Abstract
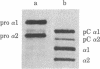
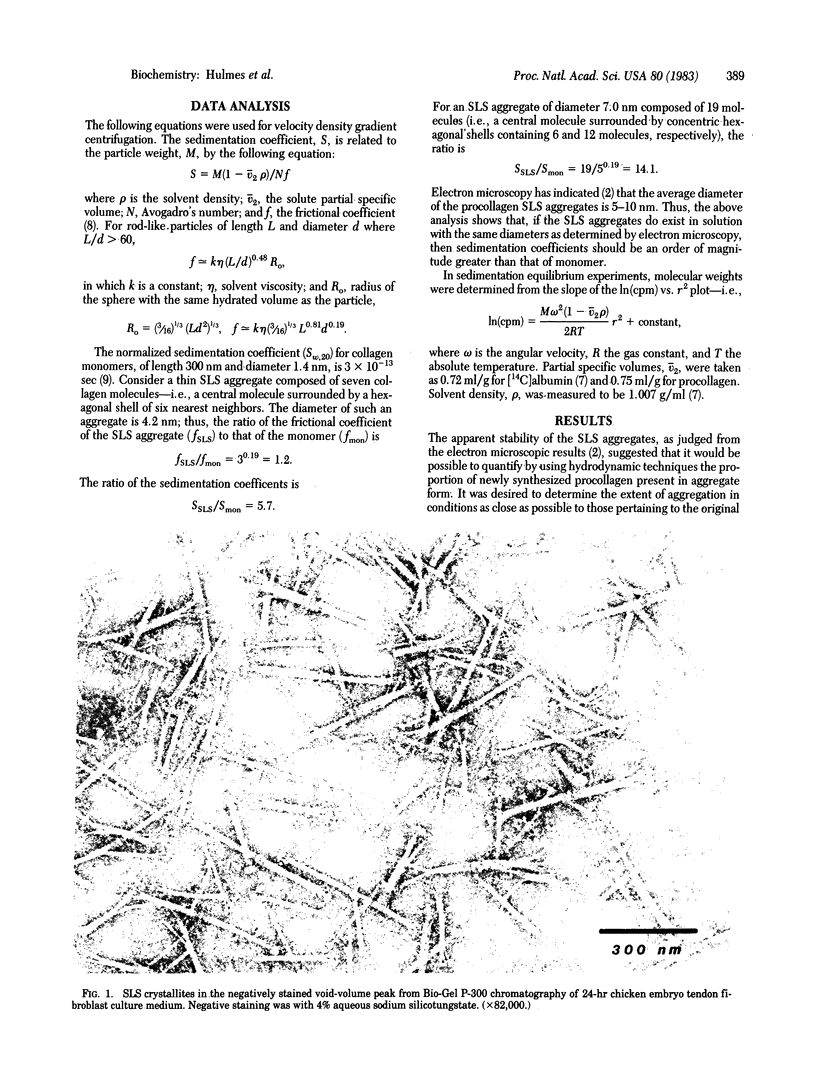
Procollagen and partially processed procollagen from cultures of primary chicken embryo tendon cells appeared as segment-long-spacing (SLS)-like aggregates when drops of medium were negatively stained and examined by electron microscopy. Similar aggregates were obtained after negative staining of medium partially purified by gel filtration and also after staining thin sections of fixed, dehydrated, and embedded pellets formed by prolonged ultracentrifugation of whole culture medium. In contrast to results from electron microscopy, analysis by velocity density gradient sedimentation or sedimentation equilibrium indicated the exclusive presence of procollagen or partially processed procollagen monomers in solution. These contradictory data can be reconciled if procollagen exists in monomeric form when greatly diluted (as in culture medium), and in specific aggregated form (SLS) at high concentration. We believe that cells in vivo secrete procollagen in high, local concentration packaged in the SLS form. We propose that such zero-D arrayed packages are the precursors of native collagen fibrils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. R., Hulmes D. J., Therrien S. F., Gross J. Procollagen segment-long-spacing crystallites: their role in collagen fibrillogenesis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):313–317. doi: 10.1073/pnas.76.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Intermediates in the limited proteolytic conversion of procollagen to collagen. Biochemistry. 1975 Nov 18;14(23):5188–5194. doi: 10.1021/bi00694a026. [DOI] [PubMed] [Google Scholar]
- Davison P. F. Bovine tendons. Aging and collagen cross-linking. J Biol Chem. 1978 Aug 25;253(16):5635–5641. [PubMed] [Google Scholar]
- Fernandez-Madrid F., Noonan S., Riddle J., Karvonen R., Sasaki D. Intracellular processing of procollagen induced by the action of colchicine. J Anat. 1980 Mar;130(Pt 2):229–241. [PMC free article] [PubMed] [Google Scholar]
- Fessler L. I., Fessler J. H. Characterization of type III procollagen from chick embryo blood vessels. J Biol Chem. 1979 Jan 10;254(1):233–239. [PubMed] [Google Scholar]
- Gross J., Highberger J. H., Schmitt F. O. COLLAGEN STRUCTURES CONSIDERED AS STATES OF AGGREGATION OF A KINETIC UNIT. THE TROPOCOLLAGEN PARTICLE. Proc Natl Acad Sci U S A. 1954 Aug;40(8):679–688. doi: 10.1073/pnas.40.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes D. J., Jesior J. C., Miller A., Berthet-Colominas C., Wolff C. Electron microscopy shows periodic structure in collagen fibril cross sections. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3567–3571. doi: 10.1073/pnas.78.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Polymeric C-terminal cross-linked material from type-I collagen. A modified method for purification, anomalous behaviour on gel filtration, molecular weight estimation, carbohydrate content and lipid content. Biochem J. 1980 Jul 1;189(1):111–124. doi: 10.1042/bj1890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Hoffmann H., Prockop D. J. Interchain disulfide bonds at the COOH-terminal end of procollagen synthesized by matrix-free cells from chick embryonic tendon and cartilage. Arch Biochem Biophys. 1976 Jul;175(1):341–350. doi: 10.1016/0003-9861(76)90516-6. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Haase B. A., Standaert M. L. Macromolecular characterization by sedimentation equilibrium in the preparative ultracentrifuge. J Biol Chem. 1979 Jan 10;254(1):30–33. [PubMed] [Google Scholar]
- Schwartz D., Veis A. The visualization of individual collagen molecules and aggregates in collagen solution: the effect of preparation and sampling techniques. Connect Tissue Res. 1978;6(3):185–190. doi: 10.3109/03008207809152630. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Church R. L., Yaeger J. A., Wampler D. E., Park E. Procollagen: intermediate forms containing several types of peptide chains and non-collagen peptide extensions at NH2 and COOH ends. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3009–3013. doi: 10.1073/pnas.71.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Lichtenstein J. R. Removal of amino-terminal and carboxy-terminal extension peptides from procollagen during synthesis of chick embryo tendon collagen. Biochem Biophys Res Commun. 1976 Jul 12;71(1):60–67. doi: 10.1016/0006-291x(76)90249-7. [DOI] [PubMed] [Google Scholar]
- Warshawsky H. The presence of atypical collagen fibrils in EDTA decalcified predentine and dentine of rat incisors. Arch Oral Biol. 1972 Dec;17(12):1745–1754. doi: 10.1016/0003-9969(72)90238-5. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Centrosymmetrical cross-banded structures in the matrix of rat incisor predentin and dentin. J Ultrastruct Res. 1977 Nov;61(2):218–229. doi: 10.1016/s0022-5320(77)80089-0. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]