Abstract
Liver metastasis is a major cause of mortality in colorectal cancer (CRC). The current study was to investigate the ability of ulinastatin (UTI) and curcumin (CUR) to inhibit CRC liver metastases via modulating matrix metalloproteinase-9 (MMP-9) and E-cadherin expression. Human CRC HCT-116 cells were treated with compounds individually and in combination in order to understand the effect on cell migration and invasion. The HCT-116 cell line was established to stably express luciferase and green fluorescent protein (GFP) by lentiviral transduction (HCT-116-Luc-GFP). We identified an anti-metastasis effect of UTI and CUR on a CRC liver metastasis mouse model. Tumor development and therapeutic responses were dynamically tracked by bioluminescence imaging. Expression of MMP-9 and E-cadherin in metastatic tumors was detected by immunohistochemical assay. Results of wound healing and cell invasion assays suggest that treatment with UTI, CUR, and UTI plus CUR, respectively, significantly inhibit HCT-116 cell migration and invasion. Furthermore, results of CRC hepatic metastasis on a nude mouse model showed that treatment with UTI, CUR alone, and a combination notably inhibited hepatic metastases from CRC and prolonged survival of tumor-bearing mice, especially in the UTI plus CUR group. These results suggest that the combination of UTI and CUR together may offer greater inhibition against metastasis of CRC.
Keywords: hepatic metastasis, bioluminescence imaging, therapy
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in females and the third in males.1 Malignant tumor formation is a complex, multistep process that depends on tumor cells’ capacity to invade and metastasize. The loss of intercellular adhesion and the acquisition of degradation properties are the beginning for tumor cells to become metastases.2,3 E-cadherin is a membrane glycoprotein that plays an essential role in maintaining the integrity of cell-to-cell adhesion, which is significantly associated with tumorous invasiveness and metastatic dissemination.4 Dysfunction or loss of E-cadherin is associated with an increased tendency for tumor metastasis.5 In addition, degradation of extracellular matrix and basement membranes by the tumor cells is a critical step and occurs at several stages of the metastatic cascade.6,7 Matrix metalloproteinases (MMPs) contribute to tumor cells’ ability to invade and metastasize, especially MMP-9.
As the loss of E-cadherin and the overexpression of MMP-9 are common features of an invasive phenotype, there is significant interest in finding agents with the potential to restrain the prerequisites and thus inhibit metastasis. Curcumin (CUR) (bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), a natural phenolic yellow-colored compound, is able to suppress cancer cell proliferation, metastasis, invasion, and angiogenesis.8 It is reported that CUR acts as an agent with potential usefulness to prevent the metastasis of cancer cells via increasing cell adhesion and E-cadherin expression while decreasing the activity and expression of MMP-9.9,10 Ulinastatin (UTI) is a multivalent Kunitz-type serine protease inhibitor that is found in human urine and blood. A lot of studies suggest that UTI may have enormous potential in cancer therapy.11–15 Our previous study and works of Yoshioka et al also indicate an anti-metastasis effect of UTI on cancer cells via modulating the activity and expression of MMP-9.15,16
On the basis of these data, we investigated whether the combined use of UTI and CUR could engender a synergistic effect. To this end, in this study we monitored the effect of UTI and CUR individually and in combination on the metastasis of CRC. Using cultured cells in vitro and CRC liver metastasis mouse model, we also determined the impact of UTI and CUR on expression of MMP-9 and E-cadherin.
Materials and methods
Ethics statement
The study was carried out in compliance with the guidance suggestion of the Animal Care Committee of Guangzhou Medical University, Guangzhou, People’s Republic of China (Permit Number: SYXK [Guangdong] 2010–0104). The study protocol was approved by the ethics committee of the Guangzhou Medical University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Cell culture and maintenance
Lentivirus packaging cell line HEK-293T and CRC cell line HCT-116 were obtained from the Shanghai Institute of Cell Biology at the Chinese Academy of Sciences (Shanghai, People’s Republic of China). The cells were maintained in 25 cm3 tissue culture flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco®, Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Gibco, Life Technologies), 100 U/mL penicillin, and 100 U/mL streptomycin, and cultured in a humidified incubator at 37°C in an atmosphere of 5% CO2 and 95% air.
Animals
Male nude mice (BALB/C nu/nu) 4–6 weeks old were purchased from the Sun Yat-Sen University’s Laboratory Animal Center (Guangzhou, People’s Republic of China). All mice were maintained in the laboratory for animal experimentation in a specific pathogen-free environment in laminar air-flow conditions at a temperature of 25°C±2°C under a 12-hour light–dark cycle. All animals had free access to standard laboratory mouse food and water. All procedures were carried out in compliance with the guidance suggestion of the Animal Care Committee of the Guangzhou Medical University.
In vitro growth inhibition assay
Cell growth was assessed using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, HCT-116 cells (5 × 103) were seeded in each well of a 96-well plate. The cells were cultured with various concentrations of UTI (100 U/mL, 200 U/mL, 400 U/mL, or 800 U/mL; Techpool Bio-Pharma Co, Ltd, Guangzhou, People’s Republic of China) or various concentrations of CUR (5 μM, 10 μM, 20 μM, or 40 μM; Sigma-Aldrich, St Louis, MO, USA) in DMEM medium with 10% FBS for a further 24 hours. The Cell Counting Kit-8 reagent was added to each well according to the manufacturer’s instructions. The absorbance at 450 nm was measured in a Thermo Scientific Multiskan (Thermo Fisher Scientific, Waltham, MA, USA).
Wound healing assays
Alteration of tumor cell migration induced by UTI and CUR was estimated by an in vitro wounding healing assay. Briefly, log-phase cells were collected after trypsinization (Gibco, Life Technologies) and inoculated into a six-well plate at 5 × 105 cells per well. The plates were cultured in DMEM containing 10% FBS for 24 hours to ~100% confluency in a monolayer. The growth medium was then removed and the wells were cross-scratched with a 200 μL yellow pipette tip and washed three times with serum-free DMEM to remove the cell debris. Cells were incubated with 800 U UTI, 10 μM CUR, 800 U UTI plus 10 μM CUR, and phosphate buffered saline (PBS), respectively, for 0 hours or 24 hours and 48 hours and inspected under an inverted microscope. The distance from one side of the scratch to the other was measured at different intervals using Image Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
HCT-116 cell invasion assay
Transwell® membrane (8 μm pore size; Corning Incorporated, Tokyo, Japan) coated with Matrigel® (2.5 mg/mL; BD Biosciences Discovery Labware, Durham, NC, USA) was used for invasion assay. Briefly, log-phase HCT-116 cells were collected, centrifuged, and resuspended at 2 × 106 cells/mL. Cells were seeded on to the upper wells of precoated Transwell chambers, 2 × 105 cells per well. Cells were incubated with 800 U UTI, 10 μM CUR, 800 U UTI plus 10 μM CUR, and PBS, respectively, and 500 μL DMEM containing 10% FBS was placed in the lower chamber as a chemoattractant. After 24 hours of incubation, cells in the upper chamber were removed with a cotton swab, fixed with methanol, stained with 0.1% crystal violet solution, and washed with PBS. Cells that attached through the Matrigel to the lower chamber were counted in five random microscope fields (200×).
Lentivirus production and labeled cell line construction
Lentivirus plasmid LV201-luc, gag, rev, and vesicular stomatitis virus G were provided by Professor Qi-Cai Liu (Guangzhou Medical University). The day before transduction, 4 × 106 low-passage and healthy 293T cells were seeded in a 10 cm dish. When cells obtained 65%–75% confluency, the transduction was carried out by introducing LV201-luc, gag, rev, and vesicular stomatitis virus into 293T cells using Lipofectamine® 2000 (Gibco, Life Technologies) according to the manufacturer’s instructions. After 60 hours post- transduction, the supernatant was collected, filtered, and stored at −80°C. HCT-116 cells were transfected with lentiviral vectors transfer of luciferase and green fluorescent protein (GFP). Puromycin (1.0 μg/mL; Sigma-Aldrich) was added at 48 hours post-transduction and maintained for ~2 weeks. Transduction efficiency was monitored by a converted fluorescence microscope (Leica, DM IRBE, Heidelberg, Germany).
In vivo experiment in CRC liver metastasis model and bioluminescence imaging to monitor tumor development and therapeutic responses
Mice were fasted for 24 hours prior to surgery and deprived of water 12 hours prior to surgery. Log-phase HCT-116-Luc-GFP cells were collected after trypsinization, and adjusted with fresh medium to a cell density of 1 × 107 cells/mL. The animals were injected with 0.2 mL cell suspension via peritoneal cavity spleen transplant. The model was set up as described in our previous report.17 A total of 28 animals were randomly divided into four groups as follows: 1) the untreated control group (n=7) was administered orally 100 μL corn oil once daily, 2) the UTI group (n=7) was intraperitoneally injected with UTI at 8,000 U/mouse once daily, 3) the CUR group (n=7) was administered orally CUR alone (1 g/kg)18 once daily, and 4) the UTI plus CUR group (n=7) was intraperitoneally injected with UTI (8,000 U/mouse) and orally administered CUR (1 g/kg). Therapy was continued for 4 weeks. Bioluminescence imaging (BLI) was conducted using bioluminescence technology (NightOWL II LB 983 NC100; Berthold Technologies, Bad Wildbad, Germany), which has a cooled charge-coupled device camera to capture images of animals in a light-tight box. Mice were anesthetized with an intraperitoneal injection of 1% pentobarbital (Gibco, Life Technologies), which was regulated to 45 mg/kg and maintained at 37°C for the duration of image acquisition. Prior to acquiring images, 200 μL of 15 mg/mL D-luciferin (bioWorld, Dublin, OH, USA) was administered by intraperitoneal injection to all mice and allowed to distribute for 10 minutes. One day after intraspleen injections, we imaged the mice in this same manner to monitor tumor development and therapeutic responses. The animals were sacrificed for sample collection 28 days after administration.
Survival assay
Mouse survival was determined by injecting HCT-116 cells into the spleens of Balb/c nu/nu mice as described previously. UTI and/or CUR were treated daily as described. The treatment lasted until the death of the animals for survival rate calculation.
Immunohistochemical assay
After 4 weeks of treatment, the liver tissue was formalin fixed and paraffin embedded for immunohistochemistry (IHC). Expression of MMP-9 and E-cadherin in hepatic metastasis tissue was detected by IHC assay. IHC staining was performed using rabbit antihuman MMP-9 monoclonal antibody (BioWorld, Atlanta, GA, USA) and rabbit antihuman E-cadherin monoclonal antibody (BioWorld), followed by incubation with corresponding secondary antibodies according to the manufacturer’s instructions. The expression of MMP-9 and E-cadherin protein was calculated and analyzed with the Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
All data were expressed as mean ± standard deviation, and all statistical analyses were performed using SPSS Version 19.0 (IBM Corporation, Armonk, NY). Differences in measurements were compared using one-way analysis of variance, and Fisher’s least significant difference method was employed to compare the means between groups. A P-value <0.05 was considered statistically significant.
Results
Effect of UTI or CUR on the growth of HCT-116 cells in vitro
The effects of UTI or CUR on cell growth were evaluated by CCK-8 assay in HCT-116 cells (Figure 1). HCT-116 cells were incubated with various concentrations of UTI or CUR for 24 hours. As shown in Figure 1A, at 100–800 U UTI did not significantly inhibit the proliferation of the HCT-116 cells (P>0.05). Therefore, we used UTI at concentrations of 800 U for the subsequent experiments in vitro. On the basis of these measurements, the IC50 values (50% cell growth inhibitory concentrations) for CUR on HCT-116 colon cancer cell viability were determined. The IC50 of CUR was 20 μM in HCT-116 cells. These results suggest that CUR has potent antiproliferative effects on CRC cells.
Figure 1.
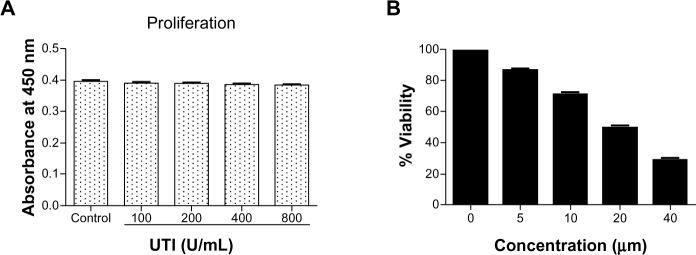
Effect of ulinastatin (UTI) or curcumin (CUR) on cell viability and proliferation of HCT-116. (A) Cytotoxicity of UTI against HCT-116 cells in vitro. Tumor cells (5.0 × 103) in each well of a 96-well culture plate were incubated for 24 hours at 37°C with or without various concentrations of UTI. CCK-8 was added to each well and, after incubation for 2 hours, the absorbance was measured at 450 nm. (B) HCT-116 cells were treated with different concentrations of CUR for 24 hours, and cell viability was measured using the CCK-8 method. Concentrations of CUR resulting in 50% growth inhibition were indicated as individual IC50 (50% cell growth inhibitory concentrations) values.
Inhibition by UTI and CUR on migration and invasion in HCT-116 cells in vitro
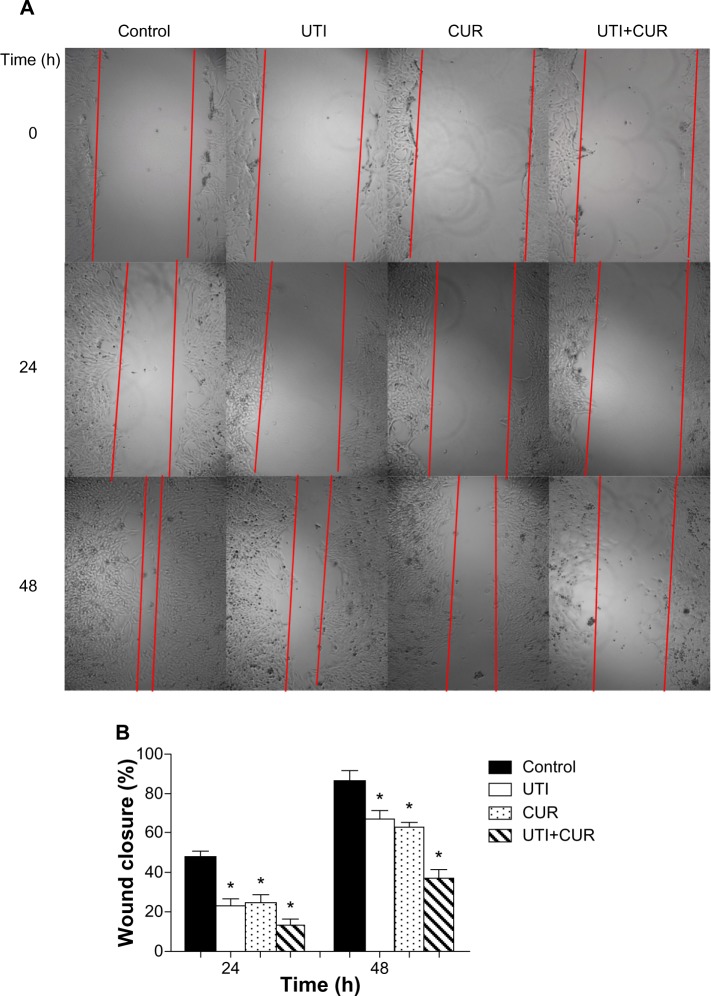
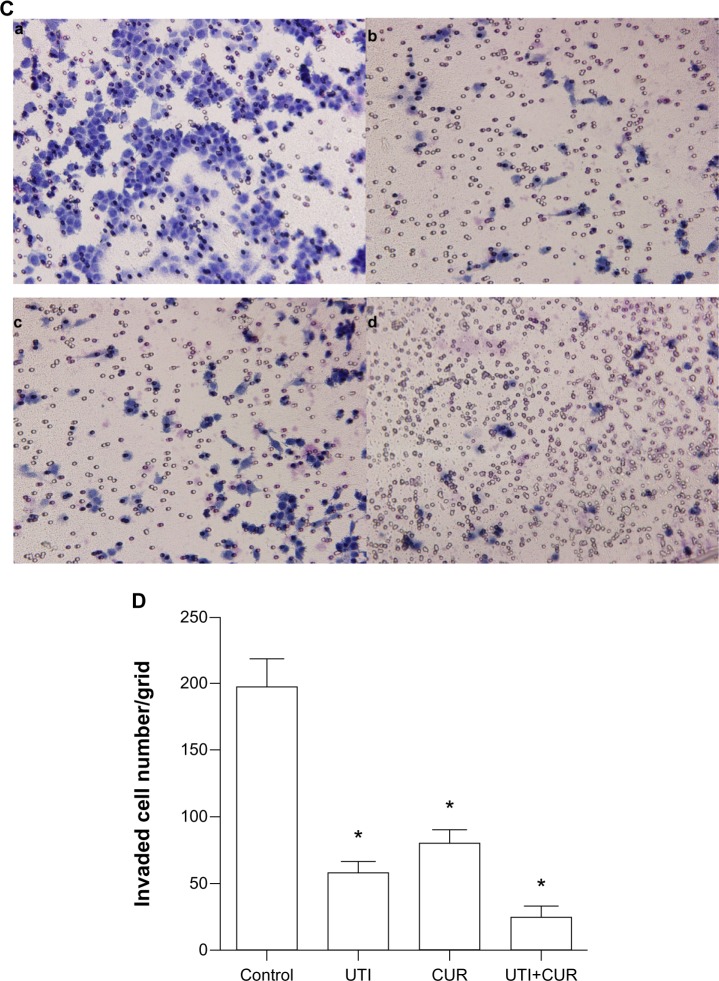
Due to the fact that CUR could reduce cell viability and the IC50 is 20 μM in HCT-116 cells (Figure 1B), the effects of CUR on migration and invasion abilities of HCT-116 cells were investigated at a concentration of 10 μM. To examine the antimetastatic mechanisms of UTI and CUR, the wound healing assay and the Matrigel-based Transwell invasion assay were performed. As shown in Figure 2, migration and invasion of HCT-116 cells were significantly inhibited by treating with UTI, CUR, and UTI plus CUR, respectively, especially the UTI plus CUR group.
Figure 2.
Effect of ulinastatin (UTI) and curcumin (CUR) on cell migration and invasion. (A) Migration of HCT-116 was assayed by wound healing assay. Cells were cultured to nearly confluent cell monolayer. A scratch wound was created on the cell surface using a micropipette tip. The monolayer was washed with phosphate buffered saline, and then UTI (800 U) or CUR (10 μM) was added or not. The cultures were incubated at 37°C for 0 hours, 24 hours, and 48 hours, respectively, and pictures were taken using light microscopy (×100). (B) The width of the wound was measured and the wound closure rate was calculated. (C) Transwell in vitro invasion assay detects the effect of UTI and CUR on the invasive ability of colon cancer cells. (a) cells treated with PBS; (b) cells treated with UTI; (c) cells treated with CUR; (d) cells treated with UTI and CUR. (D) The invaded cell numbers were measured and compared.
Note: *P<0.05 versus control.
Characteristics of the transformed CRC cell line HCT-116-Luc-GFP and in vitro bioluminescence
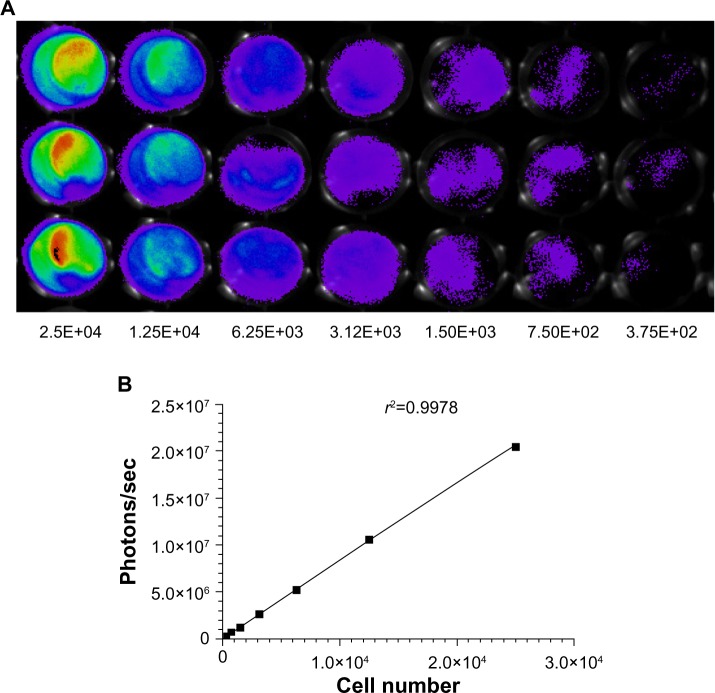
The stable expression of luciferase and GFP in the CRC cell line is a prerequisite for intensive research with an advanced molecular imaging approach. After being sequentially infected by lentivirus, the CRC cell line HCT-116-Luc-GFP was developed. To determine whether or not the cell characteristics were influenced by the virus infection process, we compared cell proliferation and morphological differences between the transformed clones and their correspondent parental strains. No obvious changes were observed in the in vitro growth pattern (data not shown). Furthermore, we serially diluted the cell concentrations, ranging from 5 × 104 to 4 × 105 cells, and assessed the accuracy of BLI as well as fluorescent imaging as indicators of cell number (Figure 3A). The light emission by region of interest analysis was highly correlated with cell number. Correlation analysis revealed a linear relationship between bioluminescent intensity and cell number (r2=0.9978), with a minimum detection of 375 cells per well (Figure 3B).
Figure 3.
Measurement of bioluminescence in HCT-116-Luc-GFP cells. HCT-116-Luc-GFP cells were counted with a hemocytometer and plated in a 96-well plate at various concentrations. The plate was imaged to verify that the cells bioluminesced in a concentration-dependent manner. (A) Quantitation of bioluminescence (photons/sec) was graphed. (B) Linear regression analysis showed a good correlation between cell number and mean bioluminescence imaging or fluorescent intensity (r2=0.9978).
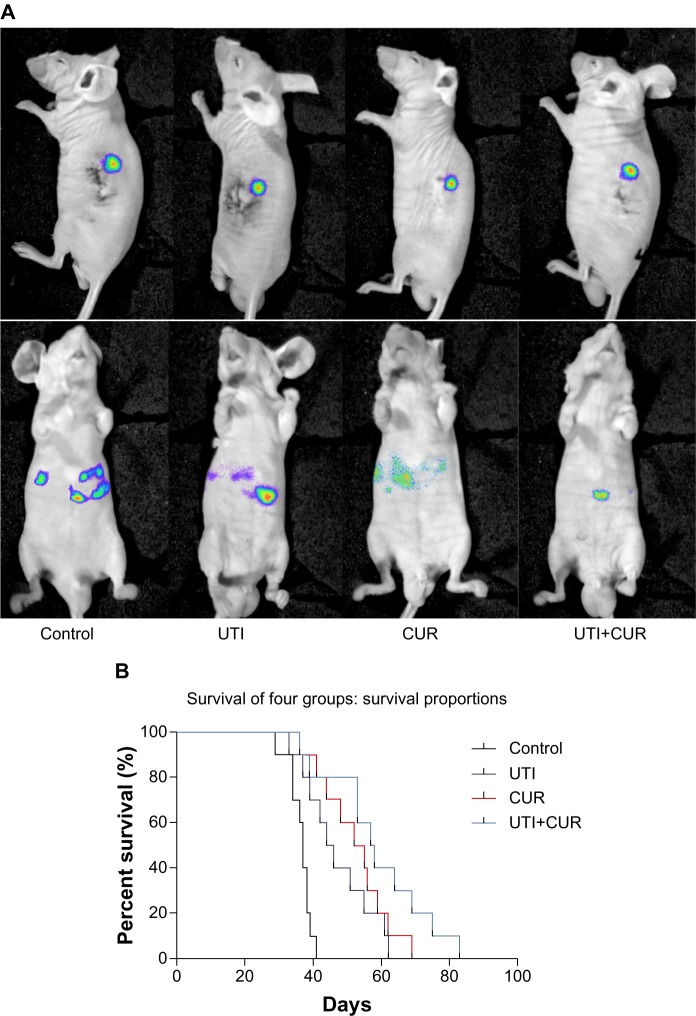
UTI and CUR treatment reduces CRC liver metastasis and prolongs the survival of metastatic tumor-bearing mice
To detect the therapeutic potential of UTI and CUR in vivo, we established an experimental mice model for CRC liver metastasis by splenic injection of HCT-116-Luc-GFP cells. After injection of the cancerous cells, the mice were imaged by bioluminescence technique. As showed in Figure 4A, representative images of dorsal and ventral views are presented. The dorsal view was better for spleen in situ, whereas the ventral imaging position was found to be more sensitive to detect hepatic metastases. Mice treated with UTI or CUR individually notably reduced liver metastasis compared with the control group. Furthermore, mice treated with UTI plus CUR showed markedly reduced liver metastases compared with treatment with either UTI or CUR alone, as monitored by BLI. Using the CRC liver metastasis mice models, we determined the effect of UTI and/or CUR on survival of mice models. We found that a combination of UTI and CUR treatment significantly increased the survival time of animals (Figure 4B). These data indicated that UTI and/or CUR reduced CRC liver metastasis and prolonged survival of tumor-bearing mice.
Figure 4.
Ulinastatin (UTI) and curcumin (CUR) inhibits liver metastasis and prolongs survival. (A) Luciferase-expressing HCT-116 cells were injected into the spleens of BLAB/c mice. The control group (n=7) received vehicle, the UTI group (n=7) was injected with UTI at 8,000 U/mouse once daily, the CUR group (n=7) was administered orally CUR alone (1 g/kg) once daily, and the UTI plus CUR group (n=7) was treated with a combination of UTI (8,000 U/mouse) and CUR (1 g/kg). Therapy was continued for 4 weeks. Bioluminescence imaging was used to monitor liver metastasis of HCT-116-Luc-GFP cells in vivo 1 day and 28 days after splenic injection. (B) Survival of mice treated with/without UTI and/or CUR was assayed after injecting HCT-116-Luc-GFP cells.
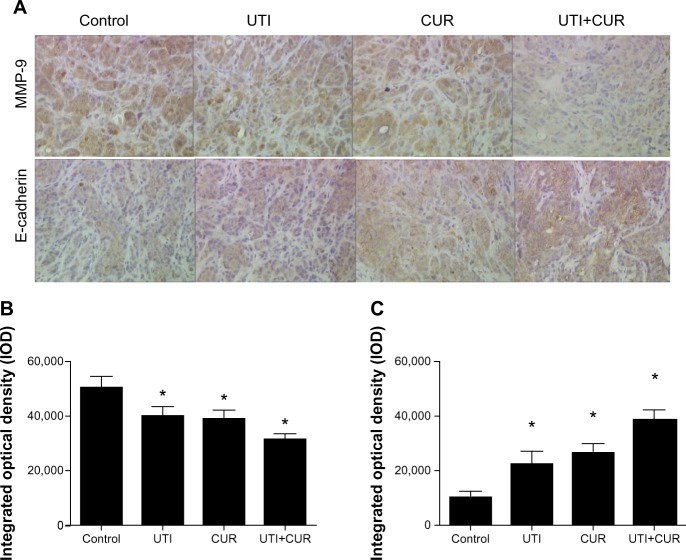
MMP-9 and E-cadherin expression in metastatic liver lesions of mice model
To investigate the role of UTI and CUR in treatment, we examined MMP-9 and E-cadherin protein expression in metastatic liver lesions by IHC. Results of IHC showed that expression of MMP-9 in UTI, CUR, and UTI plus CUR groups was remarkably decreased compared to that in the control group, whereas E-cadherin expression was significantly enhanced (Figure 5). In addition, there was a significant decrease in the expression of MMP-9 and a significant upregulation in the expression of E-cadherin after cotreatment with UTI and CUR compared with UTI or CUR treatment alone.
Figure 5.
Expression of MMP-9 and E-cadherin in metastatic tumors was detected by immunohistochemical assay (original magnification 400×). (A) Expression of MMP-9 and E-cadherin in four groups. Differences in MMP-9 (B) and E-cadherin (C) protein expression are shown.
Note: *P<0.05.
Abbreviations: CUR, curcumin; MMP-9, modulating matrix metalloproteinase-9; UTI, ulinastatin.
Discussion
CRC patients with hepatic metastasis are very common. The treatments to prevent liver metastasis from CRCs are vital to improve survival and quality of life of these patients. The overexpression of MMP-9 and loss of E-cadherin frequently contribute to metastasis and invasion of cancer cells. There is vital interest in finding ideal agents that can effectively prohibit the overexpression of MMP-9 and loss of E-cadherin. Recently, there has been increasing focus on the two drugs CUR and UTI, which show potential inhibition of tumor metastasis and invasion.
CUR is an active component of the rhizome of the perennial herb Curcuma longa.19 Several lines of evidence have shown that CUR has emerged with antiproliferation, anti-apoptosis, and anti-metastasis effects against many kinds of cancer, such as liver cancer,20 lung cancer,8,21 CRC,22–25 ovarian cancer,26 and thyroid cancer.27 However, the exact molecular mechanisms by which CUR exerts its effects are still to be determined. Cui et al28 studied the inhibitory activity of CUR against gastric and hepatic cancers using a mice model. The results showed an increase in life span and a dose-dependent decrease in tumor volume in the CUR-treated animals compared with in the untreated controls. Li et al29 indicated the comparable or greater growth inhibitory and apoptotic effects of liposomal CUR with oxaliplatin both in vitro and in vivo in CRC. Zhang et al10 indicated that CUR acts as an agent with potential usefulness to prevent the metastasis of K1 papillary thyroid cancer cells via increasing E-cadherin expression while decreasing the activity and expression of MMP-9. To identify possible sites to inhibit CRCs treated with CUR from metastasis and invasion, in this study wound healing assay and Transwell invasion assay were used. We found that CUR could significantly inhibit CRC metastasis and invasion in vitro. We also found that expression of E-cadherin was increased while MMP-9 expression was decreased in liver tissue after treatment with CUR. Collectively, CUR could inhibit migration and invasion of CRCs associated with increasing the expression of E-cadherin and decreasing the expression of MMP-9.
UTI acts as a urinary trypsin inhibitor, which has multiple potent inhibitory effects. UTI can emerge as a powerful antiproliferative agent against breast carcinoma.30,31 UTI can also enhance the effects of docetaxel on invasion of breast cancer cells via modulating uPA, uPAR, and p-ERK expression.12 In line with our previous reports,16 in the present study, by wound healing assay and Transwell invasion assay, the metastasis and invasion of cells treated with UTI were notably inhibited. Results of IHC showed that expression of MMP-9 in the UTI group was remarkably decreased while E-cadherin expression was enhanced. These data indicate that UTI could suppress migration and invasion of CRCs via decreasing MMP-9 expression and increasing E-cadherin expression.
On the basis of our data, we investigated whether combined use of UTI and CUR could engender a synergistic effect. To this end, cells treated with UTI and CUR in combination were detected. With in vitro assays the results showed that cells treated with UTI plus CUR markedly restrained the metastasis and invasion compared with treatment with either UTI or CUR alone. Results of IHC showed that expression of MMP-9 was significant reduced while E-cadherin expression was notably increased compared with monotherapy with CUR or UTI.
To detect the therapeutic potential of UTI and CUR in vivo, we used human CRC HCT-116 cells, which are aggressive in carcinogenesis and metastasize to the liver, in an experimental splenic injection mice model. We examined the effect of UTI and/or CUR on CRCs to prevent CRC liver metastases. Our findings revealed that compared with the untreated groups, those treated with UTI or CUR individually showed significantly inhibited hepatic metastasis. We also found that treatment with UTI and CUR in combination notably inhibited the hepatic metastasis compared with treatment with either UTI or CUR alone. Using the CRC liver metastasis mice models, we also found that a combination of UTI and CUR treatment significantly increased the survival time of mice.
Conclusion
We have demonstrated that synergism from the combination of UTI and CUR offers greater inhibition against CRC liver metastases and prolongs survival of tumor-bearing mice via improving expression of E-cadherin and decreasing MMP-9 expression.
Acknowledgments
This work was supported by a Guangzhou City medical science and technology key project (201102A212018) and a Guangdong Province science and technology plan project (2009B060700019 and 2010B031600189).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Nawrocki-Raby B, Gilles C, Polette M, et al. E-Cadherin mediates MMP down-regulation in highly invasive bronchial tumor cells. Am J Pathol. 2003;163:653–661. doi: 10.1016/S0002-9440(10)63692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan L, Wang H, Xia X, et al. Loss of E-cadherin promotes prostate cancer metastasis via upregulation of metastasis-associated gene 1 expression. Oncol Lett. 2012;4:1225–1233. doi: 10.3892/ol.2012.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro P, Figueiredo J, Bordeira-Carrico R, et al. Therapeutic targets associated to E-cadherin dysfunction in gastric cancer. Expert Opin Ther Targets. 2013;17:1187–1201. doi: 10.1517/14728222.2013.827174. [DOI] [PubMed] [Google Scholar]
- 5.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 6.Pal S, Ganguly KK, Chatterjee A. Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell Commun Adhes. 2013;20:105–114. doi: 10.3109/15419061.2013.833193. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Zhu Y, Zhao M, et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2) PLoS One. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H-W, Lee J-Y, Huang J-Y, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–7438. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Martinez AB, Bajo AM, Sanchez-Chapado M, Prieto JC, Carmena MJ. Vasoactive intestinal peptide behaves as a pro-metastatic factor in human prostate cancer cells. Prostate. 2009;69:774–786. doi: 10.1002/pros.20930. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C-Y, Zhang L, Yu H-X, Bao J-D, Lu R-R. Curcumin inhibits the metastasis of K1 papillary thyroid cancer cells via modulating E-cadherin and matrix metalloproteinase-9 expression. Biotechnol Lett. 2013;35:1–6. doi: 10.1007/s10529-013-1173-y. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Suzuki M, Sun GW, Hirashima Y, Terao T. Suppression of urokinase-type plasminogen activator expression from human ovarian cancer cells by urinary trypsin inhibitor. Biochim Biophys Acta. 2000;1481:310–316. doi: 10.1016/s0167-4838(00)00173-4. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Sun X, Gao F, et al. Effects of ulinastatin and docetaxel on breast cancer invasion and expression of uPA, uPAR and ERK. J Exp Clin Cancer Res. 2011;30:71. doi: 10.1186/1756-9966-30-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M, Kobayashi H, Tanaka Y, et al. Suppression of invasion and peritoneal carcinomatosis of ovarian cancer cell line by overexpression of bikunin. Int J Cancer. 2003;104:289–302. doi: 10.1002/ijc.10950. [DOI] [PubMed] [Google Scholar]
- 14.Tsui KH, Chang PL, Feng TH, Chung LC, Hsu SY, Juang HH. Down-regulation of matriptase by overexpression of bikunin attenuates cell invasion in prostate carcinoma cells. Anticancer Res. 2008;28:1977–1983. [PubMed] [Google Scholar]
- 15.Yoshioka I, Tsuchiya Y, Aozuka Y, et al. Urinary trypsin inhibitor suppresses surgical stress-facilitated lung metastasis of murine colon 26-L5 carcinoma cells. Anticancer Res. 2005;25:815–820. [PubMed] [Google Scholar]
- 16.Xu B, Li K-P, Shen F, et al. Ulinastatin reduces cancer recurrence after resection of hepatic metastases from colon cancer by inhibiting MMP-9 activation via the antifibrinolytic pathway. Biomed Res Int. 2013;2013:437950. doi: 10.1155/2013/437950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen F, Li J-L, Cai W-S, et al. Interleukin-12 prevents colorectal cancer liver metastases in mice. Onco Targets Ther. 2013;6:523. doi: 10.2147/OTT.S44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tharakan ST, Inamoto T, Sung B, Aggarwal BB, Kamat AM. Curcumin potentiates the antitumor effects of gemcitabine in an orthotopic model of human bladder cancer through suppression of proliferative and angiogenic biomarkers. Biochem Pharmacol. 2010;79:218–228. doi: 10.1016/j.bcp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Dai XZ, Yin HT, Sun LF, et al. Potential therapeutic efficacy of curcumin in liver cancer. Asian Pac J Cancer Prev. 2013;14:3855–3859. doi: 10.7314/apjcp.2013.14.6.3855. [DOI] [PubMed] [Google Scholar]
- 21.Yin HT, Zhang DG, Wu XL, Huang XE, Chen G. In vivo evaluation of curcumin-loaded nanoparticles in a A549 xenograft mice model. Asian Pac J Cancer Prev. 2013;14:409–412. doi: 10.7314/apjcp.2013.14.1.409. [DOI] [PubMed] [Google Scholar]
- 22.Lu W-D, Qin Y, Yang C, Li L. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics. 2013;68:694–701. doi: 10.6061/clinics/2013(05)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS One. 2013;8:e57218. doi: 10.1371/journal.pone.0057218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shehzad A, Lee J, Huh T-L, Lee YS. Curcumin induces apoptosis in human colorectal carcinoma (HCT-15) cells by regulating expression of Prp4 and p53. Mol Cells. 2013;35:1–7. doi: 10.1007/s10059-013-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nautiyal J, Banerjee S, Kanwar SS, et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2011;128:951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang H, Murphy CJ, Zhang B, et al. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31:7139–7149. doi: 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C-Y, Zhang L, Yu H-X, Bao J-D, Sun Z, Lu R-R. Curcumin inhibits invasion and metastasis in K1 papillary thyroid cancer cells. Food Chem. 2013;139:1021–1028. doi: 10.1016/j.foodchem.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Cui SX, Qu XJ, Xie YY, et al. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006;18:227–231. [PubMed] [Google Scholar]
- 29.Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Sun X, Gao F, Zhong B, Zhang YH, Sun Z. Effect of ulinastatin on growth inhibition, apoptosis of breast carcinoma cells is related to a decrease in signal conduction of JNk-2 and NF-kappaB. J Exp Clin Cancer Res. 2012;31:2. doi: 10.1186/1756-9966-31-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Sun X, Gao F, Luo J, Sun Z. Effects of ulinastatin and docataxel on breast tumor growth and expression of IL-6, IL-8, and TNF-alpha. J Exp Clin Cancer Res. 2011;30:22. doi: 10.1186/1756-9966-30-22. [DOI] [PMC free article] [PubMed] [Google Scholar]