Abstract
Objective
The contribution of executive cognition (EC) to the prediction of incident dementia remains unclear. This prospective study examined the predictive value of EC for subsequent cognitive decline in persons with mild cognitive impairment (MCI) over a 4-year period.
Methods
141 persons with MCI (amnestic and non-amnestic, single- and multiple-domain) received a baseline and two biennial follow-up assessments. Eighteen tests, assessing six different aspects of EC, were administered at baseline and at 2-year follow-up, together with screening cognitive and daily functioning measures. Longitudinal logistic regression models and generalized estimating equations (GEE) were used to examine whether EC could predict progression to a Clinical Dementia Rating Scale (CDR) score of 1 or more over the 4-year period, first at the univariate level and then in the context of demographic and clinical characteristics, daily functioning measures and other neurocognitive factors.
Results
Over the 4-year period, 56% of MCI patients remained stable, 35% progressed to CDR≥1, and 8% reverted to normal (CDR=0). Amnestic MCI subtypes were not associated with higher rates of progression to dementia, whereas subtypes with multiple impairments were so associated. Eight out of the 18 EC measures, including all three measures assessing inhibition of prepotent responses, predicted MCI outcome at the univariate level. However, the multivariate GEE model indicated that age, daily functioning, and overall cognitive functioning best predicted progression to dementia.
Conclusion
Measures of EC (i.e., inhibitory control) are associated with MCI outcome. However, age and global measures of cognitive and functional impairment are better predictors of incident dementia.
Keywords: mild cognitive impairment, predictors, executive cognition, dementia
Introduction
Mild cognitive impairment (MCI) is a heterogeneous clinical syndrome that sometimes signals the presence of a neurodegenerative disease such as Alzheimer's disease (AD). Persons with MCI are therefore at high risk for the development of dementia. Their annual incidence of dementia is 5–10%, in comparison to 1–2% for cognitively normal elderly (Mitchell & Shiri-Feshki, 2009). However, despite the elevated rates of incident dementia in this group, a significant proportion does not progress to dementia over very long follow-ups. Many remain stable, or even revert to normal cognitive status (Fisk & Rockwood, 2005; Ganguli et al., 2011; Visser, Kester, Jolles, & Verhey, 2006).
The prediction of which MCI individuals are destined to develop dementia is of critical importance. Specific MCI subtypes, such as those with impairments in multiple cognitive domains [multiple-domain (MD) MCI, both amnestic and non-amnestic subtypes] are considered at higher risk for progression to dementia than those with impairment in a single domain [amnestic single domain (AS); non-amnestic single domain (NAS)] (Alexopoulos, Grimmer, Perneczky, Domes, & Kurz, 2006; Aretouli, Okonkwo, Samek, & Brandt, 2011; Loewenstein et al., 2009). Although treatments to prevent or slow dementia are still not available, there is a general consensus that early intervention, before dementia is unambiguous and considerable neuronal damage has already taken place, is likely to be more effective (Petersen, 2003). Thus, knowing the specific MCI subtypes that herald dementia is useful for planning clinical trials.
One approach to the preclinical detection of neurodegenerative diseases is using neuropsychological measures. In particular, tests of executive cognition (EC) appear to decline at the earliest stages of several neurodegenerative diseases. In fact, executive dysfunction can be present in the prodromal phases of AD, Lewy body, frontotemporal dementia, Parkinson's disease and Huntington's disease (Jacobs et al., 1995; Johnson, 2009; Woods & Tröster, 2003). However, the literature remains inconclusive as to whether EC predicts future dementia among persons with MCI better than other cognitive measures or other easily obtainable information, such as demographic and clinical characteristics. It is also unclear whether specific aspects of EC are more useful for prediction than others.
Several longitudinal studies of MCI have suggested that EC measures are associated with subsequent cognitive and functional decline (Albert, Moss, Tanzi, & Jones, 2001; Amieva, Letenneur et al., 2004; Chen et al., 2000; Clark et al., 2012; Crowell, Luis, Vanderploeg, Schinka, & Mullan, 2002). However, these previous studies had methodological limitations. Often, the prognostic value of EC was not examined after controlling for more primary factors associated with outcome in MCI (i.e., demographic characteristics, non-executive cognition, and everyday functioning). In other studies, EC was assessed with only one or two measures. To complicate matters further, some studies have found no association of EC with progression of MCI. For example, Farias et al. (2009) showed that executive dysfunction was not associated with an increased risk of progression to dementia over other factors such as age, education, recruitment source, and functional status. Manly et al. (2008) found that MCI patients with isolated executive impairment were less likely to progress to dementia than those with an isolated memory or isolated language impairment. Finally, Johnson et al. (2010) found that, even in individuals with dysexecutive single-domain MCI, neither performance on EC tasks nor reported dysexecutive symptoms were predictive of MCI status after two years (Johnson et al., 2010).
In our previous study (Aretouli et al., 2011), we found that out of 18 EC tests assessing six conceptually different aspects of EC, only three measures were predictive of subsequent cognitive and functional decline over a two-year period. However, even those three measures failed to independently predict progression to dementia after adjusting for demographic, other non-EC cognitive performances, and rated competence in everyday tasks. Decline over 2 years was best predicted by informant ratings of subtle functional impairments and lower baseline scores on memory, category fluency, and constructional praxis tests.
A limitation of our previous study is the relatively short follow-up period (two years) and the fact that we performed only one re-assessment. Brief periods of observation may favor the detection of disorders with quicker progression and obscure the identification of slowly progressive conditions. Thus, a longer follow-up period might enable the detection of those MCI individuals with milder disease that take longer to progress to dementia (Cui et al., 2011; Wilson, Leurgans, Boyle, & Bennett, 2011). Although the prodromal phase of neurodegenerative disorders may last up to decades, most studies seem to suggest that decline in most cognitive domains is accelerated and may be most reliably distinguished from normal ageing 3 to 6 years (midpoint 4–5 years) before dementia diagnosis (Hall, Lipton, Sliwinski, & Stewart, 2000; Small & Backman, 2007; Wilson et al., 2011).
Although there is no consensus on how many re-assessments are required to model the change from MCI to possible dementia and examine the predictive ability of cognitive factors, multiple follow-ups have certain advantages. First, in a multiple-wave dataset, whether all change occurred immediately after the first assessment as well as the stability or instability of conversion rates (i.e., was the rate steady or did the rate increase/decrease over time?) can be evaluated. Second, multiple waves of data have the advantage of permitting the disentanglement of measurement error with true change (Singer & Willett, 2003).
The present paper describes a prospective, four-year longitudinal study with two fixed re-assessments that examined the outcome of four MCI subgroups and the ability of specific aspects of EC to predict incident dementia in persons with MCI. First, we examined whether specific MCI subtypes are associated with different rates of progression to dementia. Possible changes in rates of conversion to dementia over time were also documented. Despite some evidence to the contrary (Ramakers et al., 2010), most studies show that longer follow-up periods are associated with increased "conversion" rates to dementia (Ashford, 2004). The main aim was to investigate the contribution of EC to the prediction of dementia.
Methods
Participants
A total of 145 persons with MCI enrolled in the study. Four participants were subsequently excluded because they were younger than 55 years old or because they had fewer than 7 years of education. Most of the participants (70%) were recruited from the Johns Hopkins Alzheimer’s Disease Research Center (ADRC) and from other research studies. A smaller number of participants (30%) were referred from University clinics and physicians in the community. Ninety percent of the participants were Caucasian, and 10% were African American.
Participants were diagnosed with MCI according to Petersen criteria (Petersen, 2004; Winblad et al., 2004). The specific procedures applied to establish a diagnosis have been described in detail in our previous publications (Brandt et al., 2009). In brief, participants were required to be non-demented, as indicated by a Mini-Mental State Exam (MMSE) score at or above the 20th percentile for age and education (range: 26–30 in our sample) (Bravo & Hébert 1997), and a global score of 0.5 on the Clinical Dementia Rating Scale (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982). In addition, participants were required to perform at or below 1.5 standard deviations below the mean for age and education (i.e., 6.7th percentile), according to published norms, on one or more of the following screening tests: Logical Memory (story A) of the Wechsler Memory Scale-Revised (WMS-R) (Wechsler, 1987), the 30-item version of the Boston Naming Test (Brandt, 1989; Goodglass, 1983), word list generation [for the letters FAS (Phonemic Fluency) and the semantic categories animals and vegetables (Category Fluency)] (Rascovsky, Salmon, Hansen, Thal, & Galasko, 2007), and clock drawing to request (Rouleau, Salmon, Butters, Kennedy, & McGuire, 1992). Finally, each participant was required to have a study partner who could provide information about his/her functional abilities. Based on their performances on these screening measures participants were categorized into one of four subgroups: amnestic single-domain MCI (AS), amnestic multiple-domain MCI (AM), non-amnestic single domain MCI (NAS), non-amnestic multiple-domain MCI (NAM).
Participants were excluded if they reported any history of major mental illness, central nervous system disorder, or active systemic illness (e.g., cancer). Volunteers with past or present depression were not excluded since depression is very common in MCI and may be related to outcome (Jorm, 2001; Lyketsos et al., 2002).
Follow up status
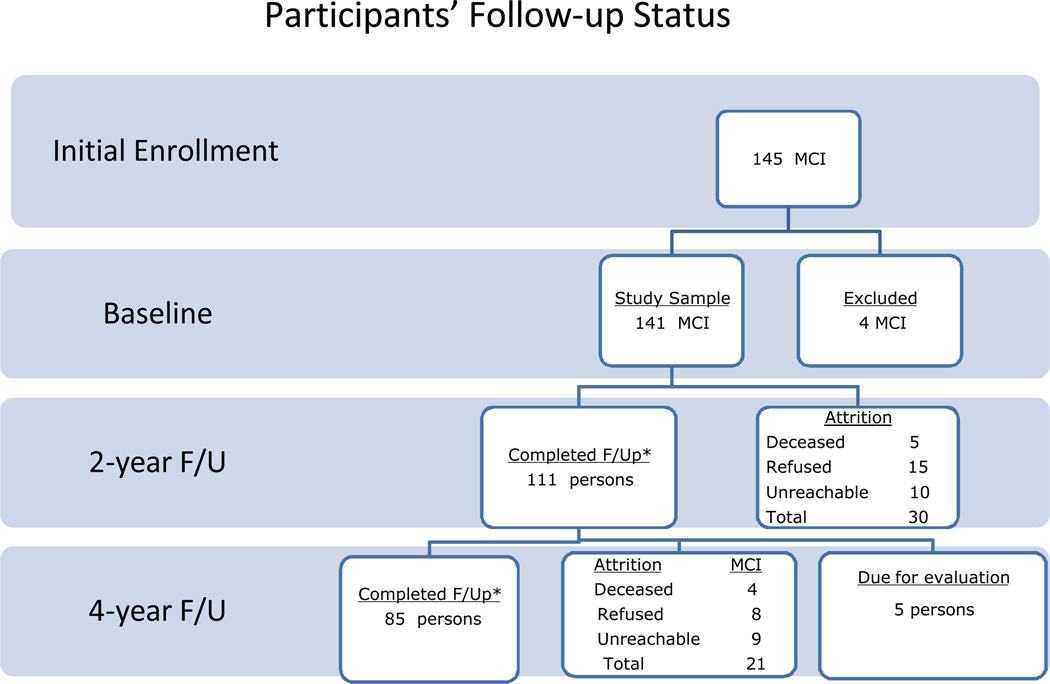
Participants received a baseline and 2 biennial follow-up evaluations. From the 141 participants tested at baseline, 111 completed the first follow-up evaluation after 2 years and 85 completed the second follow-up assessment after 4 years. The attrition rate was 21% over the first two years (annual attrition rate=10.5%) and increased to 31% over the third and fourth years of follow-up (annual attrition rate =15.5%). Reasons for attrition at the 2-year follow-up were death (5 participants; 4% of the total sample), refusal to continue participation (15 participants; 11%), or lost to follow-up/unreachable (10 participants; 7%). Similarly, at the 4-year follow-up, an additional 4 participants were deceased (4%), 8 refused participation (7%), and 9 were unreachable (8%). Figure 1 illustrates the status of the participants over time. When participants were enrolled, they were required to agree to a 2-year follow-up only. Since the 4-year follow-up was added to the research design later, participants were asked to return for this extra visit.
Figure 1.
Participants' follow-up status over the 4 years.
Note: *Numbers include also persons that reverted to CDR=0; F/U= follow-up; MCI= mild cognitive impairment
At the 2-year follow-up, the participants who were lost to follow-up did not differ from those who completed the re-evaluation with respect to demographics, subgroup classification or performance on the screening battery. At the 4-year follow-up, the participants who did not complete the re-assessment had fewer years of education compared to those who contributed data (mean=14.87 vs. 16.24, respectively; F (1,141)=10.30, p=.002). In addition, more AS MCIs completed the 4-year follow-up than were lost to follow-up (75.7% vs. 24.3% respectively), whereas more NAM were lost to follow-up than contributed data (36.8% vs. 63.2%, respectively; χ2=8.104; df=3; p=.044). There were no differences in AM and NAS with regard to the percentage of participants who had 4-year follow-ups.
Procedures
Outcome measure
All participants had a CDR =0.5 at baseline. The CDR overall score was the outcome variable, used to "diagnose" progression to dementia. Four experienced research assistants, who had completed the Brief Training & Reliability Protocol (BTRP) for the CDR through the ADRC website of the Washington University School of Medicine (http://alzheimer.wustl.edu) administered the CDR to the participants' study partners at baseline and both follow-up visits. The study partners were the same throughout the study, whenever possible. The majority of the informants were spouses of the participants (65%), 19% were adult children, 3% were siblings, 4% were other relatives, and 9% were friends. The average length of the association with the participant was 44.44 years (SD=17.04). Participants were considered to have progressed to dementia if they had a CDR global score ≥1 at either the 2- or 4-year follow-up. If they obtained a CDR global score of 0, they are considered to have reverted to normal. If they obtained a 0.5 at follow-up, they are described as still having MCI.
Executive cognition
Eighteen EC measures were derived from clinical and experimental tasks and administered at baseline and after 2 years, according to standard rules. The measures have been described in detail previously (Aretouli et al., 2011; Brandt et al., 2009) and are summarized in Table 1. The tests were chosen to represent the six following executive domains: spontaneous flexibility/generativity, inhibition of prepotent responses, planning/sequencing, concept-rule learning/set shifting, decision-making/judgment, and working memory/resource sharing.
Table 1.
Summary of tests of executive function (from Brandt et al., 2009).
| Proposed Domain | Test | Measures | Scores range |
|---|---|---|---|
| Spontaneous Flexibility and Generativity |
Alternate Uses Test (Guilford, 1978) | Total raw score | 0–36 |
| Random Number Generation (Brugger, Monsch, Salmon, & Butters, 1996) | Sum of written trial RNG and oral trial RNG |
0–2 | |
| Tinker Toy Test (Koss, Patterson, Mack, Smyth, & Whitehouse, 1998) | Total raw score | 0–13 | |
| Inhibition of Prepotent Responses |
D-KEFS Stroop Test (Delis, 2001) | Inhibition trial scaled score | 0–19 |
| Hayling Test (Burgess & Shallice, 1997) | Total scaled score | 0–10 | |
| Completions & Corrections Test (Manning, 2006) |
Total correct score | 0–12 | |
| Planning and Sequencing |
Porteus Maze Test (Porteus, 1965) | Test Age score | 0–17 |
| D-KEFS Tower Test (Delis, 2001) | Total achievement scaled score |
0–19 | |
| Tic-Tac-Toe (Brandt et al., 2009) | Total correct score | (−16)−16 | |
| Concept/Rule Learning and Set Shifting |
D-KEFS Sorting Test (Delis, 2001) | Confirmed sorts scaled score |
0–19 |
| Brixton Spatial Anticipation Test (Burgess & Shallice, 1997) |
Total scaled score | 0–10 | |
| Verbal Concept Attainment Test (Bornstein, 1982) |
Total correct raw score | 0–24 | |
| Decision-Making and Judgment |
Stanford Binet Absurdities Test (Thordike, 1986) |
Total raw score | 0–32 |
| Iowa Gambling Test (Bechara, Damasio, Tranel, & Anderson, 1998) | Advantageous selections (C+D deck responses) on block 5 minus block 1 |
(−20)−20 | |
| Experimental Judgment Test (Brandt et al., 2009) | Mean percent deviation | – | |
| Working Memory and Resource- Sharing |
Trail Making Test (Reitan, 1958) | Time on Part B minus time on Part A |
≤ 600 |
| Brief Test of Attention (Schretlen, Bobholz, & Brandt, 1996) | Total correct raw score | 0–20 | |
| TEA Telephone Search While Counting (Robertson, Ward, Ridgeway, & Nimmo-Smith, 1994) |
Dual task decrement score | – |
Functional status measures
Three functional status measures were administered to the study partners of the participants at all three assessments. Since daily functioning has been shown to be predictive of subsequent decline in many studies (Dickerson, Sperling, Hyman, Albert, & Blacker, 2007), for the purposes of the present analysis, the ratings at the baseline and the 2-year follow-up assessment of the following measures were used as potential predictors of MCI outcome: the Activities of Daily Living-Prevention Instrument (ADCS ADL-PI) (Ferris et al., 2006; Galasko et al., 2006), the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm & Jacomb, 1989) and the Dysexecutive Questionnaire (DEX) (Wilson, 1996). Higher ratings indicate more difficulties in cognitive activities of daily living.
The Johns Hopkins University Institutional Review Board fully reviewed and approved the study protocol. Written informed consent was obtained from all participants and their study partners.
Statistical Analysis
Statistical analyses were performed using PASW 17 (SPSS Inc., Chicago, Illinois) and Stata Version 10 (StataCorp., 2007). One-way analyses of variance (ANOVAs) and chi-square (χ2) were used to compare the four groups on demographic and clinical characteristics at baseline. The χ2 test was further used to evaluate the association between participants’ group and subgroup membership [A-MCI vs. NA-MCI; single domain MCI (SD-MCI) vs. multiple domain MCI (MD-MCI)] at baseline and the frequency of development of dementia at four-year follow-up. In addition, we compared the baseline characteristics and performances of those who progressed to a CDR global score ≥ 1 four years after study entry with the performances of those who remained at 0.5.
Because the current study is focused on predictors of decline in MCI, persons who reverted to normal were excluded from all further analyses. Thus, only participants who remained stable over time (as having mild cognitive impairment) or progressed to dementia (CDR ≥1) were considered in the analyses. At the 2-year follow-up, 82 MCI persons and 19 demented were included in the analysis (with 10 persons who reverted to normal excluded). At the 4-year follow-up, of the 101 participants at the 2-year follow-up, 2 were excluded as they reverted to normal (CDR=0); therefore the final sample was 99 persons. Participants who had missing data at 4-year follow-up were not excluded from the analysis. Of note, none of the persons who were demented at the 2-year follow-up reverted to MCI or normal cognitive status at the four-year follow-up.
We examined whether clinical characteristics and neurocognitive performances could predict progression to dementia over the 4 year period. To test this hypothesis, we used longitudinal logistic regression models using generalized estimating equations (GEE). GEE can be considered an extension of logistic regression for clustered data. This statistical technique evaluates between- and within-person changes over time, accounting for the correlations among repeated measurements on the same individual. At the same time, it increases the power of the analysis through the increased number of observations (Burton, Gurrin, & Sly, 1998; Zeger & Liang, 1986). GEE models were fitted using the exchangeable correlation structure with robust estimation of the standard errors. In the present analysis, we examined the predictive utility of both time-variant predictors (i.e., score on the MMSE at baseline and the 2-year follow-up) and time-invariant predictors (sex, education, participants’ group at study entry). Our outcome was also time-variant and was determined at both follow-up points (CDR overall score at the 2-year and 4-year follow-ups). Although we investigated the outcome of MCI for each MCI subgroup separately, due to the small sizes of the subgroups, the GEE analyses were performed on the MCI group as a whole. Stable MCI was the reference group in all analyses.
As a first step, we assessed the univariate predictive ability of each of the demographic, clinical and cognitive factors. The goal of this procedure was to reduce the number of potential predictors. As a second step, we included in a multivariate model all factors that were significantly associated (p <.05) with the outcome at the univariate level. The aim of this analysis was to test the predictive value of each factor in the context of all others.
Results
Group outcomes at the 4-year follow-up
85 MCIs completed all 3 assessments. The characteristics of all participants with 4-year follow-ups are presented in Table 2. Of the 85 MCI patients at baseline, 48 remained stable (56%), 30 progressed to CDR≥1 (35%, or 9% per year), and 7 reverted to CDR=0 (8%, or 2% per year) at 4-year follow-up. The presence of impairment in memory (i.e., A-MCI vs. NA-MCI) was not associated with progression to dementia (χ2=3.078; df=2; p>.05), whereas the presence of impairments in multiple domains (i.e., SD-MCI vs. MD-MCI), regardless of the specific domains involved, was so associated (χ2=12.87; df=2; p=.002). More specifically, of the 38 MD-MCI individuals at baseline, 21 (55%) progressed to dementia over a 4-year period, compared with only 9 of 47 (19%) SD-MCI patients. Only one of the MD-MCI patients reverted to normal (3%), whereas 6 (13%) of the SD-MCI did. The demographic and clinical baseline characteristics of the participants as a function of their status at each follow-up are summarized in Table 3.
Table 2.
Demographic and baseline clinical characteristics of all participants who had 4-year follow-up. All participants had CDR global scores of 0.5 at first visit. These numbers include the participants who returned to CDR=0 over time. Means + standard errors, unless otherwise noted. For continuously distributed variables, effect sizes are η2 and p values are based on one-way ANOVA. For frequency counts (sex), effect size is Cramer’s V and p value is based on Pearson’s chi-squared test.
| Amnestic MCI | Non-amnestic MCI | p | η2 or V | |||
|---|---|---|---|---|---|---|
| Single Domain |
Multiple Domain |
Single Domain |
Multiple Domain |
|||
| N | 28 | 31 | 19 | 7 | ||
| Age, years | 74.59 (SD=5.84) |
77.00 (SD=7.58) |
73.89 (SD=8.11) |
72.86 (SD=7.82) |
>.05 | .042 |
| Education, highest grade completed |
16.30 (SD=2.16) |
16.58 (SD=2.49) |
15.68 (SD=2.87) |
16.00 (SD=2.52) |
>.05 | .020 |
| Sex, male:female | 21:9 | 21:10 | 12:7 | 1:6 | .045 | .305 |
| Mini-Mental State Exam, score |
28.30 (0.22) |
28.29 (0.20) |
28.53 (0.26) |
27.85 (.43) |
>.05 | .023 |
| Clinical Dementia Rating, sum of boxes |
0.89 (0.15) |
1.58 (0.14) |
1.45 (0.17) |
1.57 (0.29) |
.005 | .146 |
| Geriatric Depression Scale, score |
2.37 (0.44) |
2.74 (0.41) |
1.79 (0.53) |
2.43 (0.87) |
>.05 | .025 |
Table 3.
Demographic and baseline clinical characteristics of the participants who had 2- and 4-year follow-ups. Means ± SDs.
| Baseline | Status at 2-year Follow Up | Status at 4-year Follow up | |||||
|---|---|---|---|---|---|---|---|
| MCI (CDR=.5) |
Normal (CDR=0) |
MCI (CDR=.5) |
Demented (CDR≥1) |
Normal (CDR=0) |
MCI (CDR=.5) |
Demented (CDR≥1) |
|
| N | 141 | 10 | 82 | 19 | 7 | 48 | 30 |
| Age, years | 75.40 (7.84) | 75.50 (5.97) | 75.56 (7.76) | 79.79 (6.12) | 70.86 (5.81) | 74.00 (7.20) | 78.03 (6.73) |
| Education, highest grade completed |
15.70 (2.54) | 15.60 (2.12) | 15.80 (2.63) | 16.32 (2.63) | 16.29 (2.22) | 15.79 (2.72) | 16.93 (1.95) |
| Sex, male:female | 80:61 | 4:6 | 52:30 | 11:8 | 1 :6 | 33 :15 | 20 :10 |
| Clinical Dementia Rating, sum of boxes |
1.27 (0.07) | 0.75 (0.49) | 1.28 (0.75) | 1.71 (0.95) | 1.29 (0.86) | 1.11 (0.68) | 1.68 (0.88) |
| Mini-Mental State Exam, score |
28.11 (0.18) |
28.70 (0.95) | 28.31 (1.15) | 27.79 (1.13) | 29.14 (0.69) | 28.28 (1.16) | 28.17 (1.09) |
| Geriatric Depression Scale, score |
2.35 (0.19) |
1.60 (0.97) | 2.31 (2.37) | 3.21 (2.25) | 1.57 (1.13) | 2.53 (2.52) | 2.33 (2.09) |
Rates of progression to dementia over the 4-year period
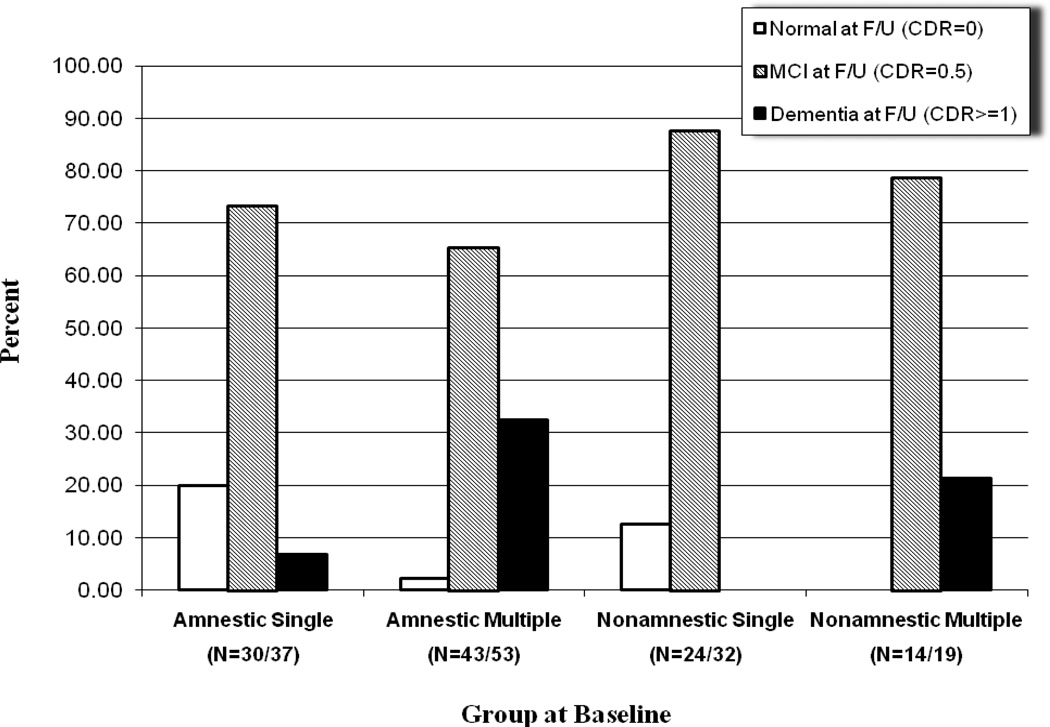
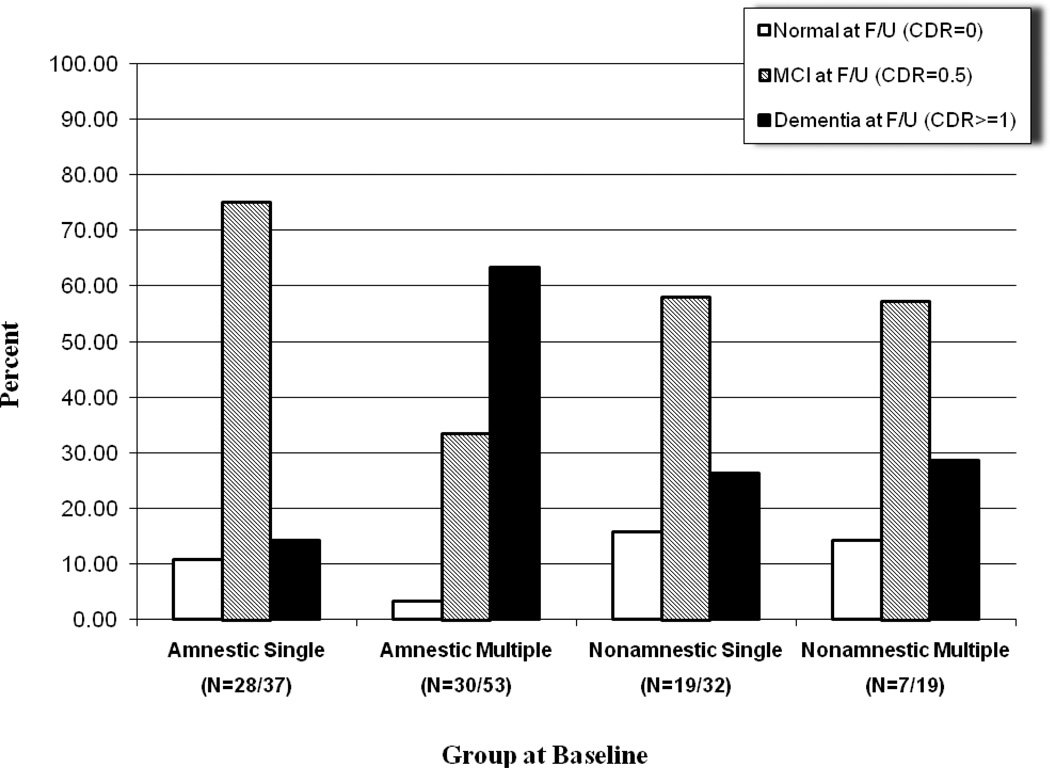
Out of 111 persons diagnosed with MCI at baseline who completed the first follow-up, 9% reverted to the normal cognitive status, 74% remained stable, and 17% progressed to a CDR≥1. The rate of conversion per year over the first two years was 9% per year. As mentioned above, of the 85 MCI patients at baseline, 30 persons had a CDR≥1 at the 4-year follow-up, which corresponds to 35% of the sample. Thus, the incidence rate of dementia increased over the third and fourth year of the study. More specifically, out of the 111 participants who completed the first follow-up, 19 cases (17%) were rated as having a CDR≥1. Out of these 19 cases, 14 received the same CDR ratings at the second follow-up, but 5 were lost to follow-up. At the second follow-up, 16 new cases of dementia were observed (out of the 71 non-demented with CDR=0.5 at the 2-year follow-up who completed the 4-year assessment), which corresponds to 23%. The detailed status of the participants at the 2- and 4-year follow-up as a function of the specific MCI subgroup at study entry is illustrated in Figures 2 and 3 respectively.
Figure 2.
Clinical status at 2-year follow up as a function of group at entry
*Number in the parentheses represent the number of the participants in each group at the specific follow-up and at baseline
Figure 3.
Clinical status at 4-year follow up as a function of group at entry
Baseline demographic, clinical and cognitive characteristics of groups defined by outcome status (for stable MCIs and those who progressed to dementia)
To further explore the outcome of the MCI patient subgroups, we compared the baseline characteristics and performances on cognitive screening measures of those who converted to dementia (n =30) with those who remained MCI (n = 48) at the 4-year follow-up. The stable MCIs were younger at baseline (stable MCI=74.0 vs. 77.9 years; F(1,72)=5.4, p=.024) and had fewer years of education (15.8 vs. 17.0 years; F(1,72)=4.7, p=.033). The two groups had equal sex distributions (χ2=0.07, df=1, p>.05) and similar baseline performances on the screening battery tests (MMSE, Clock Drawing Test, Boston Naming Test, Phonemic Fluency) with the exception of the Category Fluency (animal and vegetables), where those who developed dementia had lower initial scores than those who remained MCI after 4 years (F(1,69)=4.4, p=.040). Finally, the ratings on the CDR sum of boxes differed between the two groups (1.1 for stable MCIs vs. 1.7 for converters; F(1, 72)=12.0, p=.001). More specifically, converters received higher ratings on CDR Judgment and Problem solving domain [F(1, 72)=4.4, p=.039] and CDR Home and Hobbies [F(1, 72)=16.9, p<.001)], indicating mild difficulties on everyday cognitive tasks.
Univariate predictors of MCI outcome
Table 4 summarizes the results of the univariate GEE models. Age was a significant predictor of subsequent decline, as measured with CDR, with each year increasing by 9.4% the odds of achieving a CDR score ≥ 1. Similarly, measures of daily functioning [the ADL-PI (p < 0.001), the IQCODE (p < 0.001), the DEX (p = 0.005) and the CDR sum of boxes (p < 0.001)] were also associated with MCI outcome. More specifically, for every point increase in ADL-PI, IQCODE, and DEX, the odds ratio of progressing to CDR≥1 was 1.21, 19.99, and 1.04, respectively. Four out of the five screening measures were predictive of dementia over the four-year period. A 1-point decrease on the Boston Naming Test (p =0.027), the MMSE (p < 0.001), the Category fluency (p < 0.001) and the Clock Drawing test (p < 0.001) were associated with 8%, 35%, 11% and 34% increase, respectively, in the odds of progression to CDR ≥ 1.
Table 4.
Univariate and multivariate associations between demographic, clinical and neuropsychological characteristics and progression to dementia (CDR≥1) over the 4-year follow-up period.
| Univariate models | Multivariate model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Variables | β | p | OR | 95% CI | AICC | β | p | OR | 95% CI | ||
| low | upper | low | upper | |||||||||
| Demographics | Age | .091 | .005 | 1.094 | 1.028 | 1.166 | 202.535 | .095 | .017 | 1.100 | 1.017 | 1.190 |
| Everyday functioning measures |
ADCS-ADL- PI | .193 | <.001 | 1.212 | 1.115 | 1.318 | 181.617 | .000 | .999 | 1.000 | 0.835 | 1.198 |
| IQCODE | 2.925 | <.001 | 19.990 | 8.056 | 49.603 | 151.226 | 2.179 | .017 | 8.838 | 1.475 | 52.935 | |
| DEX | .037 | .005 | 1.038 | 1.011 | 1.065 | 179.785 | .027 | .307 | 1.027 | 0.976 | 1.081 | |
| Screening battery | BNT | −.085 | .027 | 0.919 | 0.853 | 0.990 | 201.451 | −.031 | .628 | 0.969 | 0.853 | 1.100 |
| MMSE | −.435 | <.001 | 0.647 | 0.563 | 0.743 | 171.131 | −.330 | .054 | 0.719 | 0.514 | 1.006 | |
| Animals and Vegetables | −.118 | <.001 | 0.889 | 0.838 | 0.943 | 180.048 | −.038 | .442 | 0.963 | 0.876 | 1.059 | |
| Clock Drawing Test | −.418 | <.001 | 0.659 | 0.546 | 0.794 | 187.717 | −.263 | .137 | 0.769 | 0.543 | 1.087 | |
| Executive cognition measures |
D-KEFS Stroop Test | −.144 | <.001 | 0.866 | 0.803 | 0.934 | 187.104 | .037 | .573 | 1.038 | 0.911 | 1.183 |
| Verbal Concept Attainment Test | −.175 | <.001 | 0.840 | 0.766 | 0.920 | 180.821 | −.030 | .691 | 0.970 | 0.835 | 1.127 | |
| Stanford Binet Absurdities Test | −.159 | <.001 | 0.853 | 0.782 | 0.929 | 184.345 | −.002 | .978 | 0.998 | 0.849 | 1.173 | |
| Brief Test of Attention | −.130 | <.001 | 0.878 | 0.815 | 0.945 | 182.909 | .032 | .638 | 1.033 | 0.902 | 1.183 | |
| Hayling Test | −.382 | <.001 | 0.682 | 0.552 | 0.844 | 180.813 | −.316 | .069 | 0.729 | 0.519 | 1.026 | |
| Porteus Maze Test | −.131 | .012 | 0.877 | 0.792 | 0.972 | 188.420 | .013 | .889 | 1.013 | 0.843 | 1.218 | |
| Alternate Uses Test | −.131 | <.001 | 0.878 | 0.818 | 0.942 | 181.617 | −.031 | .629 | 0.969 | 0.854 | 1.100 | |
| Corrections and Completions test | −.173 | .017 | 0.841 | 0.730 | 0.969 | 188.965 | .103 | .331 | 1.108 | 0.901 | 1.363 | |
Eight out of the 18 EC measures were also found to predict the status of the MCI participants over the 4-year period. For every point increase in the Hayling test, the odds of progressing to dementia decreased 32% (p < 0.001). One point increase in the Verbal Concept Attainment test and the Corrections and Completions test were associated with 16% (p < 0.001) and 15% (p < 0.017) lower odds of subsequent decline, respectively. One point increase in the Stanford-Binet Absurdities test, and the D-KEFS Stroop test reduced the likelihood of developing dementia by 15% (p < 0.001) and 13% (p < 0.001), respectively. Every point increase in the Brief Test of Attention (p < 0.001), the Porteus Mazes test (p < 0.012), and the Alternate Uses test (p < 0.001) was associated with 12% lower odds of progression to a CDR≥1 (Table 4).
Multivariate model of predictors of MCI outcome
The multivariate GGE model included as predictors all the variables that were significantly associated with MCI outcome at the univariate level. The goals of this analysis were, first, to test the prognostic value of the EC measures in conjunction (when entered simultaneously) with the demographic, clinical and other cognitive variables that contributed to the detection of the MCI individuals who progressed to a CDR≥1; thus, it examines whether EC measures are stronger predictors than other of the factors. The second aim was to identify the optimal subset of predictors that are uniquely associated with the outcome of MCI persons and thus yield a model of dementia prediction with no redundant predictors.
The multivariate GEE model indicated that age (p < 0.017), IQCODE (p < 0.017), MMSE (p < 0.054), and, marginally, Hayling (p < 0.069) were the best predictors of progression to dementia (Table 4). Each year of age increased by 1.1-times the likelihood of progressing to dementia. One point increase in the IQCODE ratings was associated with 8.8-fold increase in the risk of dementia, whereas each point increase in the MMSE and Hayling were associated with 28.1% and 27.1%, respectively, lower odds of subsequent cognitive decline. None of the other EC measures remained associated with the MCI outcome over the 4-year period in the multivariate model. The relative goodness of model fit of the univariate models and the multivariate model was evaluated with the corrected Akaike Information Criterion (AICc) (Akaike, 1974). Lower values indicate better model fit. AICc is recommended when the number of observations is small and the number of parameters/predictors is large. This measure decreases the risk of overfitting (selecting models with many predictors), as it adds a "penalty" when the number of predictors is increasing, resulting in the model with the fewest parameters but at the same time least loss of information. Moreover, it accounts for the specific sample size (Akaike, 1974; Burnham, 2002). The resulting multivariate model had an AICc=141.77, suggesting a better model fit than the fit of any of the univariate models (Table 4).
Discussion
In this prospective longitudinal study, we investigated the ability of EC to predict subsequent cognitive decline in persons with MCI over a four-year period using the most comprehensive battery of EC measures to date. In our previous publication, we documented the limited ability of EC to predict onset of dementia within two years (Aretouli et al., 2011). To exclude the possibility that our observed lack of predictive value of EC is due to the relatively short period of observation, we investigated in the present study the predictive ability of EC over four years.
Over the four-year observation period, 56% persons with MCI remained stable, 35% progressed to CDR≥1, and 8% reverted to CDR=0. The rate of progression to dementia would correspond to 9% per annum, if the overall rate was stable across the four-year period. However, at 2-year follow-up, the rate was 17% (corresponding to 8.5% per year), whereas it increased slightly over the third and fourth year of the study to 22% (annual rate of new cases 11.3%). The majority of persons who declined over this period were MCI individuals with impairments in multiple domains at baseline. The presence of memory impairment, in and of itself, was not associated with elevated rates of conversion to dementia.
In general, more cognitive measures were individually associated with MCI outcome at the 4-year follow-up than with MCI outcome at 2-year follow-up (at the univariate level). Out of the 18 EC measures, only three were associated with MCI outcome at the 2-year follow-up, whereas 8 were associated with outcome at the 4-year follow-up. More specifically, in addition to the Alternate Uses test (which assesses spontaneous flexibility and generativity), the Hayling test (assessing inhibition of prepotent responses) and the Verbal Concept Attainment test (assessing concept/rule learning and set shifting), five more tests --the D-KEFS Stroop test, the Stanford Binet Absurdities test, the Brief Test of Attention, the Porteus Mazes test and the Corrections and Completions test-- were also predictive of subsequent decline. These tests represented all six domains of EC we assessed. Interestingly, all three measures that assess inhibition of prepotent responses were significantly associated with MCI outcome.
Two other recent studies observed that the ability to inhibit an automatic response in favor of a controlled response is predictive of subsequent outcome in MCI (Belanger & Belleville, 2009; Clark et al., 2012), even though the definition of subsequent decline or dementia varied across studies. This is consistent with the finding that measures of inhibitory control and, in particular, semantic inhibition (measured with the Hayling test), yield the greatest in magnitude and the highest in frequency deficit in persons with MCI compared to other tests of EC (Belanger & Belleville, 2009; Johns et al., 2012). Although impairments in controlled inhibitory processes are well-documented in AD (Amieva, Phillips, Della Sala, & Henry, 2004), the mechanisms underlying this phenomenon are not clear. Despite the classical association of inhibition with the prefrontal cortex, recent neuroimaging studies suggest that an extended network subserves inhibitory processes (Bondi et al., 2002; Collette, Van der Linden, Delrue, & Salmon, 2002). The most common interpretation is that disturbed connectivity between widely distributed cortical regions may result in inhibitory failure in patients at the preclinical stage of dementia. Thus, the fact that these inhibitory operations do not rely solely on localized neural networks makes them particularly vulnerable to the earliest pathological processes involved in progressing from normal to pathological aging (Amieva, Phillips et al., 2004; Belanger & Belleville, 2009; de Haan et al., 2011).
A major finding of the present study is that although a number of EC measures were individually associated with subsequent decline, only the Hayling test showed a tendency for association with MCI outcome, in the context of other demographic, clinical and non-executive cognitive variables. Age, MMSE and IQCODE contributed uniquely to the prediction of dementia when entered in a model that considered demographic characteristics, cognitive screening tests, and everyday functioning. The limited predictive ability of EC in the context of other factors is consistent with our findings at the 2-year follow-up (Aretouli et al., 2011), but contradicts the reports of several studies that identify EC as the optimal predictor of dementia (Albert et al., 2001; Amieva, Letenneur et al., 2004; Clark et al., 2012; Elias et al., 2000). However, in none of these studies was the prognostic value of EC examined along with other factors (i.e., demographic characteristics, non-executive cognition, and everyday functioning). Another potential explanation is that some of these studies identified predictors specifically of Alzheimer's disease. However, even in this case, it has been found that MCI patients with isolated executive impairment were less likely to have underlying AD neuropathology and to be less likely to progress to dementia than those with isolated memory or language impairments (Manly et al., 2008). The limited predictive ability of the EC for subsequent dementia in our study has important implications in light of recent efforts to identify clinically homogeneous MCI subtypes with specific etiology and prognosis (Hanfelt et al., 2011; Johnson et al., 2010). Our results do not support the notion that EC-related MCI subtypes constitute a separate MCI group at increased risk for dementia. Our findings suggest that the progression from MCI to dementia is due to the degeneration of cognitive mechanisms more generally.
In addition to the EC measures, several screening tests were predictive of progression to CDR ≥1. Lower scores on MMSE, the Clock Drawing Test, Boston Naming Test and the Category Fluency were associated with increased risk for developing dementia over the four-year period, whereas episodic memory, measured with the delayed recall of the Logical Memory, was no longer significantly associated with outcome. Among these measures, MMSE contributed unique variance to the multivariate model for the prediction of dementia.
Indicators of global cognitive functioning, such as the MMSE, have been found to be at least as good as episodic memory or EC at discriminating those destined to develop dementia from those who will not (Backman, Jones, Berger, Laukka, & Small, 2005). In addition, global cognitive ability measures seem to produce larger differences than episodic memory between those who will eventually develop dementia and those who remain stable over very long follow-up periods (Backman, Jones, Berger, Laukka, & Small, 2004). This is consistent with the "multiple cognitive systems breakdown" hypothesis, which posits that not only the medial temporal lobe is affected at the earliest stage of AD (Backman et al., 2004). It can be argued that global cognitive measures, such as the MMSE, rely on several cognitive abilities that are subserved by extended neural networks. Thus, the widespread brain regions affected plausibly underlie several distinct functions and consequently contribute to lower scores on measures of global cognitive impairment.
Whereas we reported previously that memory was predictive of status at 2-year follow-up (Aretouli et al., 2011), it wasn't associated with outcome over the 4-year period. This lack of significant association could be explained partly by our operationally defining dementia by CDR scores, rather than a diagnosis of AD. Although early memory decline may be a strong predictor for AD, it might not be as prominent a feature of dementias due to other etiologies. However, this methodology allowed us to investigate predictors for all types of dementia. Furthermore, a review of the literature concluded that memory is not a stronger predictor for subsequent cognitive decline than executive functioning or perceptual speed (Backman et al., 2004). In line with this finding, we observed that amnestic MCI subtypes were not at greater risk for progression to dementia. It should be noted, however, that in the present study memory was measured with a single test, Logical Memory. In contrast to EC, which was assessed with an array of specific tasks, the predictive ability of different aspects of memory was not examined. Thus, it is possible that a more detailed and comprehensive assessment of memory would have yielded measures with higher predictive ability for subsequent cognitive decline.
All measures of everyday functioning that were administered in this study were individually associated with MCI outcome over the four-year period. In addition to the ADCS ADL-PI and the IQCODE, we also found that the DEX questionnaire, which specifically assesses daily difficulties caused by executive dysfunction, was predictive of the 4-year outcome of MCI persons. Informant-reported measures of everyday functioning share method variance with the outcome in the present study (CDR global score). This may inflate the observed association between these variables. Nonetheless, accumulating evidence shows that the degree of functional impairment at baseline has significant prognostic value for subsequent decline (Daly et al., 2000; Dickerson et al., 2007; Farias, Mungas, Reed, Harvey, & DeCarli, 2009; Jorm, 2003; Peres et al., 2006; Ravaglia et al., 2006; Rozzini et al., 2007). There are several potential explanations for this association. First, measures of general cognition predict current functional status better than of a single cognitive ability, i.e., memory (Royall et al., 2007). Second, the specific questionnaires assess cognitive change evident in everyday tasks. It is likely that prior decline predicts future decline (Aretouli et al., 2011). A third interpretation is that the multiple brain regions affected during the preclinical stage of AD produce very mild impairments in several cognitive abilities that collectively result in difficulties easily observable at the behavioral level. This latter interpretation is consistent with the finding that measures of general cognition, such as the MMSE, are strongly associated with MCI outcome in our analyses.
There are several limitations in the present study. First, since the participants were not formally evaluated by a clinician in this study, our outcome could not be validated by a clinical diagnosis. However, many of our participants were so-evaluated and diagnosed in other research studies or clinics; out of the 30 patients categorized as demented at the second follow-up 16 reported having a diagnosis of AD, 3 of frontotemporal dementia, 1 vascular dementia, 1 normal pressure hydrocephalus, 3 dementia not otherwise specified and 1 Parkinson's disease. Second, the design of the present study favored (was "biased" toward) EC over other cognitive domains. Consequently, the specific design limits the interpretation and generalizability of our findings regarding the predictive ability for dementia of non-EC cognitive abilities, e.g., memory. However, the mere fact that the predictive ability of EC for dementia was limited in a study design that clearly favored the specific cognitive domain over the rest strengthens our conclusion of the lack of differential unique contribution of EC for the prediction of dementia.
The present study improved upon prior studies in several ways. The GEE models we used included both time variant and in-variant predictors. This approach allowed us to investigate the predictive ability not only of baseline performances but performances over time, (i.e, performances at the 2-year follow-up as predictors of the 4-year status), as well as outcomes over time and not only at the 4-year year follow-up. However, because the models we tested included both time variant and in-variant predictors, it is not clear whether the effects of the time-variant factors represent between-subjects effects or within-subjects effects or both. For example, as a between-subject effect, having one-point higher IQCODE score at baseline would be associated with a 20-fold increased possibility of getting a dementia diagnosis over the 4-years. As a within-subjects effect, the same results would be interpreted as an one point increase in IQCODE in the same person over the 4 years would correspond to an average 20-fold increase in the possibility of having a CDR≥1 over the same period of time. Therefore, regression coefficients in Table 4 are interpreted as the difference of mean scores in each variable, comparing participants scoring one point higher with those scoring one point lower but also comparing participants scoring one point higher to their own baseline score over time. However, we consider more reasonable that a point difference within person over time (as opposed to a point difference between people at baseline) is predictive of subsequent decline.
In summary, the present study investigated the prognostic value of different aspects of EC for dementia in persons with MCI. Our findings show that measures of EC, and especially those that assess inhibitory control, are predictive of MCI outcome at the univariate level, but do not contribute unique variance in the context of other easily obtainable information in a routine clinical examination such as demographic characteristics, other cognitive variables and everyday functioning. Age, IQCODE and MMSE were the optimal predictors of subsequent decline. IQCODE and MMSE both represent indicators of global deterioration, rather than of a specific function that is potentially subserved by a localized neural network. Our results thus support the notion that measures that are indicative of a multiple cognitive system breakdown have high prognostic value for outcome in MCI.
Acknowledgments
The authors thank Ozioma Okonkwo, Ph.D., Jaclyn Samek, B.S., Kevin Manning, M.S., Marilyn Albert, Ph.D., Karen Bandeen-Roche, Ph.D. and the staff and participants of the Johns Hopkins Alzheimer’s Disease Research Center. This study was supported by the National Institute on Aging (J.B., grant AG-005146).
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22(1):27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- Amieva H, Letenneur L, Dartigues JF, Rouch-Leroyer I, Sourgen C, D'Alchee-Biree F. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dementia and Geriatric Cognitive Disorders. 2004;18(1):87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- Amieva H, Phillips L, Della Sala S, Henry J. Inhibitory functioning in Alzheimer's disease. Brain. 2004;127(5):949–964. doi: 10.1093/brain/awh045. [DOI] [PubMed] [Google Scholar]
- Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of the 0.5s: predictors of 2-year outcome in mild cognitive impairment. Journal of the International Neuropsychological Society. 2011;17(2):277–288. doi: 10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford JW. APOE genotype effects on Alzheimer's disease onset and epidemiology. Journal of Molecular Neuroscience. 2004;23(3):157–165. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. Journal of Internal Medicine. 2004;256(3):195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation Of working memory from decision making within the human prefrontal cortex. The Journal of Neuroscience. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger S, Belleville S. Semantic inhibition impairment in mild cognitive impairment: a distinctive feature of upcoming cognitive decline? Neuropsychology. 2009;23(5):592–606. doi: 10.1037/a0016152. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Serody AB, Chan AS, Eberson-Shumate SC, Delis DC, Hansen LA. Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer's disease. Neuropsychology. 2002;16(3):335–343. doi: 10.1037//0894-4105.16.3.335. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. A factor analytic study of the construct validity of the verbal concept attainment test. J Clin Neuropsychol. 1982;4(1):43–50. doi: 10.1080/01688638208401115. [DOI] [PubMed] [Google Scholar]
- Brandt J, Aretouli E, Neijstrom E, Samek J, Manning K, Albert MS. Selectivity of executive function deficits in mild cognitive impairment. Neuropsychology. 2009;23(5):607–618. doi: 10.1037/a0015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Mellits ED, Rovner B, Gordon B, Selnes OA, Folstein MF. Relation of age at onset and duration of illness to cognitive functioning in Alzheimer’s disease. Cognitive and Behavioral Neurology. 1989;2(2):93–101. [Google Scholar]
- Bravo G, Hébert R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. International Journal of Geriatric Psychiatry. 1997;12(10):1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brugger P, Monsch AU, Salmon DP, Butters N. Random number generation in dementia of the Alzheimer type: a test of frontal executive functions. Neuropsychologia. 1996;34(2):97–103. doi: 10.1016/0028-3932(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton Tests Manual. Bury St. Edmunds, England: Thames Valley Test Company Limited; 1997. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. New York: Springer-Verlag; 2002. [Google Scholar]
- Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Statistics in Medicine. 1998;17(11):1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55(12):1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, Bondi MW. Specific measures of executive function predict cognitive decline in older adults. Journal of the International Neuropsychological Society. 2012;18(01):118–127. doi: 10.1017/S1355617711001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Delrue G, Salmon E. Frontal hypometabolism does not explain inhibitory dysfunction in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2002;16(4):228–238. doi: 10.1097/00002093-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Crowell TA, Luis CA, Vanderploeg RD, Schinka JA, Mullan M. Memory patterns and executive functioning in mild cognitive impairment and Alzheimer's disease. Aging, Neuropsychology, and Cognition. 2002;9(4):288–297. [Google Scholar]
- Cui Y, Liu B, Luo S, Zhen X, Fan M, Liu T. Identification of conversion from mild cognitive impairment to Alzheimer's disease using multivariate predictors. PLoS One. 2011;6(7):e21896. doi: 10.1371/journal.pone.0021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Archives of Neurology. 2000;57(5):675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- de Haan W, van der Flier WM, Koene T, Smits LL, Scheltens P, Stam CJ. Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer's disease. Neuroimage. 2011;59(4):3085–3093. doi: 10.1016/j.neuroimage.2011.11.055. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System examiner's manual. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Archives of General Psychiatry. 2007;64(12):1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Archives of Neurology. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Archives of Neurology. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris SH, Aisen PS, Cummings J, Galasko D, Salmon DP, Schneider L. ADCS Prevention Instrument Project: overview and initial results. Alzheimer Disease and Associated Disorders. 2006;20(4 Suppl 3):S109–S123. doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Rockwood K. Outcomes of incident mild cognitive impairment in relation to case definition. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(8):1175–1177. doi: 10.1136/jnnp.2004.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Disease and Associated Disorders. 2006;20(4 Suppl 3):S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz BE, Saxton JA, Chang C-CH, Lee C-W, Vander Bilt J. Outcomes of mild cognitive impairment by definition: A population study. Archives of Neurology. 2011;68(6):761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Guilford JP, Christensen PR, Merryfield PR, Wilson RC. Alternate uses, Form B, Form C; manual of instructions and interpretations. Orange, CA: Sheridan Psychological Services Inc; 1978. [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Statistics in Medicine. 2000;19(11–12):1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hanfelt JJ, Wuu J, Sollinger AB, Greenaway MC, Lah JJ, Levey AI. An exploration of subgroups of mild cognitive impairment based on cognitive, neuropsychiatric and functional features: analysis of data from the National Alzheimer's Coordinating Center. Am J Geriatr Psychiatry. 2011;19(11):940–950. doi: 10.1097/JGP.0b013e31820ee9d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson's disease. Neurology. 1995;45(9):1691–1696. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- Johns EK, Phillips NA, Belleville S, Goupil D, Babins L, Kelner N. The profile of executive functioning in amnestic mild cognitive impairment: disproportionate deficits in inhibitory control. Journal of the International Neuropsychological Society. 2012:1–15. doi: 10.1017/S1355617712000069. [DOI] [PubMed] [Google Scholar]
- Johnson J. Mild cognitive impairment subgroups. In: Miller BL, Boeve BF, editors. The Behavioral Neurology of Dementia. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- Johnson JK, Pa J, Boxer AL, Kramer JH, Freeman K, Yaffe K. Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2010;30(4):344–351. doi: 10.1159/000318836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry. 2001;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF. The value of informant reports for assessment and prediction of dementia. Journal of the American Geriatric Society. 2003;51(6):881–882. doi: 10.1046/j.1365-2389.2003.51276.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychological Medicine. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Koss E, Patterson MB, Mack JL, Smyth KA, Whitehouse PJ. Reliability and Validity of the Tinkertoy Test in Evaluating Individuals with Alzheimer's Disease. The Clinical Neuropsychologist. 1998;12(3):325–329. [Google Scholar]
- Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3-year follow-up period. Dementia and Geriatric Cognitive Disorders. 2009;27(5):418–423. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Journal of the American Medical Association. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning KJ, Brandt J. Completions and Corrections as a test of executive control; Boston, Massachusetts. Paper presented at the 34th Annual Meeting of the International Neuropsychological Society.2006. [Google Scholar]
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. [Article] Acta Psychiatrica Scandinavica. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Peres K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67(3):461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment clinical trials. [Article] Nature Reviews Drug Discovery. 2003;2(8):646–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- Porteus SD. Porteus Maze Test. Fifty year's application. New York, NY: Psychological Corporation; 1965. [Google Scholar]
- Ramakers IH, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FR. Affective symptoms as predictors of Alzheimer's disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychological Medicine. 2010;40(7):1193–1201. doi: 10.1017/S0033291709991577. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2007;21(1):20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dementia and Geriatric Cognitive Disorders. 2006;21(1):51–58. doi: 10.1159/000089515. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The Test of Everyday Attention (TEA) Manual. Bury St. Edmunds, England: Thames Valley Test Company; 1994. [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain and Cognition. 1992;18(1):70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M. Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. International Journal of Geriatric Psychiatry. 2007;22(12):1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the Brief Test of Attention. The Clinical Neuropsychologist. 1996;10(1):80–89. [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York: Oxford University Press; 2003. [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer's disease: a growth mixture modeling analysis. Cortex. 2007;43(7):826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Thordike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale: Fourth Edition, Guide for Administration and Scoring. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67(7):1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Weschler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wilson BA, Alderman N, Burgess P, Emslie H, Evans J. Behavioural Assessment of the Dysexecutive Syndrome. Bury St. Edmunds: Thames Valley Test Co; 1996. [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Archives of Neurology. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Tröster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson's disease. Journal of the International Neuropsychological Society. 2003;9(1):17–24. doi: 10.1017/s1355617703910022. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]