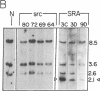
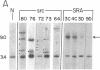
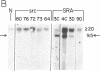
Abstract
The in vitro transforming potential of Rous sarcoma viruses has been shown to reside in a gene segment, v-src. To provide direct proof that the isolated v-src sequence contains all the information necessary for sarcoma induction in vivo, we have injected subgenomic and genomic cloned DNA carrying v-src directly into chickens. We report that v-src, without the complements of other viral genes, can effectively induce sarcomas in the host within weeks after injection. The sarcoma tumors generated by subgenomic v-src DNA are of mono- or oligoclonal origin. They have acquired v-src sequences in the genome and expressed high levels of src messages. Intact long terminal repeats do not seem to be essential in the tumorigenic process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S. Mechanism of transfection of chicken embryo fibroblasts by Rous sarcoma virus DNA. J Virol. 1978 Oct;28(1):45–52. doi: 10.1128/jvi.28.1.45-52.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Copper G. M. Transformation by subgenomic fragments of Rous sarcoma virus DNA. Cell. 1980 Apr;19(4):863–870. doi: 10.1016/0092-8674(80)90077-x. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Eagen D. A., Gulvas F. A. Assays for endogenous and exogenous lymphoid leukosis viruses and chick helper factor with RSV(--) cell lines. Infect Immun. 1979 May;24(2):379–386. doi: 10.1128/iai.24.2.379-386.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. Avian lymphoid leukosis virus infection and DNA integration in the preleukotic bursal tissues: a comparative study of susceptible and resistant lines. Virology. 1982 Jun;119(2):411–421. doi: 10.1016/0042-6822(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5842–5846. doi: 10.1073/pnas.78.9.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Huttner K. M., Juricek D. K., Ruddle F. H. Deoxyribonucleic acid-mediated gene transfer in mammalian cells: molecular analysis of unstable transformants and their progression to stability. Mol Cell Biol. 1981 Feb;1(2):111–120. doi: 10.1128/mcb.1.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H., Junghans R. P., Ju G., Skalka A. M. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. doi: 10.1128/mcb.1.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Tereba A., Astrin S. M. Chromosomal localization of ev-1, a frequently occurring endogenous retrovirus locus in white Leghorn chickens, by in situ hybridization. J Virol. 1980 Sep;35(3):888–894. doi: 10.1128/jvi.35.3.888-894.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]