Abstract
Injection of human pathogenic bacteria (Pseudomonas aeruginosa, Serratia marcescens, Salmonella enterica, Staphylococcus aureus, and Listeria monocytogenes) into the hemocoel of honeybee (Apis mellifera L.) workers kills the infected bees. The bee-killing effects of the pathogens were affected by temperature, and the LD50 values at 37°C were more than 100-fold lower than those at 15°C. Gene-disrupted S. aureus mutants of virulence genes such as agrA, saeS, arlR, srtA, hla, and hlb had attenuated bee-killing ability. Nurse bees were less susceptible than foragers and drones to S. aureus infection. Injection of antibiotics clinically used for humans had therapeutic effects against S. aureus infections of bees, and the ED50 values of these antibiotics were comparable with those determined in mammalian models. Moreover, the effectiveness of orally administered antibiotics was consistent between honeybees and mammals. These findings suggest that the honeybee could be a useful model for assessing the pathogenesis of human-infecting bacteria and the effectiveness of antibiotics.
Introduction
Bacterial infections represent one of the greatest threats to human health, and overcoming infectious diseases is a clinically important issue. The recent emergence of multi-drug resistant pathogens further increases the mortality and morbidity of bacterial infection [1]. Therapeutic effects of antibiotics against bacterial infections are defined not only by the antimicrobial mechanisms but also by the pharmacokinetics of the drugs in the host body [2]. Thus, in addition to ordinary in vitro screenings for antimicrobial substances, evaluations of therapeutic effects using whole animals is essential for the discovery of novel antibiotics that will benefit infected human patients. For in vivo assessment of candidate chemicals, mammals such as mice and rats have long been used as model animals. Over the last several years, however, infection experiments using a large number of mammals have been difficult to perform due to the high costs involved and the ethical issues regarding animal welfare [3]. Therefore, overcoming these problems by establishing novel lower animal models of in vivo bacterial infections has become an important goal.
Insects have many advantages as an infection model, such as low cost, few ethical issues, and ease of handling in pharmaceutical and infection experiments. We previously demonstrated that silkworms, Bombyx mori, are killed by injection of human pathogens into the bloodstream [4]–[9]. The silkworm infection model is useful for assessing the contribution of virulence factors, such as the two-component system regulators [10]–[14] and cytotoxins [12], to bacterial pathogenesis [5], [15]. We identified novel virulence genes conserved among several pathogenic bacteria using the silkworm as a host animal for screening [5]. Moreover, silkworms infected with bacteria are efficiently cured by administration of the same antibiotics used clinically to treat human patients [16], [17]. The amounts of antibiotics per animal weight required to prevent death (ED50) are comparable between infected silkworms and mammals [16], [17]. Moreover, basic mechanisms of the innate immune systems are conserved between vertebrates and insects [18], and we have identified a novel innate immune pathway involving the activation of an insect cytokine paralytic peptide in silkworms [19]–[21]. Thus, we consider that insects, including silkworms, are useful as alternative animal models for examining the therapeutic potential of drug candidates against human infectious microbes.
The honeybee, A. mellifera L., is a social insect that lives in a colony comprising individuals with varying tasks. In particular, worker honeybees shift their labors from nursing the blood by secreting royal jelly to guarding the hives and then to foraging for nectar and pollen according to their age after eclosion (i.e., the emergence of an adult insect from the pupal case) [22]. Workers exhibit grooming, removal of mites from the body surfaces, and cleaning dead bees and feces inside the nests, which are important tasks for maintaining the hygienic environment [23]. On the other hand, although honeybees possess a battery of innate immune pathways similar to those found in B. mori and Drosophila melanogaster, the number of immune-related genes in the honeybee genome is lower than that in other insects [24]. Thus, the development of hygienic behaviors seems to be evolutionarily important for honeybees given the limited repertoire of immune factors within each individual [24]. Although silkworms and flies have been established as infection models, there are no previous reports describing the use of honeybees for quantitative assessment of pathogenesis of human-infecting pathogens and the effects of antibiotics. Taking advantage of well-established rearing methods of bees that enable us to easily obtain large numbers of animals, here we describe the establishment of an experimental system of bacterial infection and antibiotic treatment using the honeybee.
Materials and Methods
Insects, Bacteria, and Reagents
European honeybees, Apis mellifera L., were purchased from a local distributer (Kumagaya Honeybee Farm, Saitama, Japan) and maintained at the University of Tokyo as previously reported [25]. Beehives were placed outside at a temperature ranging from 25∼35°C in summer and 0∼10°C in winter. Nurse bees, foragers, and drones were selected as previously described [26].
Escherichia coli strain KP7600, Pseudomonas aeruginosa strain PAO1, Salmonella enterica serovar Typhimurium strain ATCC 14028s, Staphylococcus aureus strain Newman, and Listeria monocytogenes strain 104035 were grown in LB10 medium at 37°C for 12 to 18 h. Serratia marcescens strain 2170 was grown in LB10 medium at 30°C for 12 to 18 h. S. aureus disruption mutants of parent strain NCTC8325-4 were constructed as previously described [15]. Bacterial cultures were centrifuged and the cells were suspended in saline (0.9% NaCl).
Vancomycin hydrochloride, gentamycin sulfate, and kanamycin sulfate were purchased from Wako and stock solutions were dissolved in Milli-Q water. Tetracycline was purchased from Sigma and dissolved in dimethyl sulfoxide. Teicoplanin was purchased from Hoechst Marion Roussel and dissolved in dimethyl sulfoxide. All reagents were diluted in saline before use.
Infection Experiments
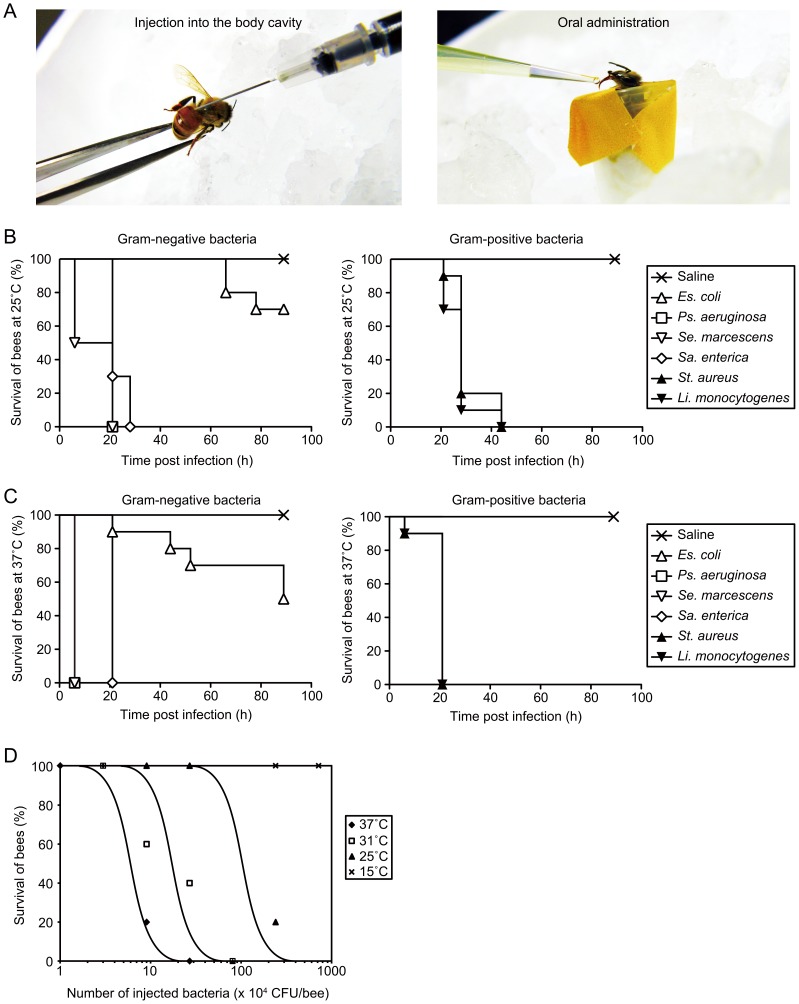
In time-course assays, overnight bacterial cultures were diluted with saline by 100- or 200-fold, and the OD600 value was adjusted to 0.05∼0.10. Two-fold serial dilutions of bacterial cultures were prepared to determine the LD50 values. For injection experiments, bees anesthetized on ice were injected with 25 µl of each sample into the abdomen using a 1-ml syringe equipped with a 27-gauge needle (Terumo). For oral administration, bees anesthetized on ice were placed in 1.5-ml tubes and fixed with paper tape (Fig. 1A). Bees were recovered from anesthesia at room temperature and fed 10 µl of each sample containing 1 mol l−1 sucrose and antibiotics at various concentrations using a micropipette (Fig. 1A; [26]). Treated bees were maintained in a plastic dish and fed with 1.4 mol l−1 sucrose-soaked cotton at either 15°C, 25°C, 31°C, or 37°C. Infected honeybees were judged to be dead if no movement was observed in any parts of the body, antennae, or legs when touched by pipette tips. The LD50 values of bees at 24 h after infection were determined using Reed-Muench method, as previously described [8]. Survival data were plotted using Prism 5 (GraphPad Software, Inc.), and statistically significant differences between the survival curves were analyzed by the log-rank test.
Figure 1. Establishment of a bacterial infection model in the honeybee.
A, Images of sample injection into the body cavities of bees (left) and oral administration (right). In the left panel, bees anesthetized on ice were injected into the abdomen with 25 µl of each sample using a 1-ml syringe. In the right panel, anesthetized bees were placed in 1.5-ml tubes and fixed with paper tape. Ten microliters of sample containing sucrose was directly administered to bees extending the proboscis. B–C, Survival of in-hive worker bees (n = 20) after infection with Gram-negative (left) and Gram-positive (right) bacteria. Overnight culture of each bacterium was diluted 100-fold with saline. Survival numbers of bees were monitored at 25°C (B) or 37°C (C). The data shown are representative of three experiments with similar results. D, Effect of incubation temperature on survival of in-hive worker bees after S. aureus infection. LD50 values were determined 24 h after injection with a live bacterial suspension of S. aureus (n = 5).
Results
Killing of Honeybees by Injection of Human Pathogenic Bacteria
We first examined whether various bacteria known to be pathogenic against humans kill honeybees. Injection of the bacteria into the hemocoel of bees seems to mimic the pathology of sepsis in humans. Each suspension of Gram-negative (Pseudomonas aeruginosa, Serratia marcescens, and Salmonella enterica) and Gram-positive bacteria (Staphylococcus aureus and Listeria monocytogenes) was injected into the hemocoel of nurse bees anesthetized on ice (Fig. 1A). After the bees were transferred and maintained in plastic cages at either 25°C or 37°C, survival of the bees was monitored. Bees infected with either of the five pathogenic bacteria were killed within 2 days (Fig. 1B, C). In contrast, it took more than 3 days to kill 50% of bees infected with an avirulent laboratory strain of E. coli (KP7600) (Fig. 1B, C). Moreover, the amount of incubation time required for killing bees by infection with either of the five pathogens was lower at 37°C than at 25°C (Fig. 1B, C). We further examined the effect of temperature on killing of bees by S. aureus infection at 15°C, 25°C, 31°C, and 37°C. The LD50 value of S. aureus at 25°C was 5×105 colony forming unit (CFU)/bee, whereas that at 31°C and 37°C was 2×105 CFU/bee and 5×104 CFU/bee, respectively (Fig. 1C). Bees incubated at 15°C were not killed even when infected with 8×106 CFU/bee of S. aureus (Fig. 1C). Based on these results, we concluded that the LD50 values of S. aureus against honeybees decreased in response to an increase in temperature.
Assessment of Pathogenesis of S. aureus Mutant Strains of Virulence Genes Using the Honeybee Infection Model
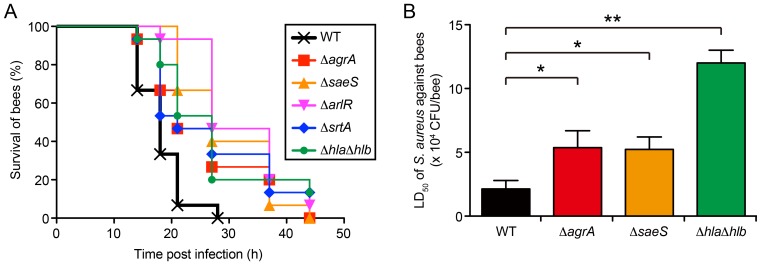
We tested the killing ability of S. aureus gene-disrupted mutants of several virulence genes in the honeybee infection model. We focused on major virulence factors required for pathogenesis in mammals; the two-component systems that globally regulate virulence gene expression (Agr [11], [12], Sae [13], and Arl [10], [14]), the cell-surface modifier sortase A (encoded by the srtA gene) [27], and extracellular hemolysins (Hla and Hlb) [12]. S. aureus mutants of the agrA, saeS, arlR, srtA genes, and a double mutant of the hemoylsin genes (ΔhlaΔhlb) showed delayed killing of honeybees compared with the parent strain (Fig. 2A). Moreover, we demonstrated that LD50 values of ΔagrA, ΔsaeS, and ΔhlaΔhlb strains increased by 3- to 6-fold (Fig. 2B). These results suggest that the honeybee model is suitable for assessing the contribution of virulence genes in bacterial pathogenesis.
Figure 2. Killing of honeybees by S. aureus gene-disrupted mutants of virulence genes.
A, Time-course of honeybee killing by S. aureus gene-disrupted mutants. Overnight culture of S. aureus NCTC8325-4 strain (WT; wild-type) or mutants (ΔagrA, ΔsaeS, ΔarlR, ΔsrtA, and ΔhlaΔhlb) was diluted 200-fold with saline. Survival of in-hive worker bees (n = 15) after infection with S. aureus mutants was monitored at 25°C. Statistical differences between the wild-type and each mutant were determined to be significant (p<0.05) by log-rank test. B, Determination of LD50 values of S. aureus mutants against honeybees. Survival of in-hive worker bees (n = 15) after 24 h of infection with S. aureus wild-type (WT) strain or mutants (ΔagrA, ΔsaeS, and ΔhlaΔhlb) was monitored at 25°C, and the LD50 values were calculated. Data represent mean ± SDs of three experiments. Statistical analysis was performed by one-way ANOVA, and differences compared with the wild-type (WT) strain-infected group were analyzed by Dunnett’s multiple comparison tests (*, p<0.05; **, p<0.005).
Bacterial Killing of Honeybees According to Division of Labor
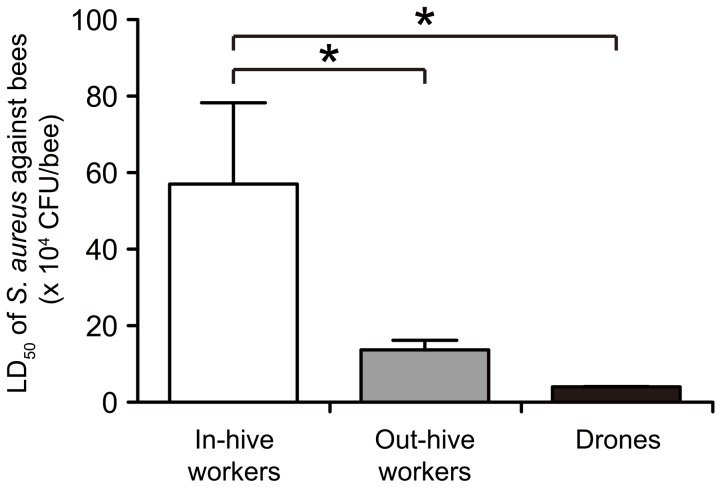
Honeybee colonies comprise female workers engaged in different labors such as nurse bees, foragers, queens, and drones [22]. The variations in labors may lead to the emergence of differences in pathogen types that bees contact, as well as the immune reactiveness of infected bees. We tested the difference in host resistance against bacterial infections among the division of labors. The LD50 values of S. aureus were determined 24 h after injection into the hemocoel of either nurse bees, foragers, or drones. The LD50 value was much higher in nurse bees than in foragers and drones (Fig. 3). The high resistance to pathogens observed in nurse bees is reasonable for avoiding group infection within the nests. On the other hand, worker bees foraging outside the hives exhibit attenuated host resistance, possibly due to senescence (i.e., old age) and alterations in their physiologic state.
Figure 3. Comparison of susceptibilities to bacterial infection between honeybees in different labor divisions.
LD50 values of S. aureus were determined 24 h after infection in either in-hive workers, out-hive workers, or drones (n = 5–8). Data represent mean ± SDs of three experiments. Statistical analysis was performed by one-way ANOVA, and differences compared with the in-hive workers group were analyzed by Dunnett’s multiple comparison tests (*, p<0.05).
Therapeutic Effects of Antibiotics against Bacterial Infection in the Honeybee
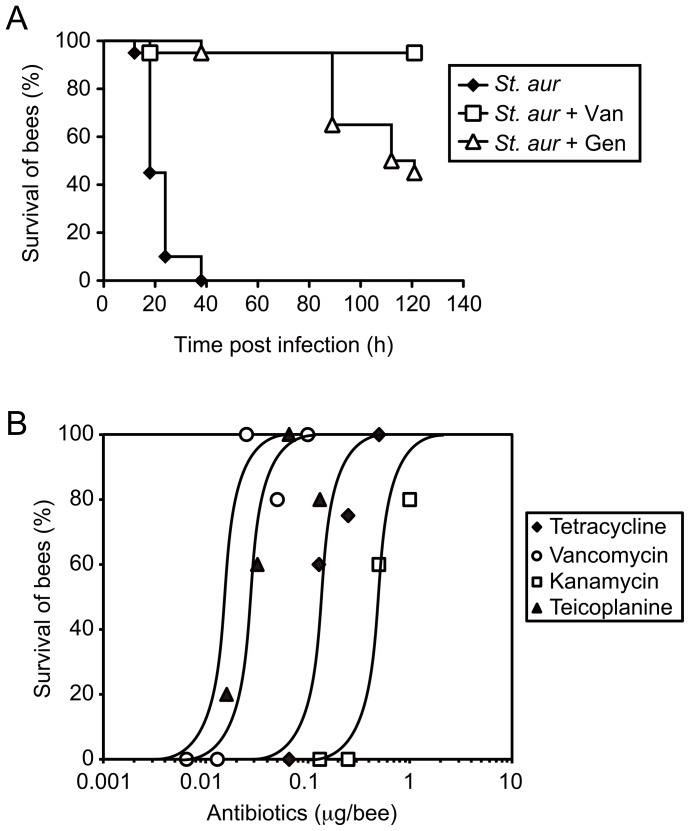
We next tested if the above infection model using the honeybee as a host animal would be useful for in vivo evaluation of the therapeutic effects of antibiotics against pathogens. While more than half of the in-hive worker bees infected with S. aureus died within 24 h, bees co-injected with S. aureus and clinically effective antibiotics such as vancomycin or gentamycin lived longer than the control group (Fig. 4A). Various antibiotics, such as tetracycline, vancomycin, kanamycin, and teicoplanin showed therapeutic effects against S. aureus infection in honeybees in a dose-dependent manner (Fig. 4B), and the ED50 values were determined (Table 1). We previously examined the therapeutic effects of sev show effects by oral administration and injection ineral antibiotics in a silkworm infection model and demonstrated that the pharmacokinetics parameters of the drugs in silkworms were similar to those reported in mammals [16], [17]. The above ED50 values determined in the honeybee infection model were comparable with those reported in other models using silkworms and mice (Table 1, [16]). We then examined the effects of antibiotics administered orally in the honeybee-S. aureus infection model. Antibiotics with large molecular masses, such as vancomycin and kanamycin, are poorly absorbed from the intestines and therefore do not show therapeutic effects by oral treatment in mammals. We previously reported that these antibiotics failed toto the midgut of silkworms [16]. Here we demonstrated that tetracycline, which is efficiently absorbed from the digestive tract in silkworms and mammals [16], delayed the killing of bees by oral administration, whereas vancomycin and kanamycin failed to show therapeutic, effects even at doses as high as 330 µg/insect (Table 1). This finding is in good agreement with previous reports of the relationship between the enteric absorption and effectiveness of antibiotics in other model animals. Thus, we concluded that the honeybee infection model was suitable for in vivo assessment of therapeutic effects of antibiotics reflecting the pharmacokinetic aspects.
Figure 4. Therapeutic effects of antibiotics against bacterial infection in honeybees.
A, Effect of co-injection of vancomycin or gentamycin on survival of in-hive worker bees infected with S. aureus (n = 20). B, Dose-dependency of therapeutic effects of antibiotics on S. aureus infection in in-hive worker bees. Survival numbers were determined 24 h after infection (n = 5).
Table 1. ED50 values of antibiotics in the honeybee S. aureus infection model.
| Antibiotics | A. mellifera L. | A. mellifera L. | B. mori a | B. mori a |
| i.c. | p.o. | i.h. | p.o. | |
| Tetracycline | 0.2b | 2 | 0.4 | 8 |
| Vancomycin | 0.02 | >330 | 0.3 | >400 |
| Kanamycin | 0.4 | >330 | 3 | >500 |
ED50 values of tetracycline, vancomycin, and kanamycin against S. aureus infection in in-hive worker bees (n = 5) were determined 24 h after infection. Antibiotics were injected into the cavities (i.c.) or orally administered (p.o.), and live bacterial suspension of S. aureus was injected immediately after antibiotic treatment.
ED50 values of B. mori were previously reported [16].
µg/insect.
Discussion
Temperature is a critical factor that affects both the infection processes of pathogens and the physiologic state of the infected hosts. In D. melanogaster, the mortality of flies infected with P. aeruginosa and Lactococcus lactis is higher at 29°C than at 17°C [28]. In addition, the mRNA levels of antimicrobial peptides (AMP) in infected flies are higher at a low temperature, supporting the findings from infection experiments [28]. In the present study, we found that the susceptibility of honeybees against S. aureus was increased by an upshift in the incubation temperature. Under high temperature conditions, the effects of both the promotion of bacterial growth and the suppression of innate immune responses in bees seem to result in attenuated host resistance against bacterial infections, consistent with previous reports using other insect models such as D. melanogaster [28].
In our experimental conditions, host resistance against S. aureus infection was higher in nurse bees than in foragers and drones. The labors of worker honeybees change from nursing to foraging, dependent on their age [22]. Recently, Laughton et al. [29] reported that lipopolysaccharide challenge-dependent AMP production levels in old-aged foragers were lower than those in young bees. They also demonstrated that the AMP levels were lower in drones than in foragers at any age [29]. Moreover, both the induction levels of melanization, another humoral immune response in insects, and the ability of hemocytes to encapsulate foreign substances significantly decrease with age in worker bumblebees [30]. Although the direct cause of immunosenescence in old-aged foragers and drones was not determined, the authors of the above reports speculated that a trade-off between immune responses and energy costs for foraging behaviors and sperm production might explain the differences. Based on both our results and previous reports, we considered that an attenuated ability to induce innate immune responses such as AMP production and encapsulation after infection in old-aged foragers and drones lowers the resistance to pathogenic bacteria.
Recent reports demonstrated that honeybees possess immune systems, such as humoral and cellular immunity, similar to those found in other insects [24], [29], [31]–[33]. Basic reactions and pathways of innate immunity are conserved among species, whereas some of the repertoires differ between honeybees and other insects [24], [34]. Under our experimental conditions, S. aureus mutants of the two-component system regulators (agr, sae, and arl), sortase A (srtA) which is required for cell wall-protein anchoring, and hemolysins (hla and hlb) showed attenuated killing activity against honeybees. The critical roles of these genes for S. aureus pathogenesis are reported in various mammalian infection models [10]–[14], [27]. In addition, the findings in honeybee are consistent with those in other invertebrate infection models; the two-component system regulators Agr and Sae are required for virulence in both silkworm [15] and Caenorhabditis elegans [35], [36], and Hla contributes to host-killing in C. elegans [35], [36]. Our results showing consistency between honeybees and other models regarding the susceptibility to pathogens and the therapeutic effects of antibiotics seem to reflect the similarity in innate immunity among insects.
Social animals, including humans, are able to suppress the spread of pathogens among group members via social behaviors. In rats, for example, individuals whose immune systems are activated by microbial stimulants tend to be isolated or attacked by other healthy members within the group [37], [38]. Honeybee nestmates display aggressive behaviors toward infected individuals to eliminate them from the colony [39]. These behavioral alterations are thought to be induced by signals transmitted from infected individuals to the other members; the underlying mechanisms, however, have yet to be clarified. In the present study, we examined the susceptibility of isolated individual bees, but further studies are needed to examine the alterations in immune responses and hygiene behaviors in grouped colonies. To date, studies of infectious diseases using honeybees have focused mainly on diseases caused by pathogens that naturally infect bees, such as Paenibacillus larvae and Nosema [40]–[42]. In the present study, we established a quantitative model in honeybees for assessing bacterial infections and the therapeutic effects of antibiotics. In addition to their advantageous features as an infection model similar to other non-social insects, honeybees may provide a useful model in future studies of the relationship between infection and social behaviors.
Acknowledgments
We are grateful to Dr. Takeo Kubo and Dr. Hideaki Takeuchi (University of Tokyo) for their helpful suggestions and their critical reading of the manuscript.
Funding Statement
This work was supported by JSPS KAKENHI grant number 24-11042 (Grant-in-Aid for JSPS Fellows to KI) and 24689008 (Grant-in-Aid for Young Scientists (A) to HH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Master RN, Deane J, Opiela C, Sahm DF (2013) Recent trends in resistance to cell envelope-active antibacterial agents among key bacterial pathogens. Ann N Y Acad Sci 1277: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Andes D, Craig WA (2002) Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19: 261–268. [DOI] [PubMed] [Google Scholar]
- 3. Baumans V (2004) Use of animals in experimental research: an ethical dilemma? Gene Ther 11 Suppl 1S64–66. [DOI] [PubMed] [Google Scholar]
- 4. Kaito C, Akimitsu N, Watanabe H, Sekimizu K (2002) Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb Pathog 32: 183–190. [DOI] [PubMed] [Google Scholar]
- 5. Kaito C, Kurokawa K, Matsumoto Y, Terao Y, Kawabata S, et al. (2005) Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol Microbiol 56: 934–944. [DOI] [PubMed] [Google Scholar]
- 6. Kaito C, Usui K, Kyuma T, Sekimizu K (2011) Isolation of mammalian pathogenic bacteria using silkworms. Drug Discov Ther 5: 66–70. [DOI] [PubMed] [Google Scholar]
- 7. Ishii K, Hamamoto H, Imamura K, Adachi T, Shoji M, et al. (2010) Porphyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J Biol Chem 285: 33338–33347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishii K, Adachi T, Imamura K, Takano S, Usui K, et al. (2012) Serratia marcescens induces apoptotic cell death in host immune cells via a lipopolysaccharide- and flagella-dependent mechanism. J Biol Chem 287: 36582–36592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii K, Adachi T, Hamamoto H, Sekimizu K (2014) Serratia marcescens Suppresses Host Cellular Immunity via the Production of an Adhesion-Inhibitory Factor Against Immunosurveillance Cells. J Biol Chem in press. [DOI] [PMC free article] [PubMed]
- 10. Benton BM, Zhang JP, Bond S, Pope C, Christian T, et al. (2004) Large-scale identification of genes required for full virulence of Staphylococcus aureus. J Bacteriol 186: 8478–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heyer G, Saba S, Adamo R, Rush W, Soong G, et al. (2002) Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect Immun 70: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, et al. (1997) Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun 65: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang X, Yu C, Sun J, Liu H, Landwehr C, et al. (2006) Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infect Immun 74: 4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, et al. (2005) Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J Bacteriol 187: 5486–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyazaki S, Matsumoto Y, Sekimizu K, Kaito C (2012) Evaluation of Staphylococcus aureus virulence factors using a silkworm model. FEMS Microbiol Lett 326: 116–124. [DOI] [PubMed] [Google Scholar]
- 16. Hamamoto H, Kurokawa K, Kaito C, Kamura K, Manitra Razanajatovo I, et al. (2004) Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob Agents Chemother 48: 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamamoto H, Sekimizu K (2005) Evaluation of the therapeutic effects of antibiotics using silkworm as an animal model. Res Adv Antimicrob Agents Chemother 5: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann JA, Reichhart JM (2002) Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3: 121–126. [DOI] [PubMed] [Google Scholar]
- 19. Ishii K, Adachi T, Hamamoto H, Oonishi T, Kamimura M, et al. (2013) Insect cytokine paralytic peptide activates innate immunity via nitric oxide production in the silkworm Bombyx mori. Dev Comp Immunol 39: 147–153. [DOI] [PubMed] [Google Scholar]
- 20. Ishii K, Hamamoto H, Kamimura M, Nakamura Y, Noda H, et al. (2010) Insect cytokine paralytic peptide (PP) induces cellular and humoral immune responses in the silkworm Bombyx mori. J Biol Chem 285: 28635–28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishii K, Hamamoto H, Kamimura M, Sekimizu K (2008) Activation of the silkworm cytokine by bacterial and fungal cell wall components via a reactive oxygen species-triggered mechanism. J Biol Chem 283: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 22.Winston ML (1987) The Biology of the Honey Bee. Cambridge: Harvard University Press. 281 p.
- 23. James RR, Xu J (2012) Mechanisms by which pesticides affect insect immunity. J Invertebr Pathol 109: 175–182. [DOI] [PubMed] [Google Scholar]
- 24. Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, et al. (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 15: 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uno Y, Fujiyuki T, Morioka M, Takeuchi H, Kubo T (2007) Identification of proteins whose expression is up- or down-regulated in the mushroom bodies in the honeybee brain using proteomics. FEBS Lett 581: 97–101. [DOI] [PubMed] [Google Scholar]
- 26. Hori S, Takeuchi H, Arikawa K, Kinoshita M, Ichikawa N, et al. (2006) Associative visual learning, color discrimination, and chromatic adaptation in the harnessed honeybee Apis mellifera L. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 192: 691–700. [DOI] [PubMed] [Google Scholar]
- 27. Weiss WJ, Lenoy E, Murphy T, Tardio L, Burgio P, et al. (2004) Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal models of infection. J Antimicrob Chemother 53: 480–486. [DOI] [PubMed] [Google Scholar]
- 28. Linder JE, Owers KA, Promislow DE (2008) The effects of temperature on host-pathogen interactions in D. melanogaster: who benefits? J Insect Physiol 54: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laughton AM, Boots M, Siva-Jothy MT (2011) The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J Insect Physiol 57: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 30. Doums C, Moret Y, Benelli E, Schmid-Hempel P (2002) Senescence of immune defence in Bombus workers. Ecol Entomol 27: 138–144. [Google Scholar]
- 31. Albert S, Gatschenberger H, Azzami K, Gimple O, Grimmer G, et al. (2011) Evidence of a novel immune responsive protein in the Hymenoptera. Insect Biochem Mol Biol 41: 968–981. [DOI] [PubMed] [Google Scholar]
- 32. Schmid MR, Brockmann A, Pirk CW, Stanley DW, Tautz J (2008) Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J Insect Physiol 54: 439–444. [DOI] [PubMed] [Google Scholar]
- 33. Wilson-Rich N, Tarpy DR, Starks PT (2012) Within- and across-colony effects of hyperpolyandry on immune function and body condition in honey bees (Apis mellifera). J Insect Physiol 58: 402–407. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka H, Ishibashi J, Fujita K, Nakajima Y, Sagisaka A, et al. (2008) A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol 38: 1087–1110. [DOI] [PubMed] [Google Scholar]
- 35. Bae T, Banger AK, Wallace A, Glass EM, Aslund F, et al. (2004) Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A 101: 12312–12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sifri CD, Begun J, Ausubel FM, Calderwood SB (2003) Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect Immun 71: 2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hart BL (1988) Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12: 123–137. [DOI] [PubMed] [Google Scholar]
- 38. Dantzer R (2001) Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 15: 7–24. [DOI] [PubMed] [Google Scholar]
- 39. Richard FJ, Aubert A, Grozinger CM (2008) Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aliferis KA, Copley T, Jabaji S (2012) Gas chromatography-mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J Insect Physiol 58: 1349–1359. [DOI] [PubMed] [Google Scholar]
- 41. Behrens D, Forsgren E, Fries I, Moritz RF (2010) Lethal infection thresholds of Paenibacillus larvae for honeybee drone and worker larvae (Apis mellifera). Environ Microbiol 12: 2838–2845. [DOI] [PubMed] [Google Scholar]
- 42. Chan QW, Melathopoulos AP, Pernal SF, Foster LJ (2009) The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genomics 10: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]