Abstract
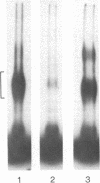
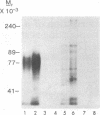
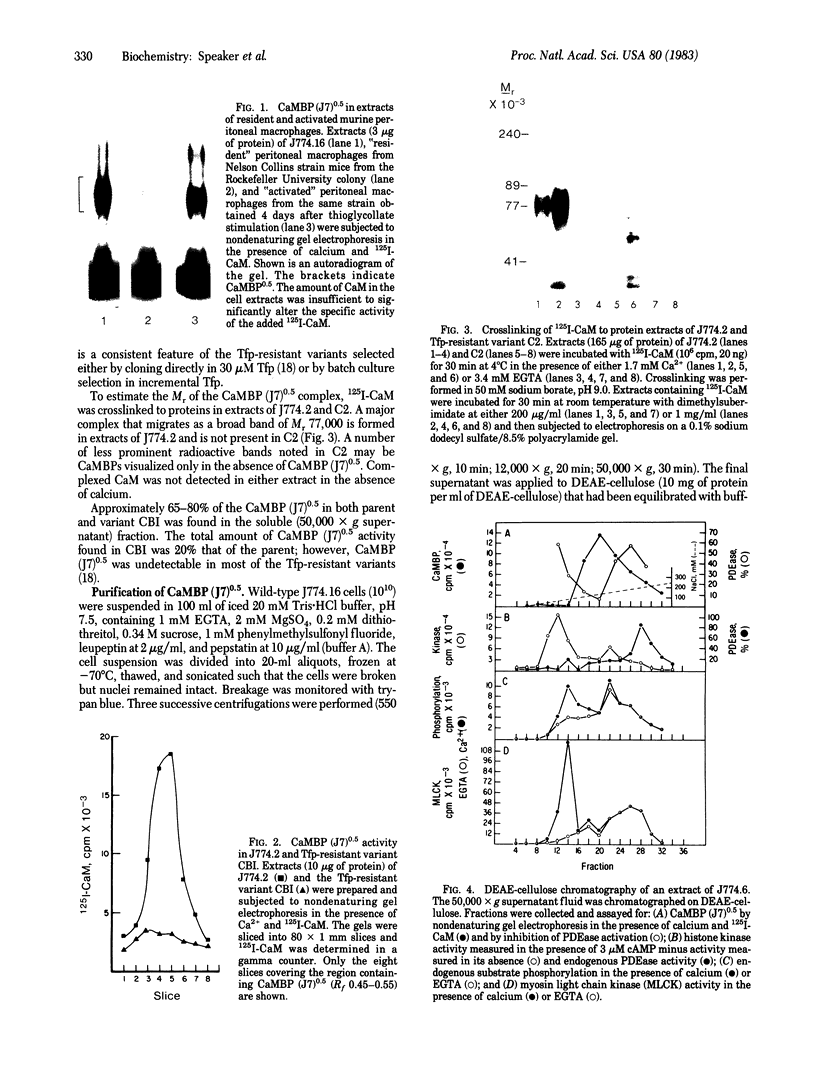
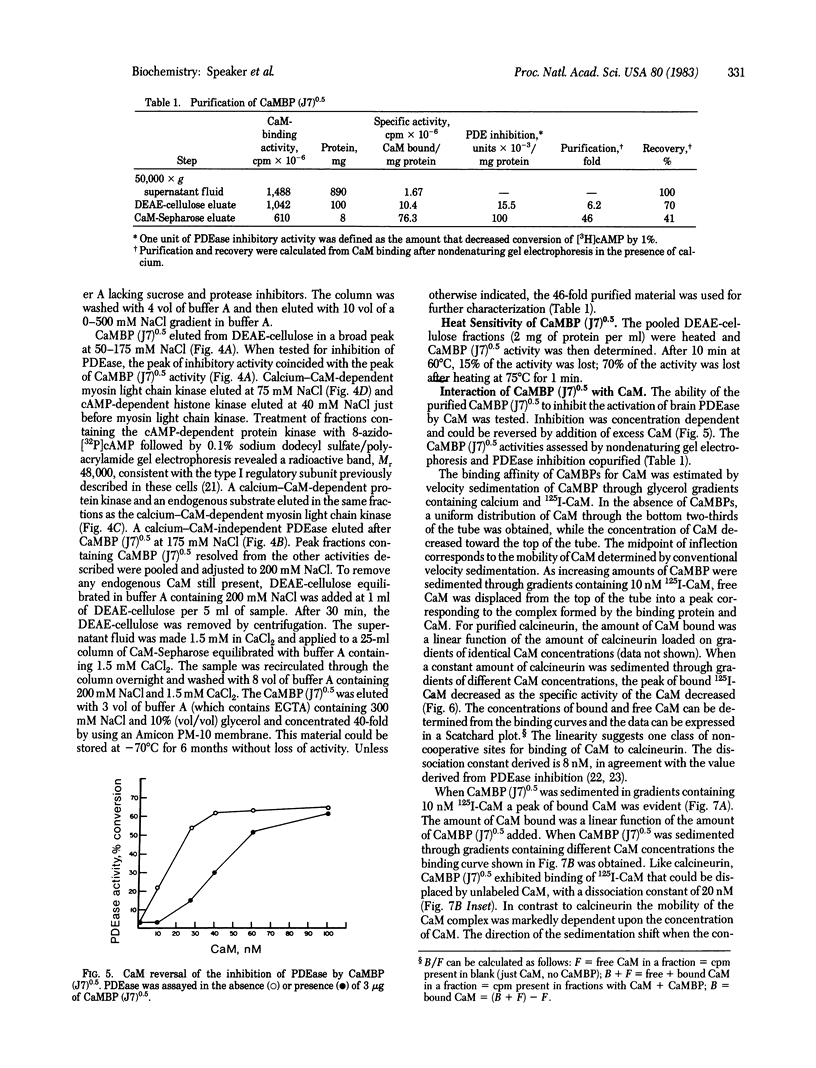
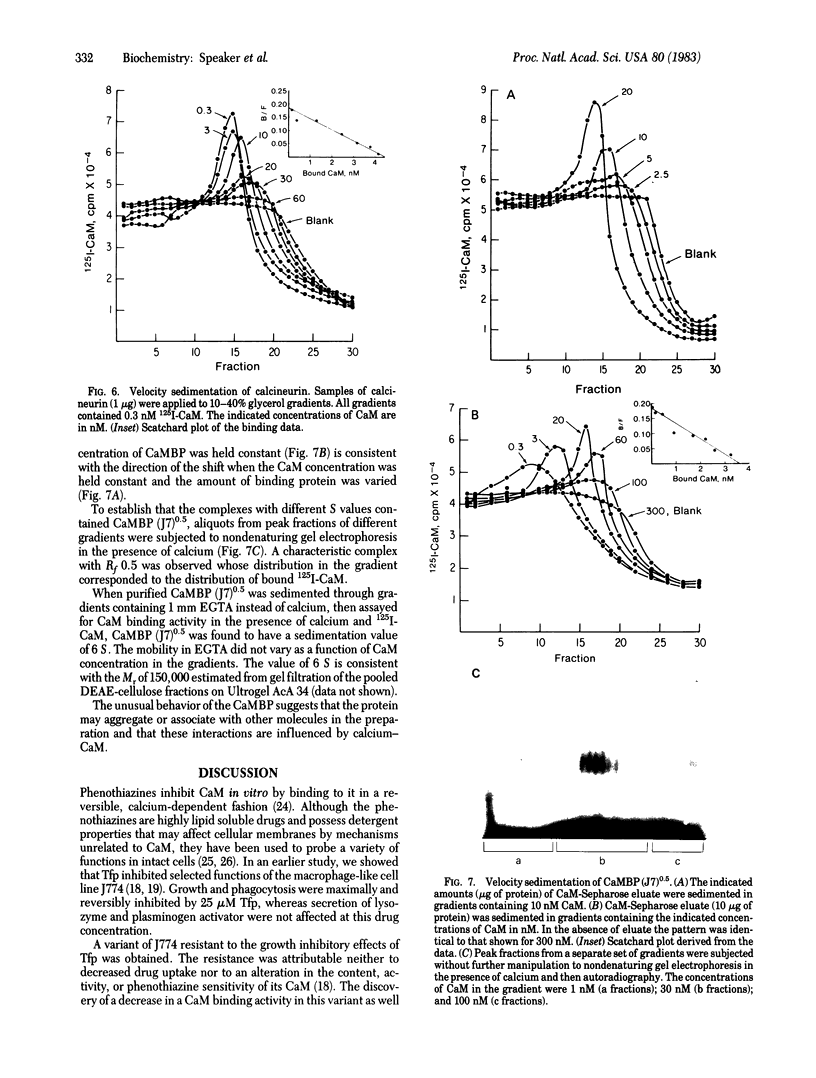
A calmodulin-binding protein is present in extracts of the macrophage-like mouse cell line J774 and in extracts of thioglycollate-stimulated mouse peritoneal macrophages; it is deficient in variants of J774 resistant to trifluoperazine and in resident peritoneal macrophages. The calmodulin-binding protein [CaMBP (J7)0.5] was purified from J774 and resolved from endogenous cyclic nucleotide phosphodiesterase and protein kinase activities. The protein has an apparent native Mr of 125,000-150,000 and binds calmodulin in a calcium-dependent manner with a Kd of 20 nM. It inhibits the ability of calmodulin to activate phosphodiesterase. Its sedimentation constant in glycerol gradients containing calmodulin was dependent upon the relative concentrations of calmodulin and the calmodulin-binding protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthélemy A., Paridaens R., Schell-Frederick E. Phagocytosis-induced 45calcium efflux in polymorphonuclear leucocytes. FEBS Lett. 1977 Oct 15;82(2):283–287. doi: 10.1016/0014-5793(77)80603-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz S. B., Chia G. H., Harracksingh C., Orlow S., Pifko-Hirst S., Schneck J., Sorbara L., Speaker M., Wilk E. W., Rosen O. M. Trifluoperazine inhibits phagocytosis in a macrophagelike cultured cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):798–802. doi: 10.1083/jcb.91.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Kanda K., Morimoto K. Purification of a 155 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1982 Feb 22;138(2):173–177. doi: 10.1016/0014-5793(82)80434-1. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Subunit structure and catalytic properties of bovine brain Ca2+-dependent cyclic nucleotide phosphodiesterase. Biochemistry. 1979 Feb 20;18(4):722–729. doi: 10.1021/bi00571a026. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Storm D. R. Detection of calcium-dependent regulatory protein binding components using 125I-labeled calcium-dependent regulatory protein. J Biol Chem. 1978 May 25;253(10):3374–3377. [PubMed] [Google Scholar]
- Larsen F. L., Vincenzi F. F. Calcium transport across the plasma membrane: stimulation by calmodulin. Science. 1979 Apr 20;204(4390):306–309. doi: 10.1126/science.155309. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- Means A. R. Calmodulin: properties, intracellular localization, and multiple roles in cell regulation. Recent Prog Horm Res. 1981;37:333–367. doi: 10.1016/b978-0-12-571137-1.50011-6. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Smith J. R., Poste G. Effects of local anesthetics on cell morphology and membrane-associated cytoskeletal organization in BALB/3T3 cells. J Cell Biol. 1976 Feb;68(2):395–402. doi: 10.1083/jcb.68.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershadsingh H. A., Landt M., McDonald J. M. Calmodulin-sensitive ATP-dependent Ca2+ transport across adipocyte plasma membranes. J Biol Chem. 1980 Oct 10;255(19):8983–8986. [PubMed] [Google Scholar]
- Rosen N., Piscitello J., Schneck J., Muschel R. J., Bloom B. R., Rosen O. M. Properties of protein kinase and adenylate cyclase-deficient variants of a macrophage-like cell line. J Cell Physiol. 1979 Jan;98(1):125–136. doi: 10.1002/jcp.1040980114. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Desai R., Waisman D. M., Wang J. H. Purification and subunit structure of bovine brain modulator binding protein. J Biol Chem. 1979 May 25;254(10):4276–4282. [PubMed] [Google Scholar]
- Sobieszek A. Ca-linked phosphorylation of a light chain of vertebrate smooth-muscle myosin. Eur J Biochem. 1977 Mar 1;73(2):477–483. doi: 10.1111/j.1432-1033.1977.tb11340.x. [DOI] [PubMed] [Google Scholar]
- Sobue K., Morimoto K., Kanda K., Maruyama K., Kakiuchi S. Reconstitution of Ca2+-sensitive gelation of actin filaments with filamin, caldesmon and calmodulin. FEBS Lett. 1982 Feb 22;138(2):289–292. doi: 10.1016/0014-5793(82)80463-8. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker M. G., Sturgill T. W., Orlow S. J., Chia G. H., Pifko-Hirst S., Rosen O. M. The effects of trifluoperazine on the macrophage-like cell line, J774. Ann N Y Acad Sci. 1980;356:162–178. doi: 10.1111/j.1749-6632.1980.tb29609.x. [DOI] [PubMed] [Google Scholar]
- Stoclet J. C. An ubiquitous protein which regulates calcium-dependent cellular functions and calcium movements. Biochem Pharmacol. 1981 Jul 1;30(13):1723–1729. doi: 10.1016/0006-2952(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. C., 2nd, Raynor S. T. The effects of trifluoperazine, an inhibitor of calmodulin, on platelet function. Thromb Res. 1980 Apr 1;18(1-2):279–284. doi: 10.1016/0049-3848(80)90194-2. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]