Abstract
Background
Sexually transmitted infections (STIs), including Hepatitis B and C virus, are emerging public health risks in China, especially among men who have sex with men (MSM). This study aims to assess the magnitude and risks of STIs among Chinese MSM.
Methods
Chinese and English peer-reviewed articles were searched in five electronic databases from January 2000 to February 2013. Pooled prevalence estimates for each STI infection were calculated using meta-analysis. Infection risks of STIs in MSM, HIV-positive MSM and male sex workers (MSW) were obtained. This review followed the PRISMA guidelines and was registered in PROSPERO.
Results
Eighty-eight articles (11 in English and 77 in Chinese) investigating 35,203 MSM in 28 provinces were included in this review. The prevalence levels of STIs among MSM were 6.3% (95% CI: 3.5–11.0%) for chlamydia, 1.5% (0.7–2.9%) for genital wart, 1.9% (1.3–2.7%) for gonorrhoea, 8.9% (7.8–10.2%) for hepatitis B (HBV), 1.2% (1.0–1.6%) for hepatitis C (HCV), 66.3% (57.4–74.1%) for human papillomavirus (HPV), 10.6% (6.2–17.6%) for herpes simplex virus (HSV-2) and 4.3% (3.2–5.8%) for Ureaplasma urealyticum. HIV-positive MSM have consistently higher odds of all these infections than the broader MSM population. As a subgroup of MSM, MSW were 2.5 (1.4–4.7), 5.7 (2.7–12.3), and 2.2 (1.4–3.7) times more likely to be infected with chlamydia, gonorrhoea and HCV than the broader MSM population, respectively.
Conclusion
Prevalence levels of STIs among MSW were significantly higher than the broader MSM population. Co-infection of HIV and STIs were prevalent among Chinese MSM. Integration of HIV and STIs healthcare and surveillance systems is essential in providing effective HIV/STIs preventive measures and treatments.
Trial Registration
PROSPERO No: CRD42013003721
Introduction
Men who have sex with men (MSM) is a high-risk population for HIV and sexually transmitted infections (STIs) in China and internationally [1]–[6]. Although MSM only accounts for 2–4% of the total Chinese sexually-active male population [7], nearly a third of new HIV infections were attributable to homosexual contact in 2011 [8]. Statistics showed that the national HIV prevalence among Chinese MSM increased rapidly from 1.2% in 2001 to 6.3% in 2011 [8], [9], and its transmission has been substantially facilitated by the co-existing STI epidemics [10]. For instance, syphilis prevalence among Chinese MSM increased from 6.8% to 13.5% during 2003–2008 [11] and prevalence of HIV/syphilis co-infection doubled from 1.4% in 2005–2006 to 2.7% in 2007–2008 [11]. MSM commonly practice anal and oral sex [12], but other sexual practices such as anilingus, fisting and rimming are prevalent [12], [13]. The diverse types of sexual intercourse and low rate of condom use elevated the risks of STIs transmission [14]–[17]. Also, social stigma and internalised homophobia have led to common psychological disorders, substance abuse and unintended high-risk behaviours [18], [19], which fuel the wide spread of HIV/STIs syndemics [19]–[21].
Co-infection of STI and hepatitis is a major risk for HIV transmission. Prevalence of human papillomavirus (HPV) is extremely high among MSM worldwide (63.9% in HIV-negative and 92.6% in HIV-positive MSM) [22], and the presence of HPV-related high-grade squamous intraepithelial lesions can destroy anogenital mucosal tissues and facilitates the intracellular transmission of HIV [23]. MSM infected with any of herpes simplex virus type 2 (HSV-2), rectal chlamydia, gonorrhoea and syphilis are associated with 3–8-folds higher risk of HIV acquisition [24]–[27]. Co-infection with viral hepatitis enhances the progression of liver diseases; significantly increasing the risk of morbidity and mortality among people living with HIV [28]. Patients with dual HIV/HBV or HIV/HCV infection have a nineteen and three times higher risk of liver death than mono-infected patients, respectively [29]–[31].
Epidemics of STIs and hepatitis infections are largely under-reported among Chinese MSM [32]. Apart from HIV, only syphilis and gonorrhoea are notifiable by law in China [33]. Since the current STIs surveillance in China mainly relies on the passive hospital-based reporting system [34], [35], it is estimated that the reported cases of STIs in China only account for 10% of the actual number of infections [36], [37]. Numerous scattered studies reported prevalence estimates for STIs and hepatitis infections among Chinese MSM, but none provided national estimates for these infections. A comprehensive data synthesis on these prevalence estimates is valuable in providing an overview of the extent of STIs and hepatitis infections disease burden and risk of infections among Chinese MSM. Building on findings of previous studies [9], [11], this study aims to assess the current disease burdens and risks of STIs and hepatitis infections among the broader MSM, HIV-infected MSM and male sex workers (MSW) in China.
Methods
Systematic Review and Meta-analysis
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement issued in 2009 (Checklist S1) [38]. The protocol for this review has been prospectively registered (CRD42013003721) with International Prospective Register of Systematic Reviews (PROSPERO) [39].
Search Strategy
We searched PubMed, Embase, Wanfang Data, VIP Chinese Journal Database (VIP) and China National Knowledge Infrastructure (CNKI) for studies reported the prevalence of STIs and hepatitis infections among MSM in China from January 2000 to February 2013. The search included Medical Subject Headings (MeSH) terms for ‘China’, ‘Chinese’, ‘hepatitis’, ‘sexually transmitted diseases’ and ‘sexually transmitted infections’, and other keywords associated with each STI: ‘chlamydia’, ‘Chlamydia trachomatis’, ‘gonorrhoea’, ‘Neisseria gonorrhoeae’, ‘genital wart’, ‘hepatitis’, ‘HBV’, ‘hepatitis B’, ‘HCV’, ‘hepatitis C’, ‘HSV’, ‘herpes simplex virus’, ‘HPV’, ‘human papillomavirus’ and ‘Ureaplasma urealyticum’. Truncation and wildcard operators were used in the search strategy. Hand searching from the reference lists of the retrieved articles in the above databases was also included. Our search strategy was limited to English and Chinese language. Two reviewers (EPFC, YW) independently screened all retrieved abstracts from the five aforementioned databases against the selection criteria. Discrepancies on which articles met the inclusion criteria were resolved by a third reviewer (LZ).
Selection Criteria
Type of studies
We preferentially looked for quantitative epidemiological studies, including cohort, before-and-after, and cross-sectional studies. Editorials, newspapers, review articles, conference abstracts, modelling studies and case reports were excluded. We only included studies conducted in mainland China. Studies conducted in Hong Kong, Macau and Taiwan were excluded.
Type of participants
We only included studies with MSM who self-reported having any homosexual intercourse in the past 12 months. There were no restrictions on age, marital status, educational level, ethnicity, residency and self-identified sexual orientation of the participants. Male sex workers (MSW) are a subpopulation in the Chinese MSM population who commercially sell sex to MSM (also known as ‘money boys’ in China), we only included studies in which MSW constituted <20% of the study sample. Studies targeted HIV-positive MSM were included to investigate the prevalence of co-infection. Studies with sample size of the MSM <30 and HIV-positive MSM population <10 were excluded in this review.
Type of outcome measures
We included studies measured the prevalence of the most common STIs among MSM (including chlamydia, genital wart [Condyloma acuminatum], gonorrhoea, HPV, HSV-2 and Ureaplasma urealyticum). We also included the two rampant and sexually transmissible hepatitis infections (i.e. HBV and HCV). Studies were included if the infection was diagnosed and confirmed by a molecular biology-based method. HBV infection was defined by the presence of hepatitis B surface antigen (HBsAg) as HBsAg is a serologic marker represents either acute or chronic HBV infections rather than due to other cases such as HBV immunity. HCV infection was diagnosed by the presence of anti-HCV antibodies. Enzyme immunoassay (EIA), polymerase chain reaction (PCR) or recombinant immunoblot assay (RIBA) was used to detect HCV. HPV infection was tested by PCR. Chlamydia, genital wart, gonorrhoea and Ureaplasma urealyticum were tested by PCR, EIA or cell culture. HSV-2 was diagnosed by the presence of Immunoglobulin G (IgG) by EIA. Self-reported infections were excluded from this review.
Quality Assessment
The quality assessment of eligible studies was measured according to the checklist tools for assessing quality in observational studies [40]. Six domains were used to assess the risk of bias: (1) methods for selecting study participants; (2) methods for measuring exposure and outcome variables; (3) design-specific source of bias; (4) method of control confounding; (5) statistical methods; and (6) other biases (including conflict of interest and disclosure of funding sources). The quality of each item was categorized as either ‘Low risk (+)’, ‘High risk (−)’ or ‘Unclear (?)’ in accordance with the guideline recommended by the Cochrane Collaboration [41].
Data Extraction and Management
Data were extracted and entered into a predesigned electronic data collection form in Microsoft Access database (Version 2010, Microsoft Corp., Redmond, WA, USA). Each study was given by a unique ID and the data collection form included information on: (1) study design: location, sampling methods/venues, type of study, sample size, and study year; (2) demographic characteristics of the study participants: age, marital status, and educational level; (3) epidemiology of STIs and hepatitis infections: prevalence estimates of STIs, HBV or HCV, method of laboratory diagnosis, and biomarkers examined.
Definition of Outcomes
The primary outcome measure was the prevalence of STIs and hepatitis infections among Chinese MSM, and it was expressed as a percentage of the number of infections divided by the number of individuals tested for the infection. To estimate the risk of STIs acquisition in MSM, the pooled STIs prevalence estimates among MSM were compared with MSW and the general population. Data on MSW were collected from a recent published meta-analysis study [42]. The prevalence of the general population was estimated among the Chinese adults of reproductive age (i.e. ≥15 years), and data were collected from the latest cross-sectional study across the country.
Statistical Analysis
Meta-analyses were performed using the Comprehensive Meta-Analysis software (Version 2.2, Biostat, Englewood, NJ, ISA) [43]. The pooled prevalence estimates for each STI were calculated by combining the weighted prevalence for each study. A standard continuity correction of 0.5 was added to the studies with prevalence of zero [44]. The effect rates of pooled estimates and 95% confidence intervals (CI) for each STI were graphically presented in the form of forest plots.
Heterogeneity tests across studies were detected by the chi-squared based Cochran Q-test (p<0.10 indicates statistically significant heterogeneity) and I 2 statistic [45]–[47]. I 2 was calculated as I 2 = [(Q – degree of freedom)/Q] ×100, where Q is the Cochran’s statistic. I 2 values of 25, 50 and 75 representing low, medium and high heterogeneity, respectively [47]. If high and significant heterogeneity (I 2>75) was detected across studies [47], the random-effect model was used to calculate the summary of pooled prevalence estimates [48], [49]. Otherwise, a fixed-effect model was used when low heterogeneity was observed across studies. Sampling sizes of the study were taken into account in both models. Evidences on HBV and HCV prevalence estimates were further stratified by six administrative Chinese regions and study years to explore the potential factor(s) that contributed to the heterogeneity. The geographical and temporal trends of STIs could not be investigated in this review due to insufficient studies.
The potential presence publication bias was examined by the inspection of funnel plot [50], and the significant of funnel plot asymmetry was statistically tested using the Begg and Mazumdar rank correlation method (p<0.05 represents statistically significant publication bias) [51], [52]. Test of publication bias was performed if the meta-analysis consisted of more than 10 studies [53], [54].
We performed additional analyses to compare the STIs prevalence among Chinese MSM with estimated prevalence among adults in the general population and MSW. Odds ratio (OR) and its 95% CI were calculated to measure the risk of STIs acquisition in MSM/MSW.
Results
Characteristics of Included Studies
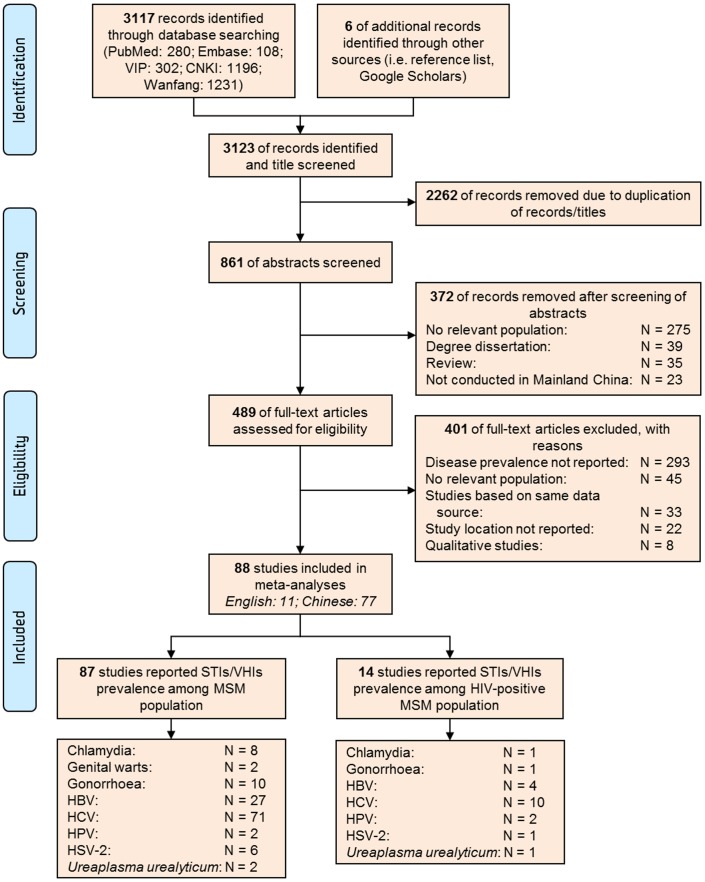
We identified 3,123 records based on our search strategy, of which 861 were unique records. After the initial screening of abstracts, we reviewed 489 articles in full and 401 articles were excluded based on our selection criteria. A total of 88 studies were included (Figure 1). The sample size of MSM in the eligible studies ranged from 59 to 1,462 (median: 273; interquartile range: 156–456) and the overall sample consisted of 35,203 MSM. The weighted mean age of MSM was 27.4 years (range: 14–84 years). The majority of studies were cross-sectional observational studies (n = 85); but two were before-and-after studies and one was cohort study. Seventy-one studies reported on HCV, twenty-seven on HBV, ten on gonorrhoea, eight on chlamydia, seven on HSV-2, two on HPV and genital wart, respectively. Most of studies (n = 23) recruited study participants at MSM hotspot venues; however, the majority of remaining studies (n = 43) utilised a combination of recruitment methods (Table S1). Fourteen studies reported the prevalence of STIs among HIV-positive MSM (Table S2).
Figure 1. PRISMA flow chart for selection of studies.
N represents the number of studies identified.
Hepatitis Infections
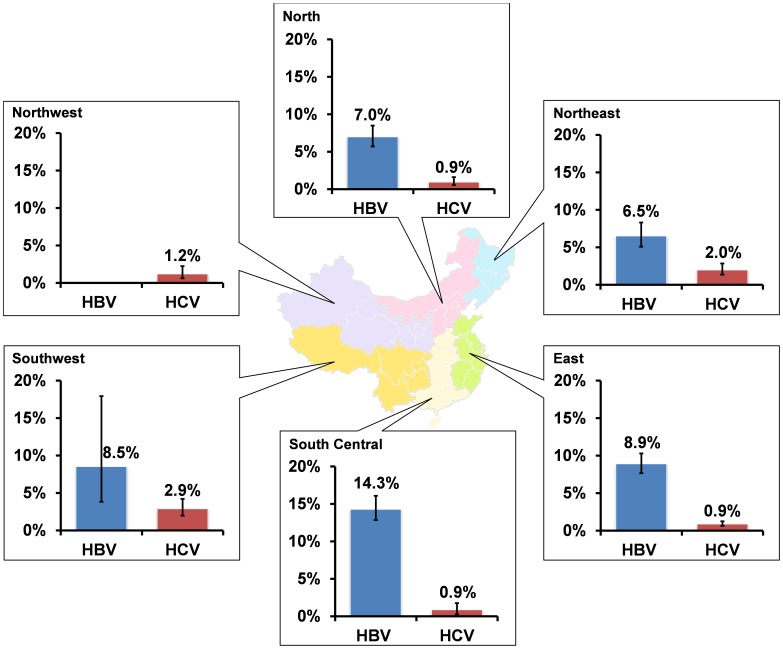
The estimated HBV and HCV prevalence among MSM were 8.9% (95% CI: 7.8–10.2%) (Figure S1) and 1.2% (1.0–1.6%) (Figure S2) over the period 2003–2011 and heterogeneities across the studies were substantial (HBV: χ2 = 166.0, p<0.001; I 2 = 79.5; HCV: χ2 = 335.4, p<0.001; I 2 = 76.2) (Table 1, Table S3). Study location stratification substantially reduced heterogeneities (Table S3). HBV prevalence varied extensively across geographical regions (χ2 = 56.6, p<0.001), ranged from 6.5% (5.1–8.3%) in the Northeast to 14.3% (12.6–16.2%) in the South Central (Figure 2). No HBV prevalence was reported in the Northwest. In comparison, South Central had the lowest HCV prevalence (0.9% [0.5–1.5%]); while prevalence in the Southwest was the highest (2.9% [2.0–4.2%]) (Figure 2). Among HIV-positive MSM, 18.3% (9.8–31.5%) and 8.4% (3.9–17.3%) were co-infected with HBV and HCV, respectively. The temporal trends of HBV (p = 0.212) and HCV (p = 0.321) prevalence among MSM were not significant (Figure S3).
Table 1. Meta-analyses of STIs and hepatitis infections prevalence among broader MSM comparing among HIV-positive MSM and male sex workers in China.
| Diseases Burden | MSM population (Ref) | HIV-positive MSM | Male sex workers | ||||||||
| Number ofstudies (numberof estimates)φ | Totalsamplesize | EstimatedPrevalence(95% CI) | Number ofstudies(number ofestimates) | Total samplesize of HIV-positive | EstimatedPrevalence(95% CI) | Odds ratio(95% CI)∧ | Number ofstudies(number ofestimates) | Totalsamplesize | EstimatedPrevalence(95% CI) | Odds ratio(95% CI)∧ | |
| Hepatitis infections | |||||||||||
| HBV | 27 (35) [56,61,65,86,121–143] | 11,305 | 8.9%(7.8–10.2%) | 4 (4) [56,121,139,144] | 151 | 18.3%(9.8–31.5%) | 2.3(1.5–3.5)*** | 1(1) [145] | 120 | 4.2%(1.7–9.6%) | 0.4(0.2–1.1) |
| HCV | 71 (81) [55,56,61,65,79,86,121,122,124–126,128–142,146–189] | 28,684 | 1.2%(1.0–1.6%) | 10 (10) [55,121,128,139,144,152,162,178,179,183] | 363 | 8.4%(3.9–17.3%) | 7.6(5.2–11.2)*** | 2(2) [145], [190] | 625 | 2.7%(0.6–12.0%) | 2.2(1.4–3.7)** |
| Sexually transmitted infections | |||||||||||
| Chlamydia | 8 (12) [55,133,140,141,191–194] | 3,921 | 6.3%(3.5–11.0%) | 1 (1) [55] | 16 | 31.3%(13.6–56.7%) | 6.7(2.3–19.5) *** | 1(1) [195] | 82 | 14.6%(8.5–24.0%) | 2.5(1.4–4.7) ** |
| Sera | 1 (1) [55] | 753 | 5.6%(4.15–7.5%) | – | – | – | – | – | – | – | – |
| Urethral | 5 (8) [133,140,141,191,192] | 2,411 | 4.3%(2.5–7.5%) | – | – | – | – | – | – | – | – |
| Rectal | 3 (3) [192–194] | 757 | 16.5%(6.9–34.4%) | – | – | – | – | – | – | – | – |
| Genital warts | 2 (3) [56], [79] | 581 | 1.5%(0.7–2.9%) | – | – | – | – | – | – | – | – |
| Gonorrhoea | 10 (13) [56,79,133,140,191–194,196,197] | 3,668 | 1.9%(1.3–2.7%) | 1 (1) [56] | 24 | 4.2%(0.6–24.4%) | 2.3(0.3–17.2) | 1(1) [195] | 82 | 9.8%(5.0–18.3%) | 5.7(2.7–12.3) *** |
| Sera | 1 (1) [197] | 157 | 2.6%(1.0–6.6%) | – | – | – | – | – | – | – | – |
| Urethral | 4 (4) [133,140,191,194] | 891 | 1.4%(0.8–2.7%) | – | – | – | – | – | – | – | – |
| Rectal | 3 (3)[140], [193], [194] | 687 | 1.0%(0.1–7.2%) | – | – | – | – | – | – | – | – |
| HIV# | N/A (60) [42] | 20,729 | 4.7%(3.9–5.6%) | – | – | – | – | 16 (16) [42] | 2,278 | 6.0%(4.2–8.5%) | 1.3(1.1–1.6) ** |
| HPV | |||||||||||
| Any type | 2 (2)[198], [199] | 861 | 66.3%(57.4–74.1%) | 2 (2) [198], [199] | 79 | 96.2%(88.9–98.8%) | 12.9(4.0–41.0) *** | – | – | – | – |
| Single type | 2 (2)[198], [199] | 861 | 32.8%(29.7–36.0%) | 2 (2) [198], [199] | 79 | 40.5%(30.3–51.7%) | 1.4(0.9–2.2) | – | – | – | – |
| Multiple types | 2 (2)[198], [199] | 861 | 33.4% (25.3–42.6%) | 2 (2) [198], [199] | 79 | 56.9%(45.8–67.4%) | 2.6(1.7–4.2) *** | – | – | – | – |
| HPV16 | 2 (2)[198], [199] | 861 | 8.0%(2.8–20.9%) | 1 (1) [198] | 50 | 34.0%(22.3–48.1%) | 5.9(3.1–11.1) *** | – | – | – | – |
| HPV18 | 1 (1) [198] | 578 | 5.9%(4.2–8.1%) | 1 (1) [198] | 50 | 14.0%(6.8–26.6%) | 2.6(1.1–6.2) ** | – | – | – | – |
| HPV45 | 2 (2)[198], [199] | 861 | 4.4%(2.7–7.2%) | 1 (1) [198] | 50 | 14.0%(6.8–26.6%) | 3.5(1.5–8.3) ** | – | – | – | – |
| HSV-2 | 6 (6) [24,84,133,189,200,201] | 3,451 | 10.6%(6.2–17.6%) | 1 (1) [24] | 47 | 33.8%(26.4–42.1%) | 4.3(2.3–7.9) *** | 3(3) [202]–[204] | 598 | 10.0%(7.6–12.9%) | 0.9(0.7–1.2) |
| Syphilis# | N/A(40) [42] | 15,317 | 13.5%(11.8–15.3%) | N/A (8) [42] | 317 | 36.2%(24.3–50.0%) | 3.6(2.9–4.6) *** | 15 (15) [42] | 2,237 | 12.4%(9.9–15.3%) | 0.9(0.8–1.0) |
| Ureaplasma urealyticum | 2 (2)[55], [205] | 938 | 4.3%(3.2–5.8%) | 1 (1) [198] | 16 | 18.8%(6.2–44.8%) | 5.2(1.4–18.9) * | – | – | – | – |
Some studies reported more than one prevalence estimate (e.g. in multiple years or locations). The number of estimates represents the total number of data points analysed in meta-analysis.
The estimated prevalence for HIV, syphilis and HIV-syphilis co-infection were extracted from a published meta-analysis study by Chow EPF et al in 2011.
The broader MSM population was used as the reference group for odds ratio.
*p<0.05, **p<0.01, ***p<0.001.
Figure 2. Prevalence of HBV and HCV infection among MSM in six Chinese regions.
The risks of HBV and HCV infections among MSM were significantly higher than general Chinese adults (HBV: OR = 1.4 [1.3–1.6]; HCV: OR = 10.1 [4.5–22.8]) (Table 2). Further, the risk of HCV infection was 2.2 (1.4–3.7) times higher among MSW compared with broader MSM, but the risks of HBV infection were similar (OR = 0.4 [0.2–1.1]). Among HIV-positive MSM, the risks of HBV and HCV infection were 2.3 (1.5–3.5) and 7.6 (5.2–11.2) times higher than the broader MSM population (Table 1).
Table 2. Comparison of hepatitis infections and STIs prevalence among MSM and adults in general population in China.
| Diseases burden | Adults in general population (Ref) | MSM | ||
| Total sample size | Estimated prevalence (%) | Sources | Odds ratio (95% CI)∧ | |
| Chlamydia | 6,334 | 3.0% (2.2–4.1%) | [206]–[208] | 2.2 (1.8–2.6)*** |
| Gonorrhoea | 5,366 | 0.2% (0.1–0.4%) | [206], [208] | 13.0 (6.3–27.1)*** |
| HIV | 52,601 | 0.05% (0.04–0.08%) | [8] | 98.7 (64.3–136.4)*** |
| HBV | 20,403 | 6.4% (4.5–9.1%) | [209]–[211] | 1.4 (1.3–1.6)*** |
| HCV | 4,950 | 0.1% (0.1–0.3%) | [212] | 10.1 (4.5–22.8)*** |
| Syphilis | 17,226 | 0.5% (0.3–0.7%) | [213], [214] | 29.9 (24.1–36.9)*** |
The general population was used as the reference group for odds ratio.
*p<0.05, ** p<0.01, *** p<0.001.
Sexually Transmitted Infections
Previous meta-analysis study reported the prevalence of HIV and syphilis among MSM to be 4.7% (3.9–5.6%) and 13.5% (11.8–15.3%), respectively [11]. Approximately 36.2% (24.3–50.0%) of HIV-positive MSM were co-infected with syphilis (Table 1).
The pooled prevalence of chlamydia was 6.3% (3.5–11.0%) (Figure S4) and heterogeneity was significant (χ2 = 223.6, p<0.001; I 2 = 95.1). Subgroup analyses indicated that the prevalence substantially varied by anatomical site, ranged from 4.3% (2.5–7.5%) for urethral chlamydia to 16.5% (6.9–34.4%) for rectal chlamydia. A single study estimated that 31.3% of HIV-positive MSM were also co-infected with chlamydia [55].
The overall sero-prevalence of gonococcal infection was 1.9% (1.3–2.7%), and heterogeneity was significant (χ2 = 51.0, p<0.001; I 2 = 74.5) (Figure S5). Stratified analysis according to the anatomical site of the infection substantially reduced heterogeneity (urethral: I 2 = 67.6; rectal: I 2 = 5.8) (Table S3). A lower overall prevalence was observed in rectal gonorrhoea (1.0% [0.1–7.2%]) than that of urethral gonorrhoea (1.4% [0.8–2.7%]). One study reported 4.2% of HIV-positive MSM were co-infected with gonorrhoea [56].
The pooled prevalence of HPV infection was 66.3% (57.4–74.1%) and the heterogeneity was substantial (χ2 = 6.1, p = 0.014; I 2 = 83.6) (Figure S6). Subgroup analyses showed that about 32.8% (29.7–36.0%) of Chinese MSM were single type infected and 33.4% (25.3–42.6%) were multiple types infected. Genotype HPV16 (8.0% [2.8–20.9%]) was found to be the most frequently identified sub-type, and was followed by HPV18 (5.9% [4.2–8.1%]) and HPV45 (4.4% [2.7–7.2%]). Co-infection between HIV and any genotypes of HPV genotypes was common (96.2% [88.9–98.8%]).
The pooled estimate of HSV-2 prevalence was 10.6% (6.2–17.6%) with high heterogeneity (χ2 = 126.8, p<0.001; I 2 = 96.1) across the included studies (Figure S7). Among HIV-positive MSM, estimated 33.8% were co-infected with HSV-2. The pooled prevalence of genital wart and Ureaplasma urealyticum were 1.5% (0.7–2.9%) and 4.3% (3.2–5.8%), respectively (Figure S8–S9).
In comparison with the general Chinese population, infection risk of STIs was significantly higher in MSM (chlamydia: OR = 2.2 [1.8–2.6]; gonorrhoea: 13.0 [6.3–27.1]; syphilis: 29.9 [24.1–36.9], Table 2). On contrast, MSW were subjected to even higher risk of STIs. The odds of chlamydial and gonococcal infections were 2.5 (1.4–4.7) and 5.7 (2.7–12.3) times higher in MSW compared with MSM, but the risks of syphilis and HSV-2 infections did not differ (Table 1). Similarly, HIV-positive MSM also demonstrated consistently greater risk of STIs compared with broader MSM (chlamydia: OR = 6.7 [2.3–19.5]; HPV: 12.9 [4.0–41.0]; HSV-2: 4.3 [2.3–7.9]; syphilis: 3.6 [2.9–4.6]; and Ureaplasma urealyticum: 5.2 [1.4–18.9]).
Risk of Bias within and Across Studies
Among the 88 included studies, seven were at low risk for all six methodological quality items (Figure S10). The remaining 81 studies were at high or unclear risk of at least one of the bias items but none of them was at high risk for all the items. Overall, most studies had low risk of methods for selecting study participants, methods for measuring exposure and outcome variables, and the statistical methods used (Figure S11). Substantial publication bias was found among studies reported HCV prevalence estimates (p = 0.045), but not those reported chlamydia (p = 0.131), genital wart (p = 0.602), gonorrhoea (p = 0.154) and HBV (p = 0.081) (Table S3).
Discussion
This study illustrates the complexity, heterogeneity and diversity of STIs and hepatitis infections among MSM in China. Our findings strongly indicate disproportionately high prevalence levels of STIs among Chinese MSM. The prevalence levels of STIs among HIV-positive MSM and MSW appeared to be higher than the broader MSM population.
Our findings illustrated that the transmission of HBV and HCV among Chinese MSM has distinct geographically patterns. HBV prevalence in China is 8.9%, which is higher than developed countries such as Australia (3%) [57], and the USA (4%) [58]. South Central region has the highest HBV prevalence across the country (14.3%). This is similar to patterns in the general population which major cities in South Central have consistently higher HBV prevalence (10.4–12.5% [59], [60]) than other Chinese regions (e.g. 3.5% in Beijing [61]). HBV transmission in China is primarily attributed to perinatal transmission [62]–[64]. Individuals engaging in high-risk behaviours, in particular, MSM with a history of STIs, injecting drug use and commercial sex activities, have elevated risk of HBV infection [65]–[70]. Although vaccination program for hepatitis B has been expanded through the National Expanded Program on Immunisation (EPI) in 1992 [71] and free hepatitis B vaccines have been provided to all newborns since 2005 [72], the majority (∼61%) of Chinese MSM remains unvaccinated, especially for those above age of 30 [65]. Low HBV immunisation coverage rate among MSM is not only found in China (∼39%), but is also observed in developed countries such as the USA (25%) [68], and Australia (53%) [73]. Successful HBV vaccination program integration as part of the sexual health programs have been observed globally [74]–[76], similar strategy especially targeting MSM are recommended in China [65], [72]. In comparison, HCV prevalence pattern in MSM is similar to that of drug use in China [77], [78]. Southwest China has a distinctly greater HCV disease burden (2.89%) in comparison with other regions. Notably 1–6% of Chinese MSM are also injecting drug users [79]–[85]. However, the risk of HCV infection may be further elevated by their high-risk homosexual behaviours [86]–[89]. The recently reported HCV outbreaks among HIV-infected but non-injecting MSM in developed countries strongly indicate that HIV-infection and high-risk sexual behaviours may also contribute to HCV transmission in MSM [90], [91]. Behavioural interventions among MSM, especially HIV-positive MSM, remain a priority for prevention of STIs and hepatitis for MSM.
The consistently much higher risks of STIs and hepatitis infections among MSM are not unexpected but alarming. Many STIs, such as syphilis, HPV, chlamydia and gonorrhoea, are known to cause genital ulceration and inflammation which may significantly increase the infection risk of HIV, HBV, HCV and other STIs [92]–[99]. Co-infections of STIs and hepatitis infections also substantially complicated the provision of care and treatment for infected individuals, especially among those living with HIV [100]–[103]. HPV is the most prevalent STIs among Chinese MSM (66%), this result is consistent with the findings in countries such as Netherlands (72%) [104], Slovenian (78%) [105], and Australia (79%) [106]. In particular, our findings indicate that the odds of HPV infection among HIV-positive MSM was the highest (OR = 12.9) among the included STIs. HPV is known to be the primary etiological agent for anal cancer [107], the progression rate of high-grade anal intraepithelial neoplasia to anal carcinoma among HIV-positive MSM (1/600 per year) is much faster than that of HIV-negative MSM (1/4000 per year), suggesting HIV-positive MSM have a much higher risk of anal caner [22]. HPV also substantially facilitate the transmission of other STIs such as chlamydia, gonorrhoea [108] and HSV-2 [109], leading to complications in prevention and treatment of these STIs.
This study has a number of limitations. First, the majority of the studies included in this review employed non-probabilistic sampling approaches to recruit MSM, and studies were mostly conducted at gay-oriented venues in large urban cities. This limits the generalisability of the study as MSM who frequently attended gay venues, particularly gay saunas and bars, were known to have greater high-risk behaviours and risks of HIV and STIs [110], [111]. Second, as ∼90% of the Chinese MSM have engaged in oral sex with other men [12], [112]–[116] and more than half of these episodes (∼55%) were not protected by condoms [12], oral transmission of STIs is likely to be common and asymptomatic in MSM. However, pharyngeal infections of STIs were examined in none of our collected studies. Third, the number of studies on HIV-positive MSM are limited and the sample sizes are generally small (mean = 45.7, range = 12–149), limiting the statistical power of meta-analysis. Fourth, this study did not investigate the geographical and temporal trends of STIs among MSM due to insufficient data. Fifth, clinical diagnostic algorithms of STIs and hepatitis infections vary in different studies and study time points, which may lead to uncertainties in its sensitivity and specificity. The pooled estimates of STIs and hepatitis infections must be interpreted with cautions. Sixth, demographic characteristics (such as age, marital status, level of education) and psychosocial factors are also associated with STIs acquisition; however, we were not able to investigate these factors due to insufficient data reported in the eligible articles.
A number of priorities should be noted in response to the high disease burdens of STIs among Chinese MSM. First, an effective integration of HIV and STIs healthcare and surveillance systems is required. Although diagnosis and treatment of HIV and STIs share many common grounds, China has two parallel systems operating to provide counselling, testing and treatment for people living with HIV and STIs respectively [34], [117], [118]. As HIV-positive MSM are commonly infected with other STIs, timely referral and follow-up of HIV-positive MSM to STIs screening and treatment is necessary. Second, HPV is the most prevalence infection among all STIs in Chinese MSM. Although clinical use of quadrivalent HPV vaccine can effectively reduce the HPV infection among MSM [119], currently no country has a universal HPV vaccination program specifically targeting MSM [120]. Finally, expansion of HBV vaccination and screening and treatment for HCV-positive MSM are also priorities for curbing the viral hepatitis epidemics and reducing complications in co-infection treatments for this HIV high-risk group in China.
Supporting Information
Prevalence of hepatitis B virus infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of hepatitis C virus infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Temporal trends of HBV and HCV prevalence among MSM in China.
(PDF)
Prevalence of chlamydia among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of gonorrhoea among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of HPV infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of HSV-2 infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of genital wart ( Condyloma acuminatum ) infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of Ureaplasma urealyticum infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
(PDF)
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
(PDF)
Systematic review of 87studies reporting the prevalence of sexually transmitted infections and/or viral hepatitis infections among men who have sex with men in China.
(DOC)
Systematic review of 14 studies reporting the co-infection prevalence of sexually transmitted infections and/or viral hepatitis infections among HIV-positive men who have sex with men in China.
(DOC)
Publication bias and heterogeneity in subgroup meta-analyses.
(DOC)
PRISMA checklist.
(DOC)
Acknowledgments
The views expressed in this publication do not necessarily represent the position of the Australian Government.
Funding Statement
This publication was funded by the Australian Government Department of Health and Ageing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lau JT, Lin C, Hao C, Wu X, Gu J (2011) Public health challenges of the emerging HIV epidemic among men who have sex with men in China. Public Health 125: 260–265. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Chow EP, Wilson DP (2012) Distributions and trends in sexual behaviors and HIV incidence among men who have sex with men in China. BMC Public Health 12: 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, et al. (2012) Global epidemiology of HIV infection in men who have sex with men. Lancet 380: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayer KH, Bekker LG, Stall R, Grulich AE, Colfax G, et al. (2012) Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet 380: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Chow EP, Jing J, Zhuang X, Li X, et al. (2013) HIV prevalence in China: integration of surveillance data and a systematic review. Lancet Infect Dis 13: 955–963. [DOI] [PubMed] [Google Scholar]
- 6. Shang H, Xu J, Han X, Spero Li J, Arledge KC, et al. (2012) HIV prevention: Bring safe sex to China. Nature 485: 576–577. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Wang N, Wang L, Li D, Jia M, et al. (2009) The 2007 Estimates for People at Risk for and Living With HIV in China: Progress and Challenges. J Acquir Immune Defic Syndr 50: 414–418. [DOI] [PubMed] [Google Scholar]
- 8.State Council AIDS Working Committee Office (SCAWCO) (2012) 2012 China AIDS Response Progress Report. Beijing: Ministry of Health of the People’s Republic of China. 70 p. [Google Scholar]
- 9. Chow EP, Wilson DP, Zhang J, Jing J, Zhang L (2011) Human immunodeficiency virus prevalence is increasing among men who have sex with men in China: findings from a review and meta-analysis. Sex Transm Dis 38: 845–857. [DOI] [PubMed] [Google Scholar]
- 10. Rottingen JA, Cameron DW, Garnett GP (2001) A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis 28: 579–597. [DOI] [PubMed] [Google Scholar]
- 11. Chow EP, Wilson DP, Zhang L (2011) HIV and syphilis co-infection increasing among men who have sex with men in China: a systematic review and meta-analysis. PLoS One 6: e22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Li X, Chu Q, Wang N, Wang Z, et al. (2008) [A survey of HIV/AIDS related behaviors among 2250 MSM in nine major cities of China]. Chinese Journal of AIDS & STD 14: 541–547. [Google Scholar]
- 13. Li Y, Zhang BC, Li XF, Zang YS, Wang LX, et al. (2010) [Behavioral characteristics of men who have sex with men with sadomasochism associated with bleeding]. Zhonghua Liu Xing Bing Xue Za Zhi 31: 142–145. [PubMed] [Google Scholar]
- 14. Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, et al. (2007) Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sex Transm Infect 83: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kippax S, Noble J, Prestage G, Crawford JM, Campbell D, et al. (1997) Sexual negotiation in the AIDS era: negotiated safety revisited. AIDS 11: 191–197. [DOI] [PubMed] [Google Scholar]
- 16. Van de Ven P, Kippax S, Crawford J, Rawstorne P, Prestage G, et al. (2002) In a minority of gay men, sexual risk practice indicates strategic positioning for perceived risk reduction rather than unbridled sex. AIDS Care 14: 471–480. [DOI] [PubMed] [Google Scholar]
- 17. Chow EP, Wilson DP, Zhang L (2012) Patterns of condom use among men who have sex with men in China: a systematic review and meta-analysis. AIDS Behav 16: 653–663. [DOI] [PubMed] [Google Scholar]
- 18. Altman D, Aggleton P, Williams M, Kong T, Reddy V, et al. (2012) Men who have sex with men: stigma and discrimination. Lancet 380: 439–445. [DOI] [PubMed] [Google Scholar]
- 19. Klein H (2011) Using a syndemics theory approach to study HIV risk taking in a population of men who use the internet to find partners for unprotected sex. Am J Mens Health 5: 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer M, Clair S (2003) Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q 17: 423–441. [DOI] [PubMed] [Google Scholar]
- 21. Singer MC, Erickson PI, Badiane L, Diaz R, Ortiz D, et al. (2006) Syndemics, sex and the city: understanding sexually transmitted diseases in social and cultural context. Soc Sci Med 63: 2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, et al. (2012) Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 13: 487–500. [DOI] [PubMed] [Google Scholar]
- 23. Chin-Hong PV, Husnik M, Cranston RD, Colfax G, Buchbinder S, et al. (2009) Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS 23: 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin YP, Chen SC, Wang HC, Wei WH, Wang QQ, et al. (2012) Prevalence and risk factors of HSV-2 infection and HSV-2/HIV coinfection in men who have sex with men in China: a multisite cross-sectional study. Sex Transm Dis 39: 354–358. [DOI] [PubMed] [Google Scholar]
- 25. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, et al. (2006) Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20: 73–83. [DOI] [PubMed] [Google Scholar]
- 26. Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD (2010) Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 53: 537–543. [DOI] [PubMed] [Google Scholar]
- 27. Fleming DT, Wasserheit JN (1999) From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 75: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K (2011) Increasing burden of liver disease in patients with HIV infection. Lancet 377: 1198–1209. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann CJ, Thio CL (2007) Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis 7: 402–409. [DOI] [PubMed] [Google Scholar]
- 30. Peters MG (2007) Diagnosis and management of hepatitis B virus and HIV coinfection. Top HIV Med 15: 163–166. [PubMed] [Google Scholar]
- 31. Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR (2009) Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. World J Gastroenterol 15: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang C, Latkin C, Luan R, Wang C, Nelson K (2010) HIV, syphilis, hepatitis C and risk behaviours among commercial sex male clients in Sichuan province, China. Sex Transm Infect 86: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Standing Committee of the National People’s Congress (2004) Law of the People’s Republic of China on Prevention and Treatment of Infectious Diseases [Revised].
- 34. Zhang L, Wilson DP (2012) Trends in notifiable infectious diseases in China: implications for surveillance and population health policy. PLoS One 7: e31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Chow EP, Zhang J, Jing J, Wilson DP (2012) Describing the Chinese HIV surveillance system and the influences of political structures and social stigma. Open AIDS J 6: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong Y, Fang X, Zhou Y, Zhao R, Li X (2011) Factors associated with sexually transmitted infection underreporting among female sex workers in China. J Womens Health (Larchmt) 20: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen XS, Yin YP, Liang GJ, Gong XD, Li HS, et al. (2005) Sexually transmitted infections among female sex workers in Yunnan, China. AIDS Patient Care STDS 19: 853–860. [DOI] [PubMed] [Google Scholar]
- 38. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow EPF, Zhang L, Wang Y (2013) Burden of sexually transmissible infections and viral hepatitis among men who have sex with men in Mainland China York, United Kingdom: PROSPERO: International Prospective Register of Systematic Reviews.
- 40. Sanderson S, Tatt ID, Higgins JP (2007) Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36: 666–676. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JPT, Altman DG (2008) Assessing Risk of Bias in Included Studies. Cochrane Handbook for Systematic Reviews of Interventions: John Wiley & Sons, Ltd. 187–241.
- 42. Chow EP, Iu KI, Fu X, Wilson DP, Zhang L (2012) HIV and sexually transmissible infections among money boys in China: a data synthesis and meta-analysis. PLoS One 7: e48025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis: John Wiley & Sons Ltd.
- 44. Cox D (1970) The continuity correction. Biometrika 57: 217–219. [Google Scholar]
- 45. Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11: 193–206. [DOI] [PubMed] [Google Scholar]
- 46. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 47. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fleiss JL (1993) The statistical basis of meta-analysis. Stat Methods Med Res 2: 121–145. [DOI] [PubMed] [Google Scholar]
- 49. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 50. Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 51. Thornton A, Lee P (2000) Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 53: 207–216. [DOI] [PubMed] [Google Scholar]
- 52. Song F, Khan KS, Dinnes J, Sutton AJ (2002) Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol 31: 88–95. [DOI] [PubMed] [Google Scholar]
- 53.Rothstein HR, Sutton AJ, Borenstein M (2005) Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, England; Hoboken, NJ: John Wiley and Sons Ltd.
- 54. Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 55. Zhang X, Wang C, Hengwei W, Li X, Li D, et al. (2007) Risk factors of HIV infection and prevalence of co-infections among men who have sex with men in Beijing, China. AIDS 21 Suppl 8 S53–57. [DOI] [PubMed] [Google Scholar]
- 56.Zhou J, Zhu JJ, Bin H, Zhang L, Yao M, et al.. (2008) [A survey of HIV/STD, HBV and HCV infections and risk behaviors among MSM in two central districts of Guiyang city]. Chinese Journal of AIDS & STD 14: 47–48, 51.
- 57. MacLachlan JH, Allard N, Towell V, Cowie BC (2013) The burden of chronic hepatitis B virus infection in Australia, 2011. Aust N Z J Public Health 37: 416–422. [DOI] [PubMed] [Google Scholar]
- 58. Remis RS, Dufour A, Alary M, Vincelette J, Otis J, et al. (2000) Association of hepatitis B virus infection with other sexually transmitted infections in homosexual men. Omega Study Group. Am J Public Health 90: 1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen P, Yu C, Ruan B, Yang S, Ren J, et al. (2013) Prevalence of hepatitis B in insular regions of southeast China: a community-based study. PLoS One 8: e56444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang M, Zhang CH, Liu JH, Cai YS, Xu JM, et al. (2009) [An investigation on the epidemiological characteristics of hepatitis B in Guangzhou]. Chinese Journal of Epidemiology 30: 1317–1318. [Google Scholar]
- 61. Wu J, Zhang W, Han LL, Lin CY, Lin H, et al. (2007) [A sero-epidemiological study on hepatitis B among general population in Beijing]. Chinese Journal of Epidemiology 28: 555–557. [PubMed] [Google Scholar]
- 62. Hu Y, Zhang S, Luo C, Liu Q, Zhou YH (2012) Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: a provincial population-based study in China. BMC Infect Dis 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xi LF, XU ZY, Shen YD, Wang YJ, Liu LH, et al. (1991) [The horizontal and perinatal transmission of hepatitis B virus infection]. Chinese Journal of Virology 7: 21–24. [Google Scholar]
- 64. Yao JL (1996) Perinatal transmission of hepatitis B virus infection and vaccination in China. Gut 38 Suppl 2 S37–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang C, Wang Y, Huang X, Li X, Zhang T, et al. (2012) Prevalence and factors associated with hepatitis B immunization and infection among men who have sex with men in Beijing, China. PLoS One 7: e48219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saxton PJ, Hughes AJ, Robinson EM (2002) Sexually transmitted diseases and hepatitis in a national sample of men who have sex with men in New Zealand. N Z Med J 115: U106. [PubMed] [Google Scholar]
- 67. Seage GR 3rd, Mayer KH, Lenderking WR, Wold C, Gross M, et al. (1997) HIV and hepatitis B infection and risk behavior in young gay and bisexual men. Public Health Rep 112: 158–167. [PMC free article] [PubMed] [Google Scholar]
- 68. Diamond C, Thiede H, Perdue T, Secura GM, Valleroy L, et al. (2003) Viral hepatitis among young men who have sex with men: prevalence of infection, risk behaviors, and vaccination. Sex Transm Dis 30: 425–432. [DOI] [PubMed] [Google Scholar]
- 69. Choi KH, McFarland W, Neilands TB, Nguyen S, Secura G, et al. (2005) High level of hepatitis B infection and ongoing risk among Asian/Pacific Islander men who have sex with men, San Francisco, 2000–2001. Sex Transm Dis 32: 44–48. [DOI] [PubMed] [Google Scholar]
- 70. Lama JR, Agurto HS, Guanira JV, Ganoza C, Casapia M, et al. (2010) Hepatitis B infection and association with other sexually transmitted infections among men who have sex with men in Peru. Am J Trop Med Hyg 83: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu X, Zhang X, Wang L (2000) [National EPI Vaccination and Hepatitis B Vaccine Coverage Rate and the Related Factors: Results from the 1999 Nationwide Survey]. Chinese Journal of Vaccines and Immunization 6: 193–197. [Google Scholar]
- 72. Liang X, Bi S, Yang W, Wang L, Cui G, et al. (2009) Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 200: 39–47. [DOI] [PubMed] [Google Scholar]
- 73. Jin F, Prestage GP, Pell CM, Donovan B, Van de Ven PG, et al. (2004) Hepatitis A and B infection and vaccination in a cohort of homosexual men in Sydney. Sex Health 1: 227–237. [DOI] [PubMed] [Google Scholar]
- 74. Reiter PL, Brewer NT (2011) Hepatitis B vaccination among a national sample of gay and bisexual men. Sex Transm Dis 38: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buffington J, Jones TS (2007) Integrating viral hepatitis prevention into public health programs serving people at high risk for infection: good public health. Public Health Rep 122 Suppl 2 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harris JL, Jones TS, Buffington J (2007) Hepatitis B vaccination in six STD clinics in the United States committed to integrating viral hepatitis prevention services. Public Health Rep 122 Suppl 2 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhuang X, Liang Y, Chow EP, Wang Y, Wilson DP, et al. (2012) HIV and HCV prevalence among entrants to methadone maintenance treatment clinics in China: a systematic review and meta-analysis. BMC Infect Dis 12: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhuang X, Wang Y, Chow EP, Liang Y, Wilson DP, et al. (2012) Risk factors associated with HIV/HCV infection among entrants in methadone maintenance treatment clinics in China: a systematic review and meta-analysis. Drug Alcohol Depend 126: 286–295. [DOI] [PubMed] [Google Scholar]
- 79.Gao LM, Chen L, Ma Y, Lu JB, Li LX, et al.. (2010) [HIV/STD infection and KABP status among men who have sex with men in Yuxi city, Yunnan province, 2009–2010]. Soft Science of Health 24: 547–549, 553.
- 80. Hu Q, Lu FB, Gong JP, Li YC, Li GB, et al. (2006) [Knowledge, attitudes, and practice about STD/AIDS among the men who have sex with men in Nanchang City, Jiangxi Province]. Chinese Journal of Health Education 22: 647–649. [Google Scholar]
- 81. Lan Y, Gu Y, Wang B, Zhou D, Zhang J (2004) [Behavioral features of men who have sex with men]. Journal of Sichuan University (Medical Science Edition) 35: 372–375. [PubMed] [Google Scholar]
- 82. Zhang D, Bi P, Lv F, Zhang J, Hiller JE (2007) Changes in HIV prevalence and sexual behavior among men who have sex with men in a northern Chinese city: 2002–2006. Journal of Infectection 55: 456–463. [DOI] [PubMed] [Google Scholar]
- 83. Wang Y, Xu J, Li ZJ, Zhang GG, Li LL, et al. (2011) [Investigation on Connective Behavior Characteristics of MSM SPUSC]. Practical Preventive Medicine 18: 2072–2076. [Google Scholar]
- 84. Zheng M, Yao YM, Shen LM, Qin O, Zhou J (2011) [Survey of HIV/AIDS infection among men who have sex with men in Guiyang city of Guizhou province]. Guizhou Medical Journal 35: 830–831. [Google Scholar]
- 85.Feng JF, Lin HJ, Zhang YF, Qiu DH, Wu QH, et al.. (2008) [Investigation on the related knowledge, behavior and infection of HIV/Syphilis among men who have sex with men in Taizhou city]. Shanghai Journal of Preventive Medicine 20: 531–533, 541.
- 86. Ma X, Zhang Q, He X, Sun W, Yue H, et al. (2007) Trends in Prevalence of HIV, Syphilis, Hepatitis C, Hepatitis B, and Sexual Risk Behavior Among Men Who Have Sex With Men. Results of 3 consecutive respondent-driven sampling surveys in Beijing, 2004 through 2006. J Acquir Immune Defic Syndr 45: 581–587. [DOI] [PubMed] [Google Scholar]
- 87. He N, Detels R, Zhu J, Jiang Q, Chen Z, et al. (2005) Characteristics and sexually transmitted diseases of male rural migrants in a metropolitan area of Eastern China. Sex Transm Dis 32: 286–292. [DOI] [PubMed] [Google Scholar]
- 88. Lin H, He N, Su M, Feng J, Chen L, et al. (2011) Herpes simplex virus infections among rural residents in eastern China. BMC Infect Dis 11: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bradshaw D, Matthews G, Danta M (2013) Sexually transmitted hepatitis C infection: the new epidemic in MSM? Curr Opin Infect Dis 26: 66–72. [DOI] [PubMed] [Google Scholar]
- 90. van de Laar TJ, Matthews GV, Prins M, Danta M (2010) Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS 24: 1799–1812. [DOI] [PubMed] [Google Scholar]
- 91. van de Laar TJ, Paxton WA, Zorgdrager F, Cornelissen M, de Vries HJ (2011) Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports. Sex Transm Dis 38: 102–104. [DOI] [PubMed] [Google Scholar]
- 92. Boukli NM, Shetty V, Cubano L, Ricaurte M, Coelho-Dos-Reis J, et al. (2012) Unique and differential protein signatures within the mononuclear cells of HIV-1 and HCV mono-infected and co-infected patients. Clin Proteomics 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ward H, Ronn M (2010) Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 5: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayer KH, Venkatesh KK (2011) Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol 65: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Johnson LF, Lewis DA (2008) The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 35: 946–959. [DOI] [PubMed] [Google Scholar]
- 96. Cohen MS (2004) HIV and sexually transmitted diseases: lethal synergy. Top HIV Med 12: 104–107. [PubMed] [Google Scholar]
- 97. Galvin SR, Cohen MS (2004) The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2: 33–42. [DOI] [PubMed] [Google Scholar]
- 98.Sasadeusz J, Locarnini S, Kidd M, Bradford D, Danta M (2008) Chapter 1 - HIV, HBV, HCV and STIs: similarities and differences. In: Bradford D, Dore G, Grulich A, Kidd M, Hoy J et al.., editors. HIV/Viral hepatitis & STIs: a guide for primary care. Sydney, Australia: Australasian Society for HIV Medicine (ASHM). 9–27.
- 99. Cunningham AL, Dwyer DE (2004) The pathogenesis underlying the interaction of HIV and herpes simplex virus after co-infection. J HIV Ther 9: 9–13. [PubMed] [Google Scholar]
- 100. Soriano V, Sulkowski M, Bergin C, Hatzakis A, Cacoub P, et al. (2002) Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS 16: 813–828. [DOI] [PubMed] [Google Scholar]
- 101. Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, et al. (2001) Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 33: 562–569. [DOI] [PubMed] [Google Scholar]
- 102. Operskalski EA, Kovacs A (2011) HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 8: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Donlin MJ, Cannon NA, Aurora R, Li J, Wahed AS, et al. (2010) Contribution of genome-wide HCV genetic differences to outcome of interferon-based therapy in Caucasian American and African American patients. PLoS One 5: e9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mooij SH, van der Klis FR, van der Sande MA, Schepp RM, Speksnijder AG, et al. (2013) Seroepidemiology of high-risk HPV in HIV-negative and HIV-infected MSM: the H2M study. Cancer Epidemiol Biomarkers Prev 22: 1698–1708. [DOI] [PubMed] [Google Scholar]
- 105. Milošević M, Poljak M, Mlakar B (2010) Anal HPV infection in Slovenian men who have sex with men. Central European Journal of Medicine 5: 698–703. [Google Scholar]
- 106. Vajdic CM, van Leeuwen MT, Jin F, Prestage G, Medley G, et al. (2009) Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sex Transm Infect 85: 330–335. [DOI] [PubMed] [Google Scholar]
- 107. Stanley MA, Winder DM, Sterling JC, Goon PK (2012) HPV infection, anal intra-epithelial neoplasia (AIN) and anal cancer: current issues. BMC Cancer 12: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Quinn R, Salvatierra J, Solari V, Calderon M, Ton TG, et al. (2012) Human papillomavirus infection in men who have sex with men in Lima, Peru. AIDS Res Hum Retroviruses 28: 1734–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Houlihan CF, Larke NL, Watson-Jones D, Smith-McCune KK, Shiboski S, et al. (2012) Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS 26: 2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lau JT, Zhao JK, Wu XB, Gu J, Hao C (2013) Gay saunas and the risks of HIV and syphilis transmissions in China–results of a meta-analysis. J Sex Med 10: 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen XS, Yin YP, Jiang N, Wang B (2012) Setting typologies and HIV prevalence among men who have sex with men in China: implication for surveillance and intervention. Sex Transm Dis 39: 226–228. [DOI] [PubMed] [Google Scholar]
- 112.Wu S, Zhang B, Li X (2004) [AIDS high risk behavioral surveillance and comparison of homosexual/bisexual men in China]. Chinese Journal of AIDS & STD 10: 332–334, 328.
- 113. Zhang BC, Wu SW, Li XF, Zhu MQ, Yang LG (2004) Study on high risk behaviors among male sex workers related to STI/HIV. Chinese Journal of AIDS & STD 10: 329–337. [Google Scholar]
- 114. Zhang BC, Li XF, Shi TX, Cao NX, Hu TZ (2002) [Survey on the high risk behaviors and other AIDS/STI related factors among men who have sex with men (MSM) in mainland China (′2001)]. Chinese Journal of Dermatology 35: 214–216. [Google Scholar]
- 115. Zhang BC, Li XF, Hu TZ, Liu DC, Cao NX (2001) [“Friend Communication” Program - an Effective AIDS Intervention Program for MSM]. Chinese Journal of Health Education 17: 206–210. [Google Scholar]
- 116. Zhang BC, Liu DC, Li XF, Hu TZ, Shi TX (2001) [Study on HIV/AIDS high risk behavior and its factors among men who have sex with men in China]. Journal of China AIDS/STD Prevention and Control 7: 102–104. [Google Scholar]
- 117. Poon AN, Li Z, Wang N, Hong Y (2011) Review of HIV and other sexually transmitted infections among female sex workers in China. AIDS Care 23 Suppl 1 5–25. [DOI] [PubMed] [Google Scholar]
- 118. Tucker JD, Wong FY, Nehl EJ, Zhang F (2012) HIV testing and care systems focused on sexually transmitted HIV in China. Sex Transm Infect 88: 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, et al. (2011) HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 365: 1576–1585. [DOI] [PubMed] [Google Scholar]
- 120. Zou H, Fairley CK, Hocking JS, Garland SM, Grulich AE, et al. (2012) The prevalence of anal human papillomavirus among young HIV negative men who have sex with men. BMC Infect Dis 12: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang R, Shuai HQ, Shang XC (2011) [Investigation on the infection of HIV and Sex Transmitted Diseases in the man who have sex with man from 2006 to 2009 in Hangzhou]. Chinese Journal of Health Laboratory Technology 21: 732–733. [Google Scholar]
- 122. Zhou ZH, Li SM, Liu YJ, Li QC, Li DL, et al. (2010) [Survey of HIV and syphilis infection and influential factors assoicated with unprotected anal intercourse in men who have sex with men in Beijing]. China Tropical Medicine 10: 10–12. [Google Scholar]
- 123. Xue FH, Xia SZ, Lin SF, Jin YH, Zhu HS, et al. (2010) [Survey on high risk behaviors and HIV/STD infection status among men who have sex with men]. Disease Surveillance 25: 54–56. [Google Scholar]
- 124.Xu H, Hu W, Zhao HY (2009) [AIDS survey of MSM homosexuality population in Anshan]. Chinese Journal of Health Laboratory Technology 19: 658,713.
- 125. Shi WD, Li G, Yang T, Zhou W, Liu PL, et al. (2009) [Survey of High Risk Sexual Behaviors and HIV,Syphilis,HCV among 456 Male Homosexuals in Wuhan]. Medicine and Society 22: 42–43. [Google Scholar]
- 126. Wang CH, Yang YH, Lu GJ, Yang XX, Ge LR, et al. (2007) [Intervent male male sexual contact AIDS high dangerous behavior and evaluate effection of intervention]. Chinese Journal of Health Laboratory Technology 17: 2291–2292. [Google Scholar]
- 127. Sun ZX, Lin SF, Wen MQ (2007) [Investigation on STD and AIDS prevalence for men who have sex with men] Modern Preventive Medicine. 34: 4130–4132. [Google Scholar]
- 128. Sun ML, Li DJ, Jin W, Jiang J, Guan L (2009) [Investigation on the Infection of HIV, HCV, Syphilis and HBV among MSM in Dalian City in 2008]. Preventive Medicine Tribune 15: 1074–1075. [Google Scholar]
- 129. Ruan Y, Luo F, Jia Y, Li X, Li Q, et al. (2009) Risk factors for syphilis and prevalence of HIV, hepatitis B and C among men who have sex with men in Beijing, China: implications for HIV prevention. AIDS Behav 13: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ruan Y, Jia Y, Zhang X, Liang H, Li Q, et al. (2009) Incidence of HIV-1, syphilis, hepatitis B, and hepatitis C virus infections and predictors associated with retention in a 12-month follow-up study among men who have sex with men in Beijing, China. J Acquir Immune Defic Syndr 52: 604–610. [DOI] [PubMed] [Google Scholar]
- 131. Lu CG, Yuan F, Shi Z, Yang JZ, Li XY, et al. (2006) [The study of HIV infection and KABP about AIDS among the MSM in Guiyang city]. Guizhou Medical Journal 30: 202–204. [Google Scholar]
- 132. Lu CG, Yuan F, Shi ZH, Yang JZ, Li XY, et al. (2006) [HIV infection survey among MSM]. Chinese Journal of Public Health 2: 1320–1321. [Google Scholar]
- 133. Jiang J, Cao N, Zhang J, Xia Q, Gong X, et al. (2006) High prevalence of sexually transmitted diseases among men who have sex with men in Jiangsu Province, China. Sex Transm Dis 33: 118–123. [DOI] [PubMed] [Google Scholar]
- 134. Zhang B, Li X, Chu Q, Wang N, Wang Z, et al. (2008) [Correlation between AIDS and homosexuals: A study of 2046 male homosexuals in nine major cities of China]. The Chinese Journal of Human Sexuality 17: 6–10. [Google Scholar]
- 135. He Q, Wang Y, Lin P, Raymond HF, Li Y, et al. (2009) High prevalence of risk behaviour concurrent with links to other high-risk populations: a potentially explosive HIV epidemic among men who have sex with men in Guangzhou, China. Sex Transm Infect 85: 383–390. [DOI] [PubMed] [Google Scholar]
- 136. He Q, Wang Y, Lin P, Liu Y, Yang F, et al. (2006) Potential bridges for HIV infection to men who have sex with men in Guangzhou, China. AIDS Behav 10: S17–23. [DOI] [PubMed] [Google Scholar]
- 137. He Q, Wang Y, Lin P, Zhang Z-b, Zhao X-x, et al. (2005) [KAP study on AIDS among men who have sex with men in Guangzhou, Guangdong province]. Chinese Journal of Disease Control & Prevention 9: 106–108. [Google Scholar]
- 138. Gu Y, Qu P, Xu L, Luo M, Wang X, et al. (2004) [Survey of knowledge, attitude, behavior and practice related to STI/HIV among male homosexuality in Shenyang]. Chinese Journal of Public Health 20: 573–574. [Google Scholar]
- 139. Guo H, Wei JF, Yang H, Huan X, Tsui SK, et al. (2009) Rapidly increasing prevalence of HIV and syphilis and HIV-1 subtype characterization among men who have sex with men in Jiangsu, China. Sex Transm Dis 36: 120–125. [DOI] [PubMed] [Google Scholar]
- 140. Chen SH, Zhu JJ, Li J (2010) [Investigation on knowledge of HIV/AIDS and related behaviors character among men who have sex with men in Nanning, Guangxi]. Chinese Journal of Disease Control & Prevention 14: 130–133. [Google Scholar]
- 141. Chen SH, Zhu JQ, Yang NH (2010) [Investigation on HIV and STI Infections among Men Who Have Sex with Men in Nanning City during 2006–2008]. Occupation and Health 26: 56–58. [Google Scholar]
- 142. Chen G, Zheng JQ, Pan YJ, Zhang CY, Lin L (2010) [Serological Surveillance and Analysis among Men Having Sex with Men in a City of Fujian, China]. Strait Journal of Preventive Medicine 16: 13–16. [Google Scholar]
- 143. Cai GF, Ma QQ, Pan XH, Fu LJ, Xu WX, et al. (2008) [HIV/AIDS Related Knowledge, Attitude, Practice and HIV/STD Infection among MSM in Two Cities of Zhejiang Province]. China Preventive Medicine 9: 482–485. [Google Scholar]
- 144. Wang WH, Ma Y, Jiang SL, Yang Y, Zhang XX, et al. (2011) [Investigation on HIV and hepatitis C co-infection among men who have sex with men in Beijing]. Chinese Journal of Public Health 27: 624–625. [Google Scholar]
- 145. Yuan F, Yang JZ, Li XY, Lu ZG, Shi ZH, et al. (2009) [Investigation on HIV Infection among Many Boy (MB) and Analysis of Related Risk Behaviors]. Preventive Medicine Tribune 15: 791–793. [Google Scholar]
- 146. Zhou CX (2010) [Study on HIV infection among men who have sex with men in Zunyi city, Guizhou province]. Anhui Journal of Prevent Medicine 16: 211–212. [Google Scholar]
- 147. Zhou C, Ding XB, Feng L, Guo X, Han M, et al. (2011) [Study on the prevalence and associated factors of HIV and syphilis among 1166 men who have sex with men]. Modern Preventive Medicine 38: 815–820. [Google Scholar]
- 148. Zhou CX, Pan ZP, Chen ZY, Guo HJ (2011) [Results analysis of AIDS sentinel surveillance in Zunyi City, 2010]. Jiangsu Journal of Preventive Medicine 22: 11–13. [Google Scholar]
- 149. Zhao XH, Yang HW, He J, Jia SG, Yang H, et al. (2010) [Surveillance of AIDS Related Knowledge Awareness, Risk Behaviors and Biology Among Men Having Sex With Men in Mianyang]. Journal of Preventive Medicine Information 26: 876–878. [Google Scholar]
- 150. Zhang ZK, Wen XQ, Chen W, Jiang W, Zhou Y (2010) [Survey on HIV/AIDS high-risk behaviors among men having sex with men in Guilin]. Disease Surveillance 25: 213–215. [Google Scholar]
- 151. Zhang Y, Liu JW, Ni MJ, Dong YH (2008) [Laboratory Study on Detective Results of Male Homosexuality Population in Urumqi, Xinjiang]. Endemic Disease Bulletin 23: 26–28. [Google Scholar]
- 152.Zhang XY, Wang C, Li XX, Zhang XX, Song YH, et al.. (2006) [Study of HIV infection, co-infection with STDs and HCV and related changes in immunological indicators and viral loads among men who have sex with men in Beijing]. Chinese Journal of AIDS & STD 12: 294–296, 320.
- 153. Zhang M, Wang XD, Yang Y (2009) [Prevalence oh HIV, anti-HCV, syphilis infection and AIDS knowledge among men who have sex with men (MSM) in Urumqi]. Chinese Journal of Public Health 25: 1075–1076. [Google Scholar]
- 154. Zhang H, Chen CG, Lin FH, Xu SY, Yao X, et al. (2011) [Analysis of Comprehensive Surveillance Results of HIV/AIDS-related High-risk Groups in Fuzhou City in 2010]. Occupation and Health 27: 2406–2409. [Google Scholar]
- 155. Zhang DD, Zhang Y, Li HL, Wang JX, Zhang S, et al. (2010) [Investigation of HIV, Syphilis and HCV Infection Situation and Sexual Behavioral Characteristics among MSM in Ningbo City]. Zhejiang Preventive Medicine 22: 1–3. [Google Scholar]
- 156.Xu XY, Liu SY, Zhang JF (2011) [Analysis of AIDS sentinel surveillance in Huhhot in 2009]. Journal of Disease Monitor & Control 5: 393–394, 390.
- 157. Wu J, Ning Z, Fan HL, Yuan J (2011) Lin (2011) [Sentinel surveillance of sexually transmitted diseases among men who have sex with men in 2010]. Shanghai Journal of Preventive Medicine 23: 373–374. [Google Scholar]
- 158. Weng YQ, Bai Y (2009) [Surveillance on the high risk behaviors among 239 men who have sex with men]. Journal of Applied Preventive Medicine 15: 152–153. [Google Scholar]
- 159. Wang ZJ, Sun L, Ma XJ (2010) [Survey on AIDS/STD risk behaviors and prevalence among men who have sex with men in Guangling,Yangzhou]. Jiangsu Journal of Preventive Medicine 21: 4–7. [Google Scholar]
- 160. Wang Y, Zhang HB, Zhang GG, Yang HW, Fan J, et al. (2009) [Biological Monitoring and Social Characteristics Investigation of MSM Group in Mianyang City]. Practical Preventive Medicine 16: 375–377. [Google Scholar]
- 161. Wang X, Ma Y, Yang Y (2011) [Prevalence of HIV, syphilis and HCV among men who have sex with men in Urumqi city of Xinjiang Uyghur Autonomous Region]. Journal of Dermatology and Venereology 33: 104–105. [Google Scholar]
- 162. Wang T, Lai XH, Li L, Chen CY, He BH (2010) [Survey on AIDS/STD Risk Behaviors and Prevalence Among Men Who Have Sex with Men in Zhongshan, Guangdong]. Practical Preventive Medicine 17: 1261–1263. [Google Scholar]
- 163. Wang C, Liang HY, Yang Y, Zeng ZL, Li QC (2008) [A survey of HIV infections and related factors among men who have sex with men in Beijing]. Chinese Journal of AIDS & STD 14: 552–557. [Google Scholar]
- 164. Sun DY, Ma YM, Nie YG, Zhu Q, Wang Z (2010) [Study on HIV/HCV and syphilis infection among MSM in two cities]. Chinese journal of Practical Medicine 37: 10–12. [Google Scholar]
- 165.Shi JC, Lu QC, Zhao T (2010) [Analysis of the knowledge and infection on AIDS among the MSM in Nanyang City]. Henan J Prev Med 21: 405–406, 416.
- 166.Nie ZQ, Lin P, Li Y, Wang Y (2011) [Surveillance of AIDS high-risk people in Guangdong province, 2009]. J Trop Med 11: 29–31, 45.
- 167. Miao ZF, Li J, Lei LM, Han X, Zhang XP (2009) [Survey of AIDS-related Knowledge and Behavior in 312 MSM]. Journal of Ningxia Medical University 31: 761–762. [Google Scholar]
- 168. Miao XL, Cheng H, Zhang X, Gu J, Ji YY, et al. (2011) [Analysis on HIV/AIDS Sentinel Surveillance in Wuxi City in 2010]. Occupation and Health 27: 2599–2601. [Google Scholar]
- 169. Meng X, Xie CM, Zheng XP (2010) [Investigation on AIDS and STD Affection in MSM of Changde]. Journal of Tropical Medicine 10: 884–885. [Google Scholar]
- 170. Mei L, Han H, Che XW, Bai SM, Yang YL, et al. (2009) [A survey of the first round AIDS integrate controlling work trial spot among men who have sex with man in Taiyuan]. Chinese Journal of Epidemiology 30: 649–650. [PubMed] [Google Scholar]
- 171. Mao FY (2010) [Survey on HIV/STI high risk behaviors among 211 men who have sex with men in Yichang city, Huebi province]. World Health Digest 7: 246–247. [Google Scholar]
- 172. Ma GL, Shen LT, Su CH, Zheng HN, Lin BY, et al. (2010) [Effect evaluation of HIV/AIDS integrated intervention of MSM in Xiamen City]. Chinese Journal of Disease Control and Prevention 14: 726–728. [Google Scholar]
- 173. Lu L, Xu D, Qiu HH, Yan H, Chen RC, et al. (2011) [Investigation on STDs/AIDS infection among MSM in Nanchang]. Journal of Public Health and Preventive Medicine 22: 45–46. [Google Scholar]
- 174. Liu YY, Tian B, Song WW (2010) [Study on the prevalence of HIV, HCV and syphilis and demographic characteristics among 507 men who have sex with men]. Practical Preventive Medicine 17: 788–790. [Google Scholar]
- 175. Liu PL, Yao ZZ, Shi WD, Ding J, Li SL, et al. (2010) [Epidemiological study on the status of HIV/STD among MSM in Wuhan City]. Chinese Journal of Disease Control and Prevention 14: 917–919. [Google Scholar]
- 176. Liang L, Chen ZQ, Miao XF, Li Bj, Bai GY, et al. (2009) [An investigation of HIV infections among men who have sex with men]. Hebei Medical Journal 31: 1991–1992. [Google Scholar]
- 177. Lan GH, Liu W, Zhu QY, Liang FX, Zhou Y (2009) [To analyze the surveillance results of immigrant MSM in Guangxi]. Applied Prev Med 15: 168–170. [Google Scholar]
- 178.Huang HY, Zhang XP, Li Y, Hu ZW, Li ZR (2011) [Study on the HIV, syphilis and hepatitis C infections and sexual behavior characteristics among men who have sex with men in Hefei]. Modern Preventive Medicine 38: 1933–1935, 1938.
- 179. Hu JF, Wang YN, Li J (2011) [Study on HIV, syphilis and HCV infection and high risk behaviors among men who have sex with men in Donghu, Jiangxi Province]. World Health Digest 8: 68–69. [Google Scholar]
- 180. He H, Zhang D, Zhou L, Ye P, Bai Z, et al. (2011) [Behavioral characters & practice of behavior intervention among 135 MSM in Yueyang]. Practical Preventive Medicine 18: 1151–1153. [Google Scholar]
- 181. Han X, Zhang XP, Li J, Lei LM, Miao ZF (2012) [Investigation on high-risk behaviour and Sexually transmissible infections among men who have sex with men in Yinchuan city of Ningxia province in 2010]. Ningxia Medical Journal 34: 69–70. [Google Scholar]
- 182. Gong CT, Zhang QH (2010) [Results of AIDS monitoring of 252 MSM in Quanzhou City]. China Tropical Medicine 10: 1496–1497. [Google Scholar]
- 183. Feng LG, Ding XB, Xu J, Ou YL, Xu SM, et al. (2010) [Study on HIV, Syphilis and HCV Prevalence and Its Assoicated Factors among Internet MSM Comparison to Non-Internet MSM in Chongqing]. Journal of Tropical Medicine 10: 78–82. [Google Scholar]
- 184. Feng JF, Lin HJ, Qiu DH, Zhang YF, Wu QH (2008) [Study on infection rate of HIV,syphilis and HCV among men who have sex with men in Taizhou]. Chinese Journal of Health Laboratory Technology 18: 1885–1886. [Google Scholar]
- 185.Feng F, Wang ZQ, Huang SP, Lu JG, Lin ZW, et al.. (2009) [Investigation on aids knowledge, attitude and practice characteristics of MSM group and HIV/syphilis infection situation]. Modern Preventive Medicine 36: 2902–2903, 2909.
- 186.Chen Z, Chen XM, Huang LH, Lu MJ, You YT, et al.. (2011) [Sentinel surveillance among men who have sex with men in Dali prefecture of Yunnan province in 2010]. Soft Science of Health 25: 719–720, 728.
- 187. Cai X (2007) [The Analysis of Risk Behaviors of MSM in Liaocheng and the Serological Detection for Anti-HIV, Anti-TP, and Anti-HCV Antibodies in 2006]. Preventive Medicine Tribune 13: 888–890. [Google Scholar]
- 188. Bai Y, Feng WD, Wei QH (2009) [Survey of infectious status of HIV,HCV and syphilis in MSM in Liuzhou in 2007–2008]. China Tropical Medicine 9: 2275–2276. [Google Scholar]
- 189. Ding XB, Feng LG, Xu J, Xu SM, Guo Xj, et al. (2010) [Study on the prevalence of HIV, Syphilis, HCV and HSV-II and its associated factors among 743 men who have sex with men in Chongqing]. Chinese Journal of Disease Control & Prevention 14: 227–231. [Google Scholar]
- 190. Shi XD, Liu SC, Zhao J, Cai WD, Wang XH, et al. (2010) [Comparisons of sexual behavior and infection of HIV and syphilis among men who have sex with men in Shenzhen, Guangdong]. South China Journal of Preventive Medicine 36: 22–25. [Google Scholar]
- 191. Chen XM, Fu G, Xu X (2011) [Study on gonococcal and chlamydial infections among men who have sex with men in Nanjing]. Acta Universitatis Medicinalis Anhui 46: 569–572. [Google Scholar]
- 192. Lu HY, Ma XY, Liu YC, Zhang QY, Hei FX, et al. (2008) [A survey of HIV/STDs prevalence in 200 MSM and related factors in Beijing]. Chinese Journal of AIDS & STD 14: 467–470. [Google Scholar]
- 193. Huan XP, Yun YP, Fu GF, Jiang N, Zhang QQ, et al. (2011) [Analysis on sexually transmitted diseases and the related risk factors among men who have sex with men in Jiangsu province]. Chinese Journal of Preventive Medicine 45: 975–978. [PubMed] [Google Scholar]
- 194. Wang HL, Zhang M, Hu QH, Ding HB, Zhao B (2008) [Prevalence of HIV/STD and risk behavior among men who have sex with men in Shenyang]. Chinese Journal of Public Health 24: 995–997. [Google Scholar]
- 195. Qi SZ, Wang QQ, Zhang GC, Cao NX, Shi MQ, et al. (2006) [Urine screening of chlamydial and gonococcal infections and associated risk factors in male sex workers]. China Journal of Leprosy and Skin Diseases 22: 819–821. [Google Scholar]
- 196. Liu Y, Jiang S, Hu Y, Song L, Yu M, et al. (2011) [Characteristics of sexual behaviors and infection status of AIDS and other sexually transmitted diseases among men who have sex with men in 2009 in Beijing]. Zhonghua Yu Fang Yi Xue Za Zhi 45: 971–974. [PubMed] [Google Scholar]
- 197. Wang JX, Dong W, Li R, Zhao Z, Xiong B, et al. (2004) [A survey of HIV/STD infection and behaviors among MSM]. Chinese Journal of Public Health 20: 1377–1378. [Google Scholar]
- 198. Gao L, Zhou F, Li X, Yang Y, Ruan Y, et al. (2010) Anal HPV Infection in HIV-Positive Men Who Have Sex with Men from China. PLoS One 5: e15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Chen XX, Yu JP, Li M, Su QX (2011) [Survey on infection of HPV and HIV among men who have sex with men in Beijing]. International Journal of Virology 18: 101–105. [Google Scholar]
- 200. Feng Y, Wu Z, Detels R, Qin G, Liu L, et al. (2010) HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection. J Acquir Immune Defic Syndr 53 Suppl 1 S74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cai WD, Fen TJ, Tan JQ, Chen L, Shi XD, et al. (2005) [A Survery Of The Characteristics And STD/HIV Infection Of Homosexuality In Shenzhen]. Modern Preventive Medicine 32: 328–330. [Google Scholar]
- 202. Liao MZ, Liu XZ, Fu JH, Qian YS, Zhang XF (2006) [Analysis of data of behavioral surveillance in men who have sex with men(MSM) in Shandong Province]. Chinese Journal of AIDS & STD 12: 530–532. [Google Scholar]
- 203. Liu S, Zhao J, Rou K, Chen L, Cai W, et al. (2011) A Survey of Condom Use Behaviors and HIV/STI Prevalence Among Venue-Based Money Boys in Shenzhen, China. AIDS Behav 16: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qi SZ, Wang QQ, Zhang GC, Cao NX, Zhang JP, et al.. (2006) [Serum and urine testing for HIV/STDs prevalence and analyses of risking factors among male sex workers]. The 3rd National prevention and control on sexually transmitted disease conference. Shenzhen. 176–179.
- 205. Chen SH, Zhou J, Zhu JJ (2007) [Investigation of STI among some Men Who Have Sex with Men in Nanning City in 2006]. Preventive Medicine Tribune 13: 772–774. [Google Scholar]
- 206. Parish WL, Laumann EO, Cohen MS, Pan S, Zheng H, et al. (2003) Population-based study of chlamydial infection in China: a hidden epidemic. JAMA 289: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 207. Zhang GF (2004) [The prevalence and risk factors of urogenital chlamydia trachomatis infection in Huaibei, Anhui]. Chinese Journal of Disease Control & Prevention 8: 433–434. [Google Scholar]
- 208. Wang W, Wei C, Buchholz ME, Martin MC, Smith BD, et al. (2010) Prevalence and risks for sexually transmitted infections among a national sample of migrants versus non-migrants in China. Int J STD AIDS 21: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Xiao HW, Cao JG, Li JA, Huang HJ, Zhang WL, et al. (2007) [Prevalence of HIV, HBV and HCV among drug users and general population]. Chinese Medical Science & Health 6: 7–8. [Google Scholar]
- 210. Yu QL, Yang Y, Liu R, Chun ZM, Di L, et al. (2007) [Investigation on HBV, syphilis and HIV infection among high-risk populations in Dunhuang city of Gansu province]. Strait Journal of Preventive Medicine 13: 45. [Google Scholar]
- 211. Luo Z, Xie Y, Deng M, Zhou X, Ruan B (2011) Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol 23: 695–700. [DOI] [PubMed] [Google Scholar]
- 212. Dai S, Shen Z, Zha Z, Leng R, Qin W, et al. (2012) Seroprevalence of HIV, syphilis, and hepatitis C virus in the general population of the Liangshan Prefecture, Sichuan Province, China. J Med Virol 84: 1–5. [DOI] [PubMed] [Google Scholar]
- 213. Hesketh T, Tang F, Wang ZB, Huang XM, Williams D, et al. (2005) HIV and syphilis in young Chinese adults: implications for spread. Int J STD AIDS 16: 262–266. [DOI] [PubMed] [Google Scholar]
- 214. Hesketh T, Ye XJ, Zhu WX (2008) Syphilis in China: the great comeback. Emerging Health Threats Journal 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of hepatitis B virus infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of hepatitis C virus infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Temporal trends of HBV and HCV prevalence among MSM in China.
(PDF)
Prevalence of chlamydia among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of gonorrhoea among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of HPV infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of HSV-2 infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of genital wart ( Condyloma acuminatum ) infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Prevalence of Ureaplasma urealyticum infection among Chinese MSM. Forest plots showing unadjusted prevalence estimates (squares) with 95% confidence intervals (lines). Pooled prevalence estimate is presented as rhombus in this plot.
(PDF)
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
(PDF)
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
(PDF)
Systematic review of 87studies reporting the prevalence of sexually transmitted infections and/or viral hepatitis infections among men who have sex with men in China.
(DOC)
Systematic review of 14 studies reporting the co-infection prevalence of sexually transmitted infections and/or viral hepatitis infections among HIV-positive men who have sex with men in China.
(DOC)
Publication bias and heterogeneity in subgroup meta-analyses.
(DOC)
PRISMA checklist.
(DOC)