Abstract
Background
Recent malaria vector control measures have considerably reduced indoor biting mosquito populations. However, reducing the outdoor biting populations remains a challenge because of the unavailability of appropriate lures to achieve this. This study sought to test the efficacy of plant-based synthetic odor baits in trapping outdoor populations of malaria vectors.
Methodology and Principal Finding
Three plant-based lures ((E)-linalool oxide [LO], (E)-linalool oxide and (E)-β-ocimene [LO + OC], and a six-component blend comprising (E)-linalool oxide, (E)-β-ocimene, hexanal, β-pinene, limonene, and (E)-β-farnesene [Blend C]), were tested alongside an animal/human-based synthetic lure (comprising heptanal, octanal, nonanal, and decanal [Blend F]) and worn socks in a malaria endemic zone in the western part of Kenya. Mosquito Magnet-X (MM-X) and lightless Centre for Disease Control (CDC) light traps were used. Odor-baited traps were compared with traps baited with either solvent alone or solvent + carbon dioxide (controls) for 18 days in a series of randomized incomplete-block designs of days × sites × treatments. The interactive effect of plant and animal/human odor was also tested by combining LO with either Blend F or worn socks. Our results show that irrespective of trap type, traps baited with synthetic plant odors compared favorably to the same traps baited with synthetic animal odors and worn socks in trapping malaria vectors, relative to the controls. Combining LO and worn socks enhanced trap captures of Anopheles species while LO + Blend F recorded reduced trap capture. Carbon dioxide enhanced total trap capture of both plant- and animal/human-derived odors. However, significantly higher proportions of male and engorged female Anopheles gambiae s.l. were caught when the odor treatments did not include carbon dioxide.
Conclusion and Significance
The results highlight the potential of plant-based odors and specifically linalool oxide, with or without carbon dioxide, for surveillance and mass trapping of malaria vectors.
Introduction
Malaria continues to be a leading cause of mortality and morbidity in sub-Saharan Africa, with the latest global estimates documenting about 219 million cases in 2010 and an estimated death toll of 1.24 million [1], [2]. Children and pregnant women are the most vulnerable groups, with at least one child dying every minute from malaria in Africa [1], [3]. Though substantial gains have been made in reducing malaria transmission, the death toll from this disease still remains unacceptably high, and as such there is a renewed effort to reduce the disease burden further and move towards malaria eradication [4]. Various control measures have been put in place to help curb this disease. These include chemotherapy, development of malaria vaccines, and reduction of human-vector contact through bed nets and vector-population control. The use of artemisinin-based combination therapy (ACT) is advocated in treatment of clinical cases, but this approach is threatened by recent discovery of emergence of resistant strains of the Plasmodium parasite to artemisinin [5], [6]. Efforts have been dedicated to the development of a malaria vaccine, which is viewed as a potent tool to reduce and even eliminate malaria. However, the development of an effective vaccine has been hampered by the complexity of the parasite and its life cycle [7], [8], extensive antigenic variation [9], and a poor understanding of the interaction between Plasmodium falciparum and the human immune system [10]. In view of this situation, a multifaceted approach to malaria control is advocated [11], with vector control forming an integral component of it [12]–[14].
Some of the vector control tools that have been used widely with considerable success include long-lasting insecticide-treated nets (LLINs) and indoor residual sprays (IRSs) [15]–[17]. However, the future of vector control based on the use of LLINs or spraying insecticides indoors is uncertain and is threatened by shortage of funds, poor bed-net coverage in some communities, as well as the development of insecticide-resistant mosquitoes [12], [15], [16]. This is exemplified by the resurgence of malaria vectors in sentinel sites in Kenya and elsewhere, despite high ownership and use of LLINs, and by the discovery of outdoor biting fractions of Anopheles gambiae Giles [18]–[20]. Outdoor biting among populations of An. gambiae is particularly of a serious concern, as they are not susceptible to current indoor control tools and are thus responsible for sustained malaria transmission [13], [21]. This has prompted the need for new and more environmentally robust methods that can supplement the existing vector control methods. New scientific knowledge about the ecology and behavior of mosquitoes and their natural predators and pathogens may lead to the development of new tools that can be incorporated into integrated vector management (IVM) programs. Other approaches that have been developed and are currently being explored for their potential to reduce vector populations include larvicides such as Bacillus thuringiensis israelensis (Bti) and insect growth regulators such as methoprene and pyroproxyfen, adulticides such as entomopathogenic fungi and viruses, introduction of genetically engineered and Wolbachia-infected mosquitoes, and the sterile insect technique (SIT) [22]–[30]. Despite the availability of these arsenals against the malaria vector, the malaria burden still remains unacceptably high.
Exploiting vector ecology to improve vector surveillance and control has been proposed as a potential new target in the fight against malaria [12], [31]–[33]. Appropriate vector control solutions are highly dependent on the local behavior and ecology of malaria vectors, hydrological and microclimatic conditions, and patterns of disease transmission [34]. Following the successful reduction of tsetse fly populations in parts of Africa [35], the development of an odor-bait technology as a surveillance and control tool for mosquito vectors has been advocated as a new and viable component of IVM [36], [37]. Currently, there are efforts to develop odor-baited traps for control of outdoor biting malaria vectors [38] with the recent development of a mosquito landing box that employs the principle of lure and kill [39]. Up to now, efforts in developing odor-baits for malaria vector management have centered mainly on human/animal-derived odors [36], [40]–[44], which though effective are limited in that they mainly target blood-seeking female mosquitoes [45]. One area that is understudied is the chemical ecology of plant feeding in Anopheles mosquitoes, which offers a promising new target for vector control [12], [45]–[47]. Besides the potential to trap mosquitoes of varying physiological status and sexes, plant-odor attraction also offers a unique opportunity to catch malaria vectors outdoors and thus reduce human-vector contact [45]. Furthermore, there have been concerns about the use of carbon dioxide in mosquito traps, since the synthetic forms of CO2 supplied via gas cylinders, dry ice, propane combustion, or sugar fermentation are expensive and present logistical challenges for use in remote areas [48]. Given that plants and fruits normally release low amounts of CO2 at night as by-products of respiration [49], [50], mosquitoes are not expected to rely heavily on it for host-plant location. Hence, plant-based odors present the potential to minimize or even eliminate the reliance on CO2 for trapping, if well formulated.
In an earlier study, we described the performance of an attractive plant-odor blend for An. gambiae sensu stricto in laboratory assays [51]. We designed the current study to evaluate the attractiveness of this blend and specific components of it against host-seeking mosquitoes in western Kenya, with emphasis on the outdoor populations of the malaria vectors An. gambiae s.l. and An. funestus Giles s.l. For comparative purposes, we included a newly developed blend of attractants formulated from animal and human odor-based compounds [44] and human foot odor collected on nylon socks (highly attractive for An. gambiae s.s. [52]). This allowed us to compare the trapping efficacy of the plant-and animal/human-derived odors in catching malaria mosquitoes of different physiology and age.
Materials and Methods
Study site
Preliminary field trials were carried out at icipe's Duduville campus, Nairobi, a low-risk malaria area [53], while field evaluation of optimized blends was conducted at Ahero, located approximately 24 km south-east of Kisumu, in western Kenya (0°10′S, 34°55′E). Malaria is highly endemic in this region and transmission occurs throughout the year. Mean annual P. falciparum sporozoite inoculation rates (EIR) of 0.4 -17.0 infective bites per year have been shown by recent studies for this region[54]. The region has an annual mean temperature range of 17–32°C, average annual rainfall of between 1,000–1,800 mm, and average relative humidity of 65% [55].
Ethical considerations
Consent for homesteads to be used in the study was approved by the Ethical Review Committee at the Kenya Medical Research Institute (Protocol KEMRI/RES/7/3/1) and further from the household heads and the local administration prior to the start of the study.
Optimization of attractive blends
Six behaviorally-active plant-based compounds reported in our previous study [51] were evaluated individually for trapping wild mosquitoes at three different concentrations to obtain the most attractive concentration. They were prepared in a pentane solvent, starting with the optimal attractive concentration determined from olfactometer studies, followed by consecutive ten-fold higher concentrations (Table S1). Of these six individual components, in a preliminary field trial only (E)-linalool oxide (2 ng/µl; mean = 11.34±0.46, P<0.05) and β-ocimene (1 ng/µl: 9.62±0.36, P<0.05) caught significant numbers of mosquitoes compared to the control (solvent + carbon dioxide: 2.56±0.62). Based on this trial, three groups of compounds were formulated: (E)-linalool oxide only (0.2 ng/µl) (99.5% furanoid form; 0.5% pyranoid form by GC-MS on both methyl silicone and carbowax columns) (hereafter referred to as LO); (E)-linalool oxide (0.2 ng/µl) and β-ocimene (0.1 ng/µl) (hereafter referred to as LO + OC); and a blend consisting of all six compounds, i.e. (E)-linalool oxide (0.2 ng/µl), β-ocimene (0.1 ng/µl), hexanal (0.2 ng/µl), β-pinene (0.2 ng/µl), limonene (0.2 ng/µl) and (E)-β-farnesene (0.1 ng/µl) (hereafter referred to as Blend C) (Table S1). These blends were further evaluated at three different concentrations with ten-fold increase in concentrations (Table S1). Carbon dioxide-baited CDC traps (model 512, John W Hock, Gainesville, FL, powered by 6-V, 10-ampere-hour rechargeable gel-cell battery) without a light bulb, combined with these synthetic chemicals, were deployed in these preliminary studies. Of the three treatments, the second concentrations were the most attractive (i.e. LO [2 ng/µl (E)-linalool oxide]; LO + OC [2 ng/µl (E)-linalool oxide + 1 ng/µl β-ocimene]; and Blend C [2 ng/µl (E)-linalool oxide + 1 ng/µl β-ocimene + 2 ng/µlhexanal +2 ng/µl β-pinene + 2 ng/µl limonene + 1 ng/µl (E)-β-farnesene]; Figure S1). These concentrationswere subsequently used in a field evaluation of these plant compounds.
Field evaluation of optimized blends
Based on the preliminary studies above, only the optimal concentration of the three groups of compounds (LO, LO + OC, and Blend C) were tested in the field alongside controls (solvent only or solvent + CO2) and Blend F (developed by Tchouassi et al. [44] based on animal and human odors; heptanal = 2 µg/µl, octanal = 0.5 µg/µl, nonanal = 0.1 µg/µl and decanal = 0.1 µg/µl) and human foot odors. The human foot odors (hereafter referred to as socks) were collected by allowing two volunteers to wear nylon socks for 10–12 h during the day prior to the experiments and replaced every night. The volunteers' feet were cleaned well with non-perfumed soap before wearing the pair of socks and no diet restriction was placed on the volunteers. The synthetic standards of the following compounds were used: hexanal (Aldrich, 98%), β-pinene (Chemika, 99.5%), β-ocimene (Chemika, (Z)-β-ocimene = 27%, (E)-β-ocimene = 67% and allo-ocimene = 6%), limonene (Sigma), (E)-linalool oxide (Aldrich, furanoid form, 99.5% pyranoid form ∼0.5%), (E)-β-farnesene (Bedoukian Research, CT, USA), heptanal (Sigma-Aldrich, 98%), octanal (Sigma-Aldrich, 98%), nonanal (Sigma-Aldrich, 98%), and decanal (Sigma-Aldrich, 98%). The odor treatments were evaluated either alone or baited with CO2 released as sublimated dry ice from Igloo thermos containers (2 L; John W Hock, Gainesville, FL) with a 13-mm hole in the bottom center (41±2.3 g/h release rate). Two types of traps were used: CDC light trap without the light bulb, with the odors released from 1.5 ml centrifuge tubes (Fisherbrand Scientific, UK) with a pinhole opening; and Mosquito Magnet-X (MM-X) traps (American Biophysics Corporation, RI, USA), with the lure dispensed from a Luna dental roll (Roeko, Langenau, Germany). All field experiments were carried out for 12 h between 18:00 h and 06:00 h local time. In general, odor-baited traps (CDC without light and MM-X) were compared with traps baited with either solvent alone (pentane; for non-CO2 baited odors) or solvent + carbon dioxide (for CO2-baited odors). The odor treatments were tested for a total of 18 nights for two separate seasons (12 nights in December, 2012 and 6 nights in November 2013) with each night used as a replicate. They were tested in a series of randomized incomplete-block designs of days × sites × treatments at three different sites. Sites were 150–200 m from each other (chosen based on the distribution of potential An. gambiae s.l. breeding sites and homesteads), while inter-trap distance was arbitrarily chosen at 20 m apart. Trapping at the three sites was alternated such that every site was sampled every two nights to allow the mosquito population to stabilize. The odor treatments were also rotated within every site to account for positional bias. Carbon dioxide was added nightly by placing 1 kg of dry ice in the containers. To test the significance of CO2 in host-plant location by the malaria vectors, the trapping efficacy of CO2 only, linalool oxide only, and (E)-linalool oxide + carbon dioxide were also evaluated. Traps were removed every morning, the mosquitoes captured were morphologically identified to species or species complex using taxonomic keys [56], [57], and their counts were recorded.
Interactive effect of plant and human/animal related odors
In another set of experiments, we evaluated the interactive effect of plant and animal/human odors by comparing the trapping efficacy of LO, Blend F, socks, LO + Blend F, and LO + socks either with or without CO2 in MM-X traps. The trap captures were compared to worn socks or worn socks + CO2 as positive controls for experiments with odor treatments baited with or without CO2, respectively. Following a series of randomized incomplete blocking designs, field evaluations were performed at the three sites in a series of randomized incomplete- block designs of days × sites × treatments at three different sites. Sites were 150–200 m from each other (chosen based on the distribution of potential An. gambiae s.l. breeding sites and homesteads), while inter-trap distance was arbitrarily chosen at 20 m apart. Trapping at the three sites was alternated such that every site was sampled every two nights to allow the mosquito population to stabilize. The positions of odor treatments at each site were randomly rotated per night to account for any positional bias and each night considered a replicate (total nine replicates). Trapping at the three sites was staggered such that each site was sampled after every two nights to allow the mosquito population to stabilize. Mosquitoes were morphologically identified to species or species complex as described above and their numbers recorded.
PCR identification of member species of Anopheles gambiae complex
Thirty female An. gambiae s.l. from each trap treatment were selected and analyzed further to determine the sibling species of the complex. Genomic DNA of each individual An. gambiae s.l. mosquito was extracted by homogenizing a leg in 50 µl of sterile double-distilled water in a 1.5 ml Eppendorf tube using a sterile plastic pestle. The homogenates were then boiled for 45 min ona water bath, allowed to cool and kept at −20°C until required. The PCR method of Scott et al. [58] was used for the identification of the sibling species of the An. gambiae complex. Amplicons were visualized in ethidium bromide-stained 2% agarose gels.
Physiological state of field collected mosquitoes
Mosquitoes were scored as unfed, blood-fed or half-gravid/gravid, based on appearance of their abdominal condition as illustrated in the WHO Manual [59]. Based on the risk of disease transmission, any An. gambiae s.l. or An. funestus s.l. mosquito with prior encounter with a blood host (either blood-fed or half-gravid/gravid) were categorized as engorged and otherwise as unfed. The number of male An. gambiae s.l. and An. funestus s.l. was also recorded for each treatment. Other Anopheles species (An. coustani Laveran and An. pharoensis Theobald) were neither classified by sex nor abdominal status.
Statistical analyses
The total trap captures of MM-X and CDC traps was compared using chi-square goodness-of-fit test. The numbers of mosquitoes per treatment were analyzed using a generalized linear model (GLM) with negative-binomial error structure and log link in R 2.15.1 software [60]. This model assumes a chi square distribution suitable for count data [61]. With the solvent-only or the solvent + CO2-baited CDC or MM-X trap (control) serving as the reference category, we calculated the incidence-rate ratios (IRR), a measure of the likelihood that mosquito species chose treatments other than the control, as well as their P-values and confidence intervals, from the model. A similar model was used to compare the performance of plant- and animal/human-based odors separately and when combined with worn socks used as control. The proportions of male and engorged female An. gambiae s.l. and An. funestus s.l. caught by the different odor treatments relative to worn socks were compared using chi-square test of proportions.
Results
Field evaluation of optimized blends
Overall, MM-X traps caught 2.8 times more mosquitoes thanCDC traps (CI = 2.75–2.92; P<0.001) (Table 1). There was a significant increase in trap capture when traps were baited with plant-derived odors, animal-derived odors or worn socks compared to the control solvent or solvent + CO2. In the absence of carbon dioxide, MM-X traps baited either with plant-derived odors, Blend F or worn socks had significantly higher captures of both An. gambiae s.l. and An. funestus s.l. than the control (solvent) (χ2 = 171.85, df = 96, P<0.001 and χ2 = 47.304df = 96, P<0.001, respectively) (Table 2). Similarly, MM-X traps baited with any of these odor treatments in the presence of CO2 had significantly higher captures of both An. gambiae s.l. and An. funestus s.l. compared to traps baited with solvent + CO2 (χ2 = 348.5, df = 96, P<0.001 and χ2 = 107.12, df = 96, P<0.001, respectively) (Table 2).
Table 1. Total number of each mosquito species/genusof both sexes caught by each type of trap.
| Species | CDC | MM-X | χ2 | P-value |
| An. gambiae s.l. | 229 | 1655 | 2155.65 | <0.001 |
| An. funestus s.l. | 230 | 867 | 737.46 | <0.001 |
| Other Anopheles spp. | 11 | 1010 | 1951.03 | <0.001 |
| Culex spp. | 1205 | 7847 | 9297.50 | <0.001 |
| Mansonia spp. | 4201 | 5080 | 166.12 | <0.001 |
| Other mosquito spp. | 15 | 238 | 389.60 | <0.001 |
Table 2. Total anopheline trap captures with plant and animal odor compounds and worn socks in the presence or absence of carbon dioxide using MM-X traps.
| Species | Treatment | N | Without CO2 | With CO2 | ||||
| n | IRR (95% CI) | P-value | n | IRR (95% CI) | P-value | |||
| An. gambiae s.l. | Control | 18 | 23 | 1.0 | 47 | 1.0 | ||
| LO | 18 | 188 | 8.9 (5.85–14.48) | <0.001 | 275 | 10 (7.05–15.59) | <0.001 | |
| LO+OC | 18 | 88 | 3.8 (2.40–6.32) | <0.001 | 228 | 8.5 (5.80–12.93) | <0.001 | |
| Blend C | 18 | 64 | 3.0 (1.90–5.11) | <0.001 | 185 | 6.9 (4.66–10.49) | <0.001 | |
| Blend F | 18 | 53 | 3.6 (2.40–5.94) | <0.001 | 228 | 8.4 (5.78–12.87) | <0.001 | |
| Socks | 18 | 76 | 3.7 (2.41–6.02) | <0.001 | 248 | 9.7 (6.44–14.97) | <0.001 | |
| An. funestus s.l. | Control | 18 | 12 | 1.0 | 33 | 1.0 | ||
| LO | 18 | 61 | 5.1 (2.84–9.91) | <0.001 | 115 | 3.5 (2.40–5.21) | <0.001 | |
| LO+OC | 18 | 33 | 2.9 (1.56–5.86) | <0.01 | 127 | 3.7 (2.57–5.56) | <0.001 | |
| Blend C | 18 | 51 | 4.3 (2.35–8.36) | <0.001 | 127 | 3.8 (2.66–5.73) | <0.001 | |
| Blend F | 18 | 59 | 4.9 (2.74–9.60) | <0.001 | 86 | 2.7 (2.01–5.35) | <0.001 | |
| Socks | 18 | 35 | 3.1 (1.54–5.79) | <0.001 | 98 | 2.9 (2.04–4.70) | <0.001 | |
IRR = incidence rate ratio, CI = confidence interval, N = number of replicates, n = total number of mosquitoes caught, LO = (E)-linalool oxide and OC = β-ocimene. NB: Control (solvent or solvent + CO2) was used as reference.
The efficacy of the plant- and animal-derived odors as well as worn socks in trapping An. gambiae s.l. and An. funestus s.l. was further demonstrated in the lightless CDC traps (Table 3). While only traps baited with Blend C and Blend F registered significant increase in trap captures of An. gambiae s.l. compared to the control solvent (χ2 = 29.911, df = 107, P<0.001), none of these baited traps performed better than the control in trapping An. funestus s.l. in the absence of CO2. On the other hand, all the odor treatments had significantly higher captures of both An. gambiae s.l. and An. funestus s.l. in the presence of CO2 relative to the control (χ2 = 64.798, df = 107, P<0.001 and χ2 = 32.472, df = 107, P<0.001, respectively) (Table 3).
Table 3. Total anopheline trap captures with plant and animal odor compounds and worn socks in the presence or absence of carbon dioxide using CDC traps.
| Species | Treatment | N | Without CO2 | With CO2 | ||||
| n | IRR (95% CI) | P-value | n | IRR (95% CI) | P-value | |||
| An. gambiae s.l. | Control | 18 | 11 | 1.0 | 17 | 1.0 | ||
| LO | 18 | 19 | 1.4 (0.68–3.04) | 0.36 | 80 | 3.9 (2.34–7.05) | <0.001 | |
| LO+OC | 18 | 13 | 1.0 (0.44–2.25) | 1.00 | 85 | 4.9 (2.97–8.76) | <0.001 | |
| Blend C | 18 | 38 | 3.2 (1.71–6.33) | <0.001 | 55 | 2.9 (1.70–5.34) | <0.001 | |
| Blend F | 18 | 30 | 2.5 (1.31–5.08) | <0.01 | 80 | 5.0 (3.01–8.87) | <0.001 | |
| Socks | 18 | 9 | 0.9 (0.40–2.09) | 0.83 | 77 | 4.8 (2.89–8.55) | <0.001 | |
| An. funestus s.l. | Control | 18 | 9 | 1.0 | 10 | 1.0 | ||
| LO | 18 | 14 | 1.1 (0.49–2.41) | 0.84 | 42 | 2.7 (1.46–5.28) | <0.01 | |
| LO+OC | 18 | 13 | 0.9 (0.40–2.09) | 0.84 | 61 | 4.4 (2.48–8.37) | <0.001 | |
| Blend C | 18 | 13 | 1.0 (0.44–2.25) | 1.00 | 31 | 2.0 (1.05–4.02) | <0.05 | |
| Blend F | 18 | 15 | 1.3 (0.59–2.72) | 0.57 | 28 | 2.2 (1.14–4.30) | <0.05 | |
| Socks | 18 | 9 | 0.8 (0.35–1.93) | 0.67 | 26 | 2.3 (1.23–4.57) | <0.05 | |
IRR = incidence rate ratio, CI = confidence interval, N = number of replicates, n = total number of mosquitoes caught, LO = (E)-linalool oxide and OC = β-ocimene. NB: Control (solvent or solvent + CO2) was used as a reference.
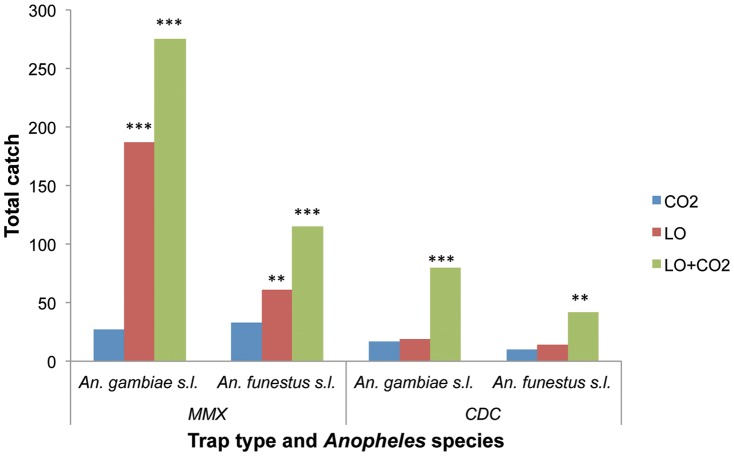
Further analysis revealed that in the absence of carbon dioxide, LO was superior than any of the other odor treatments in trapping the two malaria vectors and was as good as any of them when used together with CO2 (Tables 2 and 3). When LO was compared with CO2, it trapped 7- and 1.8-fold more An. gambiae s.l. and An. funestus s.l., respectively, than CO2 when dispensed using MM-X traps but not CDC traps (Figure 1). Combining LO and CO2 enhanced trap captures for both MM-X and CDC traps (Figure 1). The enhanced effect of CO2 with plant and animal/human odors was further confirmed for LO+OC, Blend C, Blend F and socks, which registered significantly increased captures of the two mosquito species compared to non-carbon dioxide baited traps (Tables 2 and 3).
Figure 1. Trapping efficacies of carbon dioxide and linalool oxide for An. gambiae s.l. and An. funestus s.l.
. Number of replicates = 18; bars capped with asterisks are significantly different from their respective controls as determined by general linear model with negative-binomial error structure and log link in R 2.15.1 software; ** = P<0.01, *** = P<0.001.
Interactive effect of plant and human/animal related odors
Combining LO with worn socks significantly increased the trap captures of An. gambiae s.l. both in the absence and presence of CO2 (χ2 = 2.12, df = 46, P<0.05 and χ2 = 4.35, df = 46, P<0.001, respectively) but this did not affect the number of An. funestus s.l. trapped (Table 4). On the other hand, combining LO with Blend F significantly reduced trap captures of both An. gambiae s.l. and An. funestus s.l.; with significant reduction in trap captures of An. funestus s.l. recorded in non-CO2-baited traps (χ2 = 2.38, df = 46, P<0.05) (Table 4).
Table 4. Interactive effect of plant and animal/human lures on anopheline trap captures using MM-X traps.
| Species | Treatment | N | Without CO2 | With CO2 | ||||
| n | IRR (95% CI) | P-value | n | IRR (95% CI) | P-value | |||
| An. gambiae s.l. | Socks | 9 | 48 | 1.0 | 166 | 1.0 | ||
| LO | 9 | 90 | 1.7 (1.21–2.49) | <0.01 | 237 | 1.4 (1.15–1.71) | <0.001 | |
| Blend F | 9 | 69 | 1.4 (0.95–2.02) | 0.09 | 181 | 1.1 (0.88–1.34) | 0.42 | |
| LO+Blend F | 9 | 62 | 1.3 (0.90–1.94) | 0.15 | 152 | 0.9 (0.73–1.14) | 0.43 | |
| LO+Socks | 9 | 79 | 1.4 (1.00–2.11) | <0.05 | 256 | 1.5 (1.27–1.88) | <0.001 | |
| An. funestus s.l. | Socks | 9 | 23 | 1.0 | 34 | 1.0 | ||
| LO | 9 | 26 | 1 (0.56–1.79) | 1.00 | 32 | 0.9 (0.54–1.44) | 0.62 | |
| Blend F | 9 | 36 | 1.5 (0.91–2.61) | 0.18 | 78 | 2.3(1.55–3.47) | <0.001 | |
| LO+Blend F | 9 | 11 | 0.4 (0.17–0.82) | <0.05 | 36 | 1.1 (0.66–1.70) | 0.81 | |
| LO+Socks | 9 | 28 | 1.2 (0.67–2.06) | 0.57 | 33 | 1 (0.60–1.57) | 0.90 | |
IRR = incidence rate ratio, CI = confidence interval, N = number of replicates, n = total number of mosquitoes caught, LO = (E)-linalool oxide and OC = β-ocimene. Worn socks were used as reference.
PCR identification of member species of Anopheles gambiae complex
Of the 501 An. gambiae s.l. processed for molecular species identification, 99% (n = 496) were identified as An. arabiensis with only 1% (n = 5) as An. gambiae s.s. The few An. gambiae s.s. recorded were all found in the MM-X trap baited with Blend F+CO2.
Physiological status of field collected mosquitoes
Irrespective of odor treatment, unfed females constituted the highest percentage of the total number of mosquitoes caught by the two trap types. Of the Anopheles species captured, CDC traps captured16 male and 442 female An. gambiae s.l. (89% unfed, 8% blood-fed and 3%half-gravid/gravid); and 14 male and 265 female An. funestus s.l. (95% unfed, and 5% blood-fed), while MM-X traps captured 77 male and 1575 female An. gambiae s.l. (84% unfed, 11 blood-fed % and 5% gravid); and 52 male and 822 female An. funestus s.l. (91% unfed, 2% blood-fed and 7% gravid).
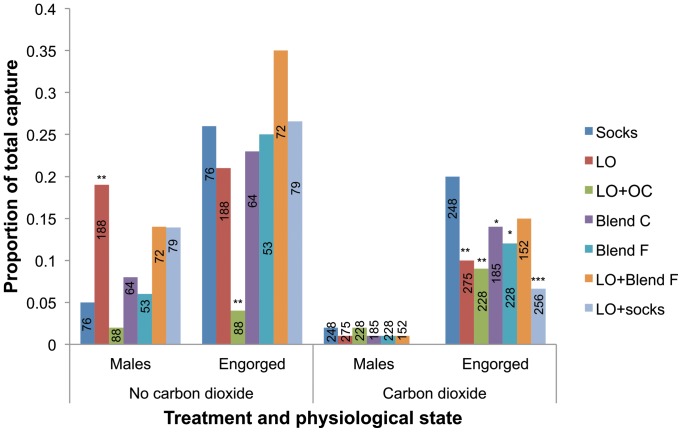
To understand the diversity of the various physiological states of mosquitoes caught by the plant- and animal/human-based odors, we analyzed the proportions of male and engorged (blood-fed + half-gravid/gravid) female An. gambiae s.l. caught by the different odor treatments. Overall, the odor treatments caught a higher proportion of male and engorged female An. gambiae s.l. in the absence of CO2 than when they were combined with carbon dioxide (Figure 2). In the absence of CO2, LO-baited traps captured a significantly higher proportion of male An. gambiae s.l. than the traps baited with worn socks (χ2 = 6.66, df = 1, P<0.01) while the trap baited with LO+OC had a significantly lower proportion of engorged females than the traps baited with worn socks (χ2 = 9.85, df = 1, P<0.01) (Figure 2). On the other hand, traps baited with worn socks caught a significantly higher proportion of engorged female An. gambiae s.l. than those baited with LO, LO+OC, Blend C and Blend F (Figure 2). Combining LO with either Blend F or worn socks did not significantly affect the performance of Blend F, but in the presence of CO2 it significantly reduced the proportion of engorged female An. gambiae s.l. trapped by the worn socks compared to when the socks were used with CO2 alone (χ2 = 18.82, df = 1, P<0.001).
Figure 2. Proportions of male and engorged female An. gambiae s.l. caught by different odor treatments.
The bars show the proportions of male and engorged (blood-fed + semi-gravid/gravid) female An. gambiae s.l.; numbers embedded in the bars are the total of mosquitoes caught by each of the odor treatment; LO = (E)-linalool oxide; OC = β-ocimene; the different treatments were compared to socks (control); bars capped with asterisks are significantly different from their respective controls as determined by chi square test of proportions in R 2.15.1 software * = P<0.05, ** = P<0.01, *** = P<0.001.
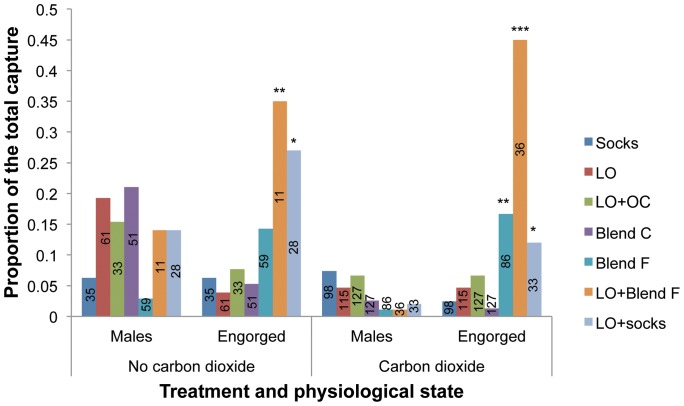
In terms of An. funestuss.l. captured, only traps baited with Blend F, LO + Blend F, and LO + socks caught a significant number of engorged females compared to socks both in the presence and absence of CO2 (Figure 3). Overall, a reduced number of males and engorged female An. funestus s.l. were captured in traps when the odors were combined with CO2.
Figure 3. Proportions of male and engorged female An. funestus s.l. caught by different odor treatments.
The bars show the proportions of male and engorged (blood-fed + semi-gravid/gravid) female An. funestus s.l.; numbers embedded in the bars are the total of mosquitoes caught by each of the odor treatment; LO = (E)-linalool oxide; OC = -β-ocimene; the different treatments were compared to socks (control); bars capped with asterisks are significantly different from their respective controls as determined by chi square test of proportions using R 2.15.1 software * = P<0.05, ** = P<0.01, *** = P<0.001.
Discussion
Our results show that the two trap types differed in the number of mosquito captures. The MM-X traps, either unbaited or baited with plant-based or animal/human odors, captured a higher number of mosquitoes than the corresponding CDC traps in terms of total mosquito captures as well as Anopheles captures. It is likely that the different dispensers used with different release rates of odors could have influenced captures in both traps. As such, these results should be interpreted with caution. However, the difference in designs for both traps supposes that the release rates are likely to be different even for the same type of dispensers. These results corroborate previous findings [62]–[64], which have shown consistently that traps operating under the counter-flow principle are generally more efficient compared to other trap types. It is not surprising therefore, that higher captures were recorded in the MM-X trap which operates using the counter-flow concept with two fans, the weaker one dispersing the odor and the stronger one sucking the mosquitoes into the trap as they are flying up the odor plume [65].
Furthermore, we found that like animal/human-derived odors, plant odors also performed well in trapping malaria vectors, compared to controls. Aside from the field trapping with ripened fruits and flowers [66], [67], this is the first field evidence of malaria vector attraction to plant-derived odors. Particularly outstanding was the performance of LO, which in the absence of CO2, had the highest Anopheles capture as well as the highest proportion of males. These findings confirm the significance of olfaction in the mosquito-host plant interactions and therefore underpin the potential for deployment of plant-derived odors in IVM. There has been widespread concern about the prospects of malaria eradication in the recent past, following the emergence of a cryptic subgroup of outdoor-biting An. gambiae, which is implicated in the sustained transmission of malaria even in communities where LLINs and IRS are regularly implemented [4], [20]. Given that sugar feeding in mosquitoes predominantly takes place outdoors and that mosquitoes use olfactory cues to locate sugar sources [47], [51], these findings are significant as they highlight the potential of deploying these plant odors in controlling sugar-seeking segments of a malaria vector population. Sugar feeding takes place at all states of an adult mosquito [68], hence a wide spectrum of the malaria vector population, including age, sex, and behavioral state, can be targeted.
Furthermore, progress has been made in the development of attractive toxic sugar-baits (ATSB) [69], [70], but these baits are still limited by the fact that they employ ripened fruits and flowering plants, and therefore their application is still restricted to available plant products [69], [70]. Thus, these findings present opportunities for deployment of attractive synthetic plant-derived odors such as linalool oxide to enhance the efficacy of ATSB. Unlike the aldehydes that constituted the animal-derived bait in the present study and can oxidize easily in air, linalool oxide is relatively stable [71]. Therefore, it can be employed in long-term field trappings in remote malaria-endemic villages in Africa where access to dry ice or CO2 generators that consume large amounts of sugar can be a problem.
This study also highlights the potential of utilizing a combination of plant-based and animal/human odors in surveillance and control of malaria vectors. While it appeared that there was an inhibitory effect on trap captures when LO was combined with Blend F, an enhanced effect was recorded in trapping An. gambiae s.l. when LO was combined with worn socks. The exclusive aldehyde components in Blend F combined with LO seemed to exhibit an antagonistic effect on each other as compared to the socks, which releases a diverse range of chemicals. This suggests that the interactive effects of plant and animal odors might be limited to a certain group or individual compounds. The interactive effect of these lures on mosquito captures has not been evaluated; yet, this approach may open up new ways for maximizing trap lures in order to minimize the use and over-dependence on CO2 in surveillance traps.
We also found that irrespective of the trap type and the odor bait, inclusion of CO2 significantly increased mosquito trap capture. Carbon dioxide and host odors both play important roles in long-range host selection and orientation, with specific host odors playing a role in close-range host recognition and acceptance [52], [72]. The receptors for CO2 are located on the maxillary palps of mosquitoes and have been shown to be sensitive to even as slight a change in atmospheric carbon dioxide concentration as 0.01% [48], [73], [74]. Mosquitoes aregenerally thought to respond to small changes in CO2 concentration by flying upwind [75], [76] and to use optomotor anemotaxis to orient to the source of the host odor [77]. These behavioral responses vary with plume structure and odor [78]. Plants normally release small amounts of CO2, constituting between 0.01 and 0.1% of atmospheric CO2 at night [49]. The combined effect found between CO2 and plant odors therefore suggests that possibly Afrotropical malaria vectors also utilize CO2 to locate their potential host plants. Alternatively, given the observed paucity of males in the collections and the large amounts of CO2 probably being released from the containers, it is likely that it also was serving as a blood-host kairomone. Taken together, these observations and results suggest the possibility that mosquitoes were responding to both plant and animal cues, thus explaining the partial additive effects of their combinations. This interpretation fits with the conclusion that, unlike most zoophilic mosquito species, the Afrotropical malaria vectors are known to rely more on the specific host odors rather than CO2 to locate a suitable host [79].
Interestingly, this study also indicates a reduction in the proportions of male and engorged female An. gambiae s.l. and An. funestus s.l. when the tested odors were combined with CO2 as compared to when used alone. While the reduction in proportion of males captured in CO2-baited traps is not surprising given that males feed entirely on plant nectar, the reduction in proportions of engorged females was not expected. A possible explanation for this is that carbon dioxide reception in engorged female Anopheles triggers an avoidance behavior as opposed to its attractive role in unfed females. This study also shows that in the absence of CO2, (E)-linalool oxide caught a significantly higher proportion of males than worn socks and compares well with it in trapping engorged females. It also increases the proportion of males caught by socks and Blend F as well as the proportion of engorged females caught by Blend F when combined. This confirms the hypothesis that plant-odor-based attractants have the potential to attract mosquitoes of more divergent physiology and sex [45]. Thus, adoption of a plant-odor-bait technology presents a potent tool for surveillance of malaria vectors of varying physiological states. However, it is important to note that the total number of males was low in both plant- and animal/human-derived odor-baited traps. This result may be explained by the locations of the traps, which were next to homesteads. Possibly, if such traps were to be placed next to breeding sites, they might capture more males, given that mating in An. gambiae s.l. and An. funestus s.l. occurs a few days after emergence within the vicinity of breeding sites or in swarms formed around specific environmental swarm markers [80]–[82].
While our lures had been optimized for An. gambiae s.s. in the laboratory, the field study was dominated by An. arabiensis in collections of An. gambiae s.l. and An. funestus s.l., both of which are important malaria vectors in western Kenya. There has been a proportionate increase in An. arabiensis compared to its closely related sibling species An. gambiaes.s. in western Kenya, presumably as a result of widespread bed net use [83]. The exophilic and anthropo-zoophilic tendencies of An. arabiensis imply it is less susceptible to bed nets. Our current study shows dominance of trap captures by An. arabiensis, which is in line with similar findings by recent studies [40], [43]. The significant response to the lures by An. funestus s.l., which together with An. arabiensis constitute important malaria vectors, suggests that our lures used here can provide a tool for the sampling of these species. Given that sugar resources are readily exploited by most mosquito species, the significantly increased captures recorded for the plant-based lure emphasizes its role as an attractant for a wide range of mosquito species as evidenced by the diversity of mosquito species collected in this study.
Conclusion
This field study has confirmed the effectiveness of plant-derived synthetic odors in trapping malaria vectors and other mosquito species. The study also highlights the potential of combining plant and human odors in trapping malaria vectors, as well as the role played by CO2 in trapping An. gambiae s.l. and An. funestus s.l. of varying physiology and sex. Traps baited with plant-derived chemicals significantly increased capture of female mosquitoes when compared to either CDC or MM-X traps baited with either solvent or solvent + CO2. Thus, the use of plant-produced kairomones has significant promise for the surveillance and integrated control of malaria vector populations in Africa.
Supporting Information
Preliminary trap captures of all species of mosquitoes using different concentrations of plant-derived synthetic blends. LO = (E)-linalool oxide, OC = β-ocimene, bars capped with asterisks are significantly different from their respective controls as detected by general linear model with negative-binomial error structure and log link in R 2.15.1 software; * = P<0.05, ** = P<0.01.
(PNG)
Composition and concentrations of blends tested in field assays.
(DOCX)
Acknowledgments
We are grateful to the icipe staff in Nairobi and Mbita, who provided support during the study. Special thanks are extended to Wolfgang R. Mukabana for logistical support, Daisy Salifu for assistance with statistical analysis, Pamela Seda for PCR analysis, Brian K. Mwashi for graphical work and Onesmus Wanyama and Paul Angina for their technical support in the course of this study.
Funding Statement
This study was funded in part by the Center for Medical, Agricultural, and Veterinary Entomology, U.S. Department of Agriculture and by the U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases, grant R01A1077722 to WAF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organisation (2013) Malaria-World Health Organisation. Available: http://www.who.int/mediacentre/factsheets/fs094/. Accessed 25 April 2013.
- 2. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet 379: 413–431. [DOI] [PubMed] [Google Scholar]
- 3. Guyatt HL, Snow RW (2001) The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Amer Trop Med Hyg 64: 36–44. [DOI] [PubMed] [Google Scholar]
- 4. Alonso PL, Brown G, Arévalo-Herrera M, Binka F, Chitnis C, et al. (2011) A research agenda to underpin malaria eradication. PLoS Med 8: e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Brien C, Henrich PP, Passi N, Fidock DA (2011) Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum . Curr Opin Infect Dis 24: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MalERA Consultative Group on Drugs (2011) A research agenda for malaria eradication: drugs. PLoS Med 8: e1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419: 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, et al. (2002) A proteomic view of the Plasmodium falciparum life cycle. Nature 419: 520–526. [DOI] [PubMed] [Google Scholar]
- 9. Scherf A, Lopez-Rubio JJ, Riviere L (2008) Antigenic variation in Plasmodium falciparum . Annu Rev Microbiol 62: 445–470. [DOI] [PubMed] [Google Scholar]
- 10. Langhorne J, Ndungu FM, Sponaas A-M, Marsh K (2008) Immunity to malaria: More questions than answers. Nature Immunol 9: 725–732. [DOI] [PubMed] [Google Scholar]
- 11. MalERA Consultative Group on Integration Strategies (2011) A research agenda for malaria eradication: Cross-cutting issues for eradication. PLoS Med 8: e1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, et al. (2010) Ecology: A prerequisite for malaria elimination and eradication. PLoS Med 7: e1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MalERA Consultative Group on Vector Control (2011) A research agenda for malaria eradication: Vector control. PLoS Med 8: e1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gravitz L (2012) The last bite: Preventing mosquitoes from transmitting the malaria parasite is a crucial piece of the eradication puzzle. Nature 484: S26–S27.22534530 [Google Scholar]
- 15. Pates H, Curtis C (2005) Mosquito behavior and vector control. Annu Rev Entomol 50: 53–70. [DOI] [PubMed] [Google Scholar]
- 16. Enayati A, Hemingway J (2010) Malaria management: past, present, and future. Ann Rev Entomol 55: 569–591. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation (2011) World Malaria Report 2010 Geneva: World Health Organization; 2010. pp 27–75.
- 18. Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, et al. (2011) Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PloS one 6: e20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation (2012) World Malaria Report 2011. pp. 23–30.
- 20. Riehle MM, Guelbeogo WM, Gneme A, Eiglmeier K, Holm I, et al. (2011) A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science 331: 596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Govella NJ, Ferguson H (2012) Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol 3: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fillinger U, Lindsay SW (2006) Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health 11: 1629–1642. [DOI] [PubMed] [Google Scholar]
- 23. Scholte E-J, Ng'habi K, Kihonda J, Takken W, Paaijmans K, et al. (2005) An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308: 1641–1642. [DOI] [PubMed] [Google Scholar]
- 24. Ren X, Hoiczyk E, Rasgon JL (2008) Viral paratransgenesis in the malaria vector Anopheles gambiae . PLoS Pathog 4: e1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agelopoulos N, Birkett MA, Hick AJ, Hooper AM, Pickett JA, et al. (1999) Exploiting semiochemicals in insect control. Pestic Sci 55: 225–235. [Google Scholar]
- 26. Riehle MA, Srinivasan P, Moreira CK, Jacobs-Lorena M (2003) Towards genetic manipulation of wild mosquito populations to combat malaria: advances and challenges. J Exp Biol 206: 3809–3816. [DOI] [PubMed] [Google Scholar]
- 27.Breman J, Alilio M, White NJ, Knols BG, Bossin HC, et al. (2007) Transgenic mosquitoes and the fight against malaria: managing technology push in a turbulent GMO world. Am J Trop Med Hyg 77: 232–242. [PubMed] [Google Scholar]
- 28. Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL (2010) Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PloS one 5: e13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffmann A, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. [DOI] [PubMed] [Google Scholar]
- 30. Bian G, Joshi D, Dong Y, Lu P, Zhou G, et al. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340: 748–751. [DOI] [PubMed] [Google Scholar]
- 31. Takken W, Knols BGJ (1999) Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol 44: 131–157. [DOI] [PubMed] [Google Scholar]
- 32. Bentley MD, Day JF (1989) Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol 34: 401–421. [DOI] [PubMed] [Google Scholar]
- 33. Takken W, Knols BG (2009) Malaria vector control: current and future strategies. Trends Parasitol 25: 101–104. [DOI] [PubMed] [Google Scholar]
- 34. Sutherst RW (2004) Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev 17: 136–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vale G (1993) Development of baits for tsetse flies (Diptera: Glossinidae) in Zimbabwe. J Med Entomol 30: 831–842. [DOI] [PubMed] [Google Scholar]
- 36. Kline DL (2007) Semiochemicals, traps/targets and mass trapping technology for mosquito management. J Amer Mosq Control Assoc 23: 241–251. [DOI] [PubMed] [Google Scholar]
- 37. Rose RI (2001) Pesticides and public health: integrated methods of mosquito management. Emerg Infect Dis 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiscox A, Maire N, Kiche I, Silkey M, Homan T, et al. (2012) The SolarMal Project: innovative mosquito trapping technology for malaria control. Malaria J 11: O45. [Google Scholar]
- 39. Matowo NS, Moore J, Mapua S, Madumla EP, Moshi IR, et al. (2013) Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: a report on design and field evaluation of the Mosquito Landing Box. Parasit Vector 6: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, et al. (2010) Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PloS one 5: e8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cork A, Park K (1996) Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol 10: 269–276. [DOI] [PubMed] [Google Scholar]
- 42. Smallegange RC, Qiu YT, van Loon JJ, Takken W (2005) Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Sens 30: 145–152. [DOI] [PubMed] [Google Scholar]
- 43. Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, et al. (2012) A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol 38: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tchouassi DP, Sang R, Sole CL, Bastos AD, Teal PE, et al. (2013) Common host-derived chemicals increase catches of disease-transmitting mosquitoes and can improve early warning systems for Rift Valley Fever Virus. PLoS Negl Trop Dis 7: e2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foster WA (2008) Phytochemicals as population sampling lures. J Am Mosq Control Assoc 24: 138–146. [DOI] [PubMed] [Google Scholar]
- 46. Townson H, Nathan M, Zaim M, Guillet P, Manga L, et al. (2005) Exploiting the potential of vector control for disease prevention. Bull World Health Org 83: 942–947. [PMC free article] [PubMed] [Google Scholar]
- 47.Nyasembe VO, Torto B (2013) Volatile phytochemicals as mosquito semiochemicals. Phytochem Lett: In press. [DOI] [PMC free article] [PubMed]
- 48. Turner SL, Li N, Guda T, Githure J, Cardé RT, et al. (2011) Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards PW (1952) The tropical rain forest: An ecological study. Cambridge: Cambridge University Press. 450 p. [Google Scholar]
- 50. Golding J, Shearer D, Wyllie S, McGlasson W (1998) Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Technol 14: 87–98. [Google Scholar]
- 51. Nyasembe VO, Teal PEA, Mukabana WR, Tumlinson JH, Torto B (2012) Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors 5: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Njiru BN, Mukabana WR, Takken W, Knols BG (2006) Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malaria J 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Noor A, Gething P, Alegana V, Patil A, Hay S, et al. (2009) The risks of malaria infection in Kenya in 2009. BMC Infect Dis 9: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, et al. (2006) Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol 43: 200–206. [DOI] [PubMed] [Google Scholar]
- 55. Atieli H, Menya D, Githeko A, Scott T (2009) House design modifications reduce indoor resting malaria vector densities in rice irrigation scheme area in western Kenya. Malaria J 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards FW (1941) Mosquitoes of the Ethiopian Region. III.-Culicine Adults and Pupae: London, Brit. Mus. (N. H.)
- 57.Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Johannesburg: South African Institute for Medical Research. 343 p. [Google Scholar]
- 58. Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Amer J Trop Med Hyg 49: 520–529. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organisation (2002) Malaria entomolgy and vector control. Learner's Guide pp. 41–46.
- 60.R Development Core Team (2010) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. pp. ISBN 3-900051-900007-900050, URL http://www.R-project.org.
- 61. White GC, Bennetts RE (1996) Analysis of frequency count data using the negative binomial distribution. Ecology 77: 2549–2557. [Google Scholar]
- 62. Kline DL (2002) Evaluation of various models of propane-powered mosquito traps. J Vector Ecol 27: 1–7. [PubMed] [Google Scholar]
- 63. Mboera LEG, Takken W, Mdira K, Pickett J (2000) Sampling gravid Culex quinquefasciatus (Diptera: Culicidae) in Tanzania with traps baited with synthetic oviposition pheromone and grass infusions. J Med Entomol 37: 172–176. [DOI] [PubMed] [Google Scholar]
- 64. Williams CR, Long SA, Russell RC, Ritchie SA (2006) Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Control Assoc 22: 296–300. [DOI] [PubMed] [Google Scholar]
- 65. Kline DL (1999) Comparison of two American Biophysics mosquito traps: the professional and a new counterflow geometry trap. J Am Mosq Control Assoc 15: 276–282. [PubMed] [Google Scholar]
- 66. Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, et al. (2010) Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malaria J 9: 262–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Müller GC, Xue RD, Schlein Y (2011) Differential attraction of Aedes albopictus in the field to flowers, fruits and honeydew. Acta Trop 118: 45–49. [DOI] [PubMed] [Google Scholar]
- 68. Foster WA (1995) Mosquito sugar feeding and reproductive energetics. Ann Rev Entomol 40: 443–474. [DOI] [PubMed] [Google Scholar]
- 69. Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y (2012) Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malaria J 11: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, et al. (2010) Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malaria J 9: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Misharina T (2001) Influence of the duration and conditions of storage on the composition of the essential oil from coriander seeds. Appl Biochem Microbiol 37: 622–628. [Google Scholar]
- 72. Gillies M (1980) The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res 70: 525–532. [Google Scholar]
- 73. Kellogg F (1970) Water vapour and carbon dioxide receptors in Aedes aegypti . J Insect Physiol 16: 99–108. [DOI] [PubMed] [Google Scholar]
- 74. Omer S, Gillies M (1971) Loss of response to carbon dioxide in palpectomized female mosquitoes. Entomol Exp Appl 14: 251–252. [Google Scholar]
- 75. Mayer M, James J (1969) Attraction of Aedes aegypti (L.): Responses to human arms, carbon dioxide, and air currents in a new type of olfactometer. Bull EntomolRes 58: 629–642. [Google Scholar]
- 76.Grant AJ, O'Connell RJ (1996) Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla; GR B, G C, editors: Chichester: J Wiley& Sons. 233–253 p. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy JS (1940) The visual responses of flying mosquitoes. Wiley Online Library. pp. 221–242.
- 78. Geier M, Bosch OJ, Boeckh J (1999) Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J Exp Biol 202: 1639–1648. [DOI] [PubMed] [Google Scholar]
- 79. Costantini C, Gibson G, Sagnon NF, DellaTorre A, Brady J, et al. (1996) Mosquito responses to carbon dioxide in a West African Sudan savanna village. Med Vet Entomol 10: 220–227. [DOI] [PubMed] [Google Scholar]
- 80. Verhoek B, Takken W (1994) Age effects on the insemination rate of Anopheles gambiae sl in the laboratory. Entomol Exp Appl 72: 167–172. [Google Scholar]
- 81. Charlwood J, Pinto J, Sousa C, Madsen H, Ferreira C, et al. (2002) The swarming and mating behaviour of Anopheles gambiae ss (Diptera: Culicidae) from Sao Tome Island. J Vector Ecol 27: 178–183. [PubMed] [Google Scholar]
- 82. Charlwood J, Thompson R, Madsen H (2003) Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malaria J 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, et al. (2010) Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria J 9: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preliminary trap captures of all species of mosquitoes using different concentrations of plant-derived synthetic blends. LO = (E)-linalool oxide, OC = β-ocimene, bars capped with asterisks are significantly different from their respective controls as detected by general linear model with negative-binomial error structure and log link in R 2.15.1 software; * = P<0.05, ** = P<0.01.
(PNG)
Composition and concentrations of blends tested in field assays.
(DOCX)