colleagues (2000) hypothesized that the enzyme is bound to the endothelial cells of blood vessels and is released in response to a changed redox environment, matched by the high expression of PRDX4 in endothelial cells. There is further sufficient evidence that augmented PRDX4 serum levels possibly results in response to pro-oxidant and pro-inflammatory signaling cascades (Yu et al. 2010). In the present study, pro-inflammatory molecules as ICAM-1 and VCAM-1 were markedly elevated congruent with PRDX4, which might explain the rise in its level. However, no correlation could be detected between PRDX4 and either ICAM-1 or VCAM-1, possibly due to limited number of heterogeneous levels, or to inclusion of unselected patients with severe atherosclerosis, and small size of population.
Finally, PRDX6 also reported higher levels in PAD patients compared to control subjects. Interestingly, PRDX6 possesses both peroxidase and phospholipase A2 activities, of which the later plays a distinct role in diabetes and atherosclerosis (Hui et al. 2012). Very recently, it was shown that besides the peroxidase activity of PRDX6, phospholipase activity also plays a substantial role in protecting cells against oxidant stress (Lien et al. 2012). Therefore, it can possibly protect cell membranes including endothelial cells during oxidative stress and its absence can account for increased toxicity as was reported in PRDX6 null cells (Lui et al. 2010). Collectively, the high levels of the circulating PRDX1, 2, 4, and 6 in PAD possibly represent a physiological adaptation against oxidative stress in atherosclerosis.
Peroxiredoxin and comorbidities in PAD
Despite intensive glycemic control, individuals with T1DM and T2DM are predisposed to developing vascular complications, including atherosclerosis (Lockhart et al. 2008). In this study, we assessed the potential associations between circulating levels of Peroxiredoxins and comorbidities that characterize diabetes. Elevated triglycerides (TG) in diabetic patients with atherosclerotic disease, as reported herein, demonstrated a novel association with circulating PRDX1. Since levels of triglycerides vary inversely with HDL cholesterol and PRDX1 has been proposed as anti-atherogenic, this relationship suggests a counter-regulatory effect by PRDX1. A previous report showed that plasma TG has an independent role in vascular inflammation (Austin et al. 1998). In addition, triglycerides-rich lipoproteins have been reported to stimulate the expression of leukocyte adhesion molecules and monocyte adherence in the presence, but not in the absence of TNF-α (Ting et al. 2007). Thus increased TG may contribute to amplify vascular inflammation. Additional studies are needed to examine the effect of PRDX1 on TG.
Insulin resistance in patients with type 2 diabetes is reflected by elevated HOMA-IR index as was previously reported (Matthews et al. 1985) and further documented in this study. Interestingly, we demonstrated a novel positive association of PRDX2 levels and HOMA-IR, suggesting a counter-regulatory role of PRDX2 under insulin resistance conditions.
Peroxiredoxins and severity of PAD
Whether the degree and localization of atherosclerosis affected the levels of PRDXS was analyzed. In the present study, the severity of PAD was determined by ABI, carotid stenosis, or both. Our population showed a significant drop in ABI and or increased carotid stenosis.
Of peroxiredoxins studied, only PRDX1 and PRDX4 showed a negative correlation with the ankle–brachial index, a measure of severity of the peripheral atherosclerotic disease. Given that low ABI has been associated with increased risk of cardiovascular disease (Doobay and Anand 2005) and that low ABI is associated with high levels of PRDX1, 2, 4, and 6 without showing significant correlation except for PRDX1 and 4, suggest that measurement of both may provide incremental information. However, it remains unclear whether this association is related to causality, predisposes to cardiovascular complications or of prognostic value.
Peroxiredoxins and endothelial dysfunction
Previous studies reported that the levels of inflammatory markers including adhesion molecules, intracellular molecule (ICAM-1), and vascular cell adhesion molecule (VCAM-1), reflect endothelial dysfunction (Brevetti et al. 2006). Our results were congruent with previously published reports that these endothelial biomarkers are significantly elevated in patients with PAD (Silvestro et al. 2003). Of the endothelial biomarkers studied here, only circulating ICAM-1 levels were positively associated with the PRDX2 and the logarithm of PRDX6 level. This is of interest because it suggests that these two members of the peroxiredoxin family might operate through different pathways to combat the systemic inflammation and endothelial dysfunction. However, the hypothesis cannot be proved from the current study and merits further attention. Interestingly, PRDX2 deficiency in apolipoprotein E-deficient mice, resulted in increased expression of VCAM-1 and ICAM-1, leading to increased immune cell adhesion and predisposition to atherosclerosis (Park et al. 2011), suggesting that PRDX2 prevents endothelial dysfunction. In our human model, where diabetes and atherosclerosis co-exist, PRDX2 levels are increased possibly in response to inflammatory condition induced by the disease process. In addition, the association of serum ICAM-1 with the logarithm of plasma PRDX6 level suggests that PRDX6 possibly compensates for the endothelial injury and oxidative stress. This speculation is supported by the observation that deletion of PRDX6 resulted in endothelial damage and expression of PCAM-1 (Kumin et al. 2007). On the other hand, we could not detect any associations among PRDX1 or PRDX4 and markers of endothelial dysfunction. Diabetes is believed to increase the risk of cardiovascular events through mechanisms that could involve low-grade inflammation, ED, and impaired insulin sensitivity. Although the mechanisms by which diabetes increases cardiovascular complications are incompletely understood, strong supportive evidence from experimental and clinical studies points to the impaired function of the vascular endothelium as a critical inducer of cardiovascular complications (Lockhart et al. 2008). While postprandial hyperglycemia has its impact on vascular endothelial dysfunction and oxidative stress (Mah and Bruno 2012), future studies are warranted to investigate whether PRDXs are affected by acute postprandial hyperglycemia. Of interest we found a negative association between PRDX2 and 6 plasma levels and HbA1c (unpublished data).
The divergent associations obtained in the current work can be explained by the divergent expressions of PRDXs where 1, 2, and 6 are cytosolic, 3 and 5 are mitochondrial, and 4 is a secretory protein. Also it might reflect different mechanistic roles of PRDXs, in response to oxidative stress. Finally the small sample size might have contributed to the lack of associations between PRDXs and some other variables.
Conclusions
This study investigated the circulating levels of peroxiredoxins with the extent of atherosclerosis in diabetic patients. The high levels of PRDXs reported in diabetic patients with atherosclerotic peripheral arterial disease and its novel association with TG, HOMA IR, ICAM-1, and ABI suggest them as a marker of atherosclerosis comorbidity and severity related. However, these results warrant further evaluation to establish the exact biomarker role of PRDXs in atherosclerosis, in order to clarify whether high circulating levels of PRDXs are a consequence of chronic atherosclerotic disease or a predisposing factor for later cardiovascular events and also the response to treatment. As peroxiredoxins are proposed to be atheroprotective (Rhee et al. 2005), novel antioxidant strategies are required to clarify the role of antioxidant intervention in vascular diseases.
Limitations to the study
Although theses pilot studies provide some new dimension to the understanding of atherosclerosis and oxidative stress in diabetic patients with peripheral arterial disease, the study results and analysis are limited by the small sample size. In addition to the study groups compared here, it would be interesting to include a control group with diabetes only. Also it would be more interesting to include patients with PAD only, or with intima media thickness only, which would be considered for further studies.
Acknowledgments
The authors extend their appreciation to Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RGP-VPP-016, entitled “Cardiovascular Research Group.” The authors are thankful for the laboratory technical support of Mr. James Chu.
Disclosures
None
References
- Austin M, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B–12B. doi: 10.1016/S0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Bast A, Wolf G, Oberbaumer I, Walther R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia. 2002;45:867–876. doi: 10.1007/s00125-002-0846-1. [DOI] [PubMed] [Google Scholar]
- Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Schiano V, Chiariello M. Cellular adhesion molecules and peripheral arterial disease. Vasc Med. 2006;11:39–47. doi: 10.1191/1358863x06vm645ra. [DOI] [PubMed] [Google Scholar]
- Conway JP, Kinter M. Dual role of peroxiredoxin1 in macrophage-derived foam cells. J Biol Chem. 2006;281:27991–28001. doi: 10.1074/jbc.M605026200. [DOI] [PubMed] [Google Scholar]
- Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis gray-scale and Doppler US diagnostic Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81:18B–25B. doi: 10.1016/S0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- Hui DY. Phospholipase A2 enzymes in metabolic and cardiovascular diseases. Curr Opin Lipidol. 2012;23(3):235–240. doi: 10.1097/MOL.0b013e328351b439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HH, Lee KO, Chi YH, et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117(5):625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Khawaja FJ, Kullo IJ. Novel markers of peripheral arterial disease. Vasc Med. 2009;14(4):381–392. doi: 10.1177/1358863X09106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisucka J, Chauhan AK, Patten IS, et al. Peroxiredoxin 1 prevents excessive endothelial activation and early atherosclerosis. Circ Res. 2008;103:598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumin A, Schafer M, Epp N, et al. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J Cell Biol. 2007;179(4):747–760. doi: 10.1083/jcb.200706090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YC, Feinstein SI, Dodia C, Fisher AB. The roles of peroxidase and phospholipase A2 activities of peroxiredoxin 6 in protecting pulmonary microvascular endothelial cells against peroxidative stress. Antioxid Redox Signal. 2012;16(5):440–451. doi: 10.1089/ars.2011.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart CJ, Hamilton PK, McVeigh KA, McVeigh GE. A cardiologist view of vascular disease in diabetes. Diabetes, Obesity and Metabolism. 2008;10(4):279–292. doi: 10.1111/j.1463-1326.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- Lui G, Feinstein SI, Wang Y, et al. Comparison of glutathione peroxidase 1 and peroxidase 6 in protection against oxidative stress in the mouse lung. Free Radic Biol Med. 2010;49(7):1172–1181. doi: 10.1016/j.freeradbiomed.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- Mah E, Bruno RS. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res. 2012;10:727–740. doi: 10.1016/j.nutres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna R, Ramos-Mozo P, Madrigal-Matute J, et al. Identification of peroxiredoxin-1 as a novel biomarker of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2011;31(4):935–943. doi: 10.1161/ATVBAHA.110.214429. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Moon EY, Noh YW, Han YH, et al. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (PrxII) gene. Immunol Lett. 2006;102:184–190. doi: 10.1016/j.imlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar sheer stress up-regulates peroxiredoxins (PRDX) in endothelial cells: PRDX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defense and tumor suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A, Matsumoto A, Fujii J, Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 2000;127(3):493–501. doi: 10.1093/oxfordjournals.jbchem.a022632. [DOI] [PubMed] [Google Scholar]
- Park JG, Oh GT. The role of peroxidases in the pathogenesis of atherosclerosis. BMB. 2011;44(8):497–505. doi: 10.5483/BMBRep.2011.44.8.497. [DOI] [PubMed] [Google Scholar]
- Park JG, Yoo JY, Jeong SJ, et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Cir Res. 2011 doi: 10.1161/CIRCRESAHA.111.245530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Schulte J, Struck J, Kohrle J, Muller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35(5):460–465. doi: 10.1097/SHK.0b013e3182115f40. [DOI] [PubMed] [Google Scholar]
- Silvestro A, Scopacasa F, Ruocco A, et al. Inflammatory status and endothelial function in asymptomatic and symptomatic peripheral arterial disease. Vasc Med. 2003;8(4):225–232. doi: 10.1191/1358863x03vm503oa. [DOI] [PubMed] [Google Scholar]
- Ting HJ, Stice JP, Schaff UY, et al. Triglycerides-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circ Res. 2007;100:381–390. doi: 10.1161/01.RES.0000258023.76515.a3. [DOI] [PubMed] [Google Scholar]
- Wang X, Phelan SA, Forsman-Semb K, et al. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- Yu S, Mu Y, Ao J, Chen X. Peroxiredoxin IV regulates pro-inflammatory responses in large yellow croaker (Pseudosciaena crocea) and protects against bacterial challenge. J Proteome Res. 2010;9(3):1424–1436. doi: 10.1021/pr900961x. [DOI] [PubMed] [Google Scholar]