Abstract
Background: In fertile women, glycodelin and glutathione peroxidase 3 (GPx3) genes expression rises during the luteal phase, with a peak occurring during the implantation window. The expression of these genes decreases in women with myomas. To determine whether myomectomy would reverse glycodelin and GPx3 expression, we evaluated the transcript levels of these genes in the endometrium of patients before and after myomectomy. Methods: Expression of glycodelin and GPx3 genes were examined prospectively during the midluteal phase in the endometrium obtained from infertile women with myoma (n = 12) before and three months after myomectomy. Endometrial expression of these genes was evaluated using quantitative real-time RT-PCR. Results: Endometrial glycodelin mRNA expression levels (normalized to 18S rRNA expression) were increased significantly in endometrium of patients after myomectomy (P = 0.02). GPx3 mRNA expression was increased insignificantly after myomectomy (P = 0.43). Conclusion: The results showed that myomectomy increased endometrial glycodelin (significantly) and GPx3 (not significantly) gene expression after 3 months. Study at different times and detecting expression of these genes can reveal more details. Iran.
Key Words: Myoma, Glutathione peroxidase 3 (GPx3), Endometrium, Glycodelin
Introduction
Uterine myomas are the most common benign tumors found in the female genital tract [1-4]. They are estimated to occur in 20 to 50% of women with an increased frequency during the late reproductive years. Myomas are present in approximately 5%–10% of women with infertility and are the sole factor identified in 1%–2.4% of the infertile patients [5, 6]. Depending on location in the uterus, intramural or submucosal, myomas have been implicated in both recurrent pregnancy loss and infertility [1].
Intramural myomas that distort the endometrial cavity, are associated with lower pregnancy, implantation, and delivery rates in women undergoing IVF compared with infertile women without myomas [7].
It is plausible that detrimental effect of myoma on implantation could be through adverse effects on the endometrium and impaired endometrial receptivity is considered to be a major limiting factor for pregnancy establishment [8].
Reproductive outcomes are improved after myo-mectomy, and the difference will be more pronounced if the myoma is the only identifiable etiology of infertility.
The molecular mechanisms implicated in the endometrium after removal of intramural myoma are still unknown. In an attempt to develop a clinically relevant and reproducible evaluation of endometrial function, a number of molecular markers specific to the implantation window were identified. For instance, expression of the epithelial endometrial glycodelin and glutathione peroxidase 3 (GPx3) genes increases during mid-secretory phase coincident with the opening of this window [9]. Decreased midluteal glycodelin and GPx3 expressions have been reported in connection with some luteal phase deficiency [10].
Glycodelin is a glycoprotein from the human lipocalins superfamily and is mainly expressed in reproductive tissues. This protein contains a number of glycosylation sites, which is found in amniotic fluid [11], endometrium, and deciduas [12]. The pro-gestagen-associated endometrial protein gene, which is an official symbol for the glycodelin gene, is located in the chromosomal region 9q34. It is composed of 7 exons and has four response putative elements for glucocorticoids/progesterone upstream from its promoter region [10].
Although the precise function of glycodelin is still unknown, it is believed that its activity could depend on the type of glycosylation that it undergoes. Some of the proposed functions are contraception, immuno-suppressant, participation in angiogenesis, and apoptosis. Moreover, there are also activities independent from glycosylation, such as glandular morphogenesis induction, epithelial differentiation, and tumor suppression [10, 13]. The glycodelin synthesis in endometrium seems to be regulated by progesterone [10, 14].
GPX3 acts to protect cells from damage from unstable reactive radicals and heavy metals [15, 16]. The glutathione peroxidases are a family of reducing agents, which function to reduce hydrogen peroxide and organohydroperoxides. Free radical damage is implicated in the pathophysiology of a number of organs including the endometrium. Glutathione peroxidases reduce these free radicals to harmless compounds. The glutathione peroxidase family is a selenium-dependent protein [17]. Selenium deficiency in women is associated with spontaneous abortion and infertility, thus the selenium-dependent GPx may play a role around the time of implantation to protect the embryo from oxidant damage and to create a safe environment for reception of a fertilized ovum [17, 18]. It has been shown that GPx3 is highly expressed in secretory phase compared with proliferative one [15].
Horcajadas et al. [19] have shown that expression of GPx3 and glycodelin is down-regulated 9 and 12 times in large intramural myoma in relation to healthy women without uterine disorders. In this study, we investigated the effect of myomectomy on expression of glycodelin and GPx3 mRNA.
MATERIALS AND METHODS
This case-control study included women of reproductive age, who had uterine myoma with a size greater than 5 cm and were infertile. The subjects were identified prior to surgery, and all of them underwent myomectomy. At the time of surgery, the following data were obtained: age (under 38), uterine size, obstetric and gynecologic history, medical conditions, medications, surgical history, and last menstrual period. Subjects had not used hormonal medications for at least 3 months prior to surgery. They also did not have any other condition demonstrated to affect endometrial receptivity, such as endometriosis, polycystic ovarian syndrome, or hydrosalpinges. The size, numbers, and location of myoma were documented by ultrasound.
Endometrial tissue biopsies were performed during 19 to 23 days of a menstrual cycle (which is overlapped to midluteal phase) before and 3 months after myomectomy using an endometrial suction catheter. Each sample was divided into two portions. The first tissue portion was fixed in 10% formalin for histopathological examination. The second portion was immediately collected in RNA extraction solution (RNX-Plus, Cinagene Company, Iran) and stored at -80ºC until further analysis. All tissue samples were obtained with full and informed patient consent. The research protocol was approved by the Medical Ethics Committee of Hamadan University of Medical Sciences (Iran). Endometrium from subjects before and after myomectomy was evaluated for mRNA expression of glycodelin and GPx3.
RNA extraction. To obtain total RNA, each sample was placed in 1 ml RNA extraction solution (RNX- Plus, Cinagene Company, Iran) and homogenized by a homogenizer. The cellular lysate was incubated, chloroform (0.2 mL) was added, and the samples were then centrifuged (17,000 ×g at 4°C for 15 min). A clear aqueous phase was collected and transferred to a new tube, and RNA was precipitated with isopropanol and washed with 75% ethanol. The RNA pellet was air dried, then resuspended with RNase-free water [14]. From all obtained RNA samples, 2 μl was analyzed using the Epoch Microplate Spectro-photometer (Biotek, USA).
Reverse transcription. Single-stranded cDNA was synthesized using AccuPower RT PreMix Kit (Bioneer, Republic of Korea) using 1 µg RNA according to the manufacturer's protocol. The transcription process included incubation of the reaction mixture at 20°C for 30 s, followed by 5 min at 44°C, 30 s at 55°C, and 5 min at 95°C. The cDNA was stored at -80°C until further use for PCR.
Quantitative real-time PCR. PCR analyses were performed using C1000 Thermocycler, CFX96 Real-Time System (BioRad, USA), and QuantiFast SYBR Green PCR Kit (Bioneer, Korea) in a final volume of 25 μl with 10 pmol of each primer. The reaction was incubated at 95°C for 5 min, followed by 40 cycles of 15 s at 95°C, 30 s at annealing temperature, 30 s at 72°C, and then fluorescence was measured. Each assay was run in triplicate with each set of primers. Primer pairs for the amplification of cDNA coding for glycodelin and GPx3 were designed from the GenBank databases using the AlleleID 6 software and checked for minimum overlap. The sequences of primers, accession number, and products length have been presented in Table 1. Annealing temperatures were 45.9°C, 43.6°C, and 53.5°C for glycodelin, GPx3, and 18S rRNA, respectively. Specificity of PCR amplifications was verified by a melting curve program (70-95°C with a heating rate of 0.5°C/s and a continuous fluorescence measurement) and analyzed by electrophoresis on a 1% agarose gel, 1× Tris-borate-EDTA buffer. To choose an appropriate housekeeping gene for normalization, we examine β-actine and 18S rRNA. Of the two genes examined, 18S rRNA was more stable in both control and mayoma endometrium. Therefore, we used 18S rRNA as internal controls for quantitative reverse transcription polymerase chain reaction analysis.
Table 1.
Primer sequences used in PCR
| Gene | Primer sequence | Product size (bp) | Accession no. |
|---|---|---|---|
| Glycodelin | Sense: 5’ CTGGTGGAGGACGATGAG 3’ Anti-sense: 5’ CTCTGGAGGTGTGGAAGG 3’ |
195 | NM_001018049 |
| GPx3 | Sense: 5’ GTCTCCAACCACACTATCTAC 3’ Anti-sense:5’ ACACACAATCACGCATACC 3’ |
162 | NM_002084.3 |
| 18S rRNA | Forward: 5’ GTAACCCGTTGAACCCCATT 3’ Reverse: 5’ CCATCCAATCGGTAGTAGCG 3’ |
152 | X03205 |
Data analyses. Cycle threshold (Ct) values were obtained through the auto Ct function. Following efficiency correction, the mean of CT value was calculated and then normalized to the reference gene (18S rRNA) using delta (Δ)CT. Changes in relative expression were calculated using the 2-∆∆ct method [20]. The specific transcripts were presented as a n-fold change relative to pre-myomectomy level.
Statistical analysis. ΔCT was reported as means ± SEM of three independent experiments. Values of P<0.05 were considered significant. Results were analyzed using t-test for comparison between pre- and post-operation.
Results
The clinical characteristics of infertile patients with myoma (n = 12) have been summarized in Table 2. In this study, the mean (± SD) age of all patients with myoma was 31.7 ± 2.65 years. The mean size of myoma was 4.02 ± 1.64 × 6.04 ± 1.44 cm. The study subjects had an average duration of infertility of 9.7 ± 6.9 years. Of 12 people, 2 got pregnant following surgery (Table 1). All samples underwent histological evaluation, and normal mid-secretory phase of the endometrium was identified.
Table 2.
Demographic characteristics of infertile patients with my
| Patient number | Age | Duration of infertility (year) | Number of myoma | Size of myoma (cm) | Type of myoma | Pregnancy after myomectomy |
|---|---|---|---|---|---|---|
| 1 | 37 | 9 | 1 | 6.0 × 6.0 | intramural | + |
| 2 | 36 | 17 | 2 | 4.6 × 6.0 3.5 × 5.0 |
intramural | - |
| 3 | 35 | 22 | 2 | 2.3 × 4.0 5.5 × 1.5 |
intramural | - |
| 4 | 36 | 10 | 2 | 4.8 × 7.0 2.0 × 2.0 |
intramural | - |
| 5 | 33 | 7 | 1 | 6.5 × 5.3 | intramural | + |
| 6 | 37 | 14 | 2 | 2.5 × 6.0 6.0 × 6.0 |
intramural | - |
| 7 | 30 | 6 | 4 | 3.8-9.0 4.0 × 4.0 |
intramural | - |
| 8 | 31 | 1 | 1 | 5.6 × 42 | intramural | - |
| 9 | 35 | 18 | 4 | 2.6-7.0 2.0 × 3.0 |
intramural | - |
| 10 | 31 | 10 | 2 | 1.5 × 7.0 1.3 × 4.0 |
intramural | - |
| 11 | 37 | 2 | 1 | 4.0 × 5.0 | intramural | - |
| 12 | 37 | 1 | 2 | 5.5 × 2.0 3.5 × 4.5 |
intramural | - |
To evaluate the effect of myomectomy on endometrial glycodelin and GPx3 mRNA expression in subjects with myoma, pre- and post-operative endometrial mRNA expression levels on each subject were analyzed by quantitative real-time RT-PCR. For each person and gene, ΔCT was computed and compared between the two groups.
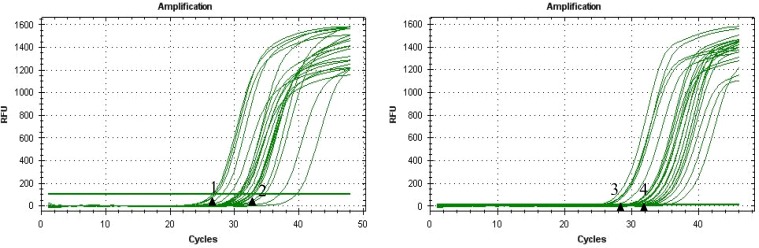
Expression of GPX3 and glycodelin mRNA before and after myomectomy in endomtrium was efficiently amplified (Fig. 1). In most cases, the Ct value of GPx3 and glycodelin before myomectomy was smaller than that after removal of myoma in the same sample. For example, No. 1 (post-operative) and No. 2 (pre-operative) were from the same patients before and after myomectomy, and their Ct values for GPx3 were 26.3 and 31.6, respectively (Fig. 1).
Fig. 1.
Amplification plots of real time PCR. (A) GPx3 and (B) glycodelin before and after myomectomy. Numbers 1 and 2 are from the same patient indicate the RT-PCR results after (26.3) and before myomectomy (31.6), respectively for GPx3. Numbers 3 and 4 are from the same patient indicate the RT-PCR results after (28.2) and before myomectomy (31.6), respectively for glycodelin
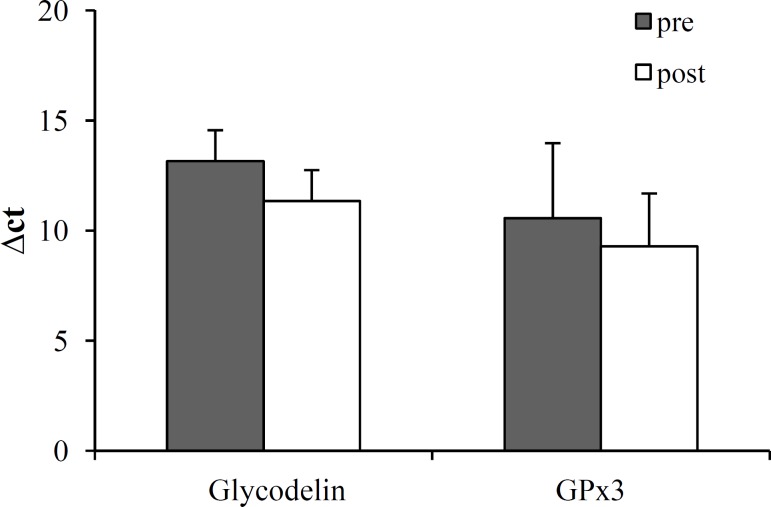
An increase in endometrial glycodelin expression was seen in all post-myomectomy endometrial samples compared with corresponding pre-myomectomy samples (ΔCT = 11.35 ± 1.61 versus 13.16 ± 1.43, P = 0.02) (Fig. 2). Glycodelin levels were increased by 3.5-fold flowing myomectomy after normalization with 18S rRNA.
Fig. 2.
Level of GPx3 and glycodelin in pre- and post-myomectomy. Values shown (ΔCt) are relative to those of 18S rRNA
Endometrial GPx3 mRNA expression (normalized to 18S rRNA expression) was higher in uteri with post-
than pre-operative samples; however, this difference was not significant (mean ΔCT = 9.29 ± 2.40 and 10.57 ± 3.36, respectively; P = 0.43) (Fig. 2). An increase in endometrial GPx3 expression was seen in 9 of the 12 post-myomectomy endometrial samples. GPx3 levels were increased by 2.4 folds after myomectomy after normalization with 18S rRNA.
Discussion
Endometrial development resulting in endometrial receptivity requires an intricate coordination of a number of architectural, cellular, and molecular events. Impaired endometrial growth and differentiation may be an important factor that contributes to infertility and recurrent pregnancy loss. It has been reported that 50% to 75% of the pregnancy loss are due to failure in implantation [21].
There is a general agreement that submucous or intramural leiomyomas protruding into the endometrial cavity are associated with decreased implantation and pregnancy rates [19]. Also multiple studies including a recent meta-analysis have shown the presence of non-cavity-distorting intramural fibroids is related to adverse pregnancy outcomes in women undergoing IVF treatment [22]. Therefore, the overall benefit of myomectomy before IVF in improving reproductive outcome has been recommended as a therapeutic option for patients with myoma [23]. The molecular mechanisms implicated in the endometrium after removal of intramural myoma are still unknown.
In this study, the effect of myomectomy on the endometrium was evaluated using two established molecular markers of endometrial receptivity: glycodeline and GPx3. It has been shown that expression of GPx3 and glycodelin are down-regulated 9 and 12 folds in large intramural myoma in relation to healthy women without uterine disorders [19].
Glycodelin, also called placental protein 14, is a member of the lipocalin family of proteins. It is synthesized in the endometrium in response to progesterone and relaxing and has immunosuppressive properties; therefore, it has been suggested as a potential receptivity marker [10]. We here demonstrate that endometrial expression of glycodelin mRNA is increased significantly at the time of embryo implantation in patients who have undergone myo-mectomy, suggesting an improvement in endometrial receptivity. These findings support the previous reports that have demonstrated detrimental effects of myoma on implantation and the benefit of its surgical removal on endometrial [22, 24, 25].
GPx3 is a selenoprotein enzyme that protects cells from oxidative damage by catalyzing the reduction of hydrogen peroxidase, lipid peroxides, and organic hydroxyperoxide by glutathione [9, 26]. In reproductive tissues of female mice, it is regulated by 17 β-estradiol and selenium [27]. Its expression increases during the receptive compared with the pre-receptive phase [9]. According to our result, GPx3 mRNA expression in endometrium of post-myomectomy subjects shows a higher level in pre-operative patients; however, the differences failed to reach statistical significance.
These two genes (glycodelin and GPx3) were down-regulated during the window of implantation in some non-optimal conditions, such as in controlled ovarian stimulation with GnRH agonists [28], endometriosis [29], myoma [30], and in the presence of an intrauterine device [31].
The effect of intramural leiomyomas on molecular determinants of endometrial receptivity is controversial [21]. Horcajadas et al. [19] using functional genomics of the endometrium during the window of implantation suggested that endometrial receptivity should not be affected by the presence of intramural myomas not distorting the uterine cavity. In a search for differences in gene expression between women with and without leiomyomas, they found that of 25 genes that could be relevant to endometrial receptivity, 3 genes, namely GPx3, glycodelin, and aldehyde dehydrogenase 3 family member B2, were down-regulated in the presence of large leiomyomas [19]. In agreement with Horcajadas et al. study [19], both glycodelin and GPx3 gene expressions were increased in the endometrium after removal of myoma. However, the increased level of GPx3 expression is not significant after 3 months.
Our results are in line with the Horcajadas et al. [31] study that have shown GPx3 mRNA expression remains dysregulated one year after the intrauterine device removal.
In the current study, myomectomy was performed for infertile women who had liomyoma with average size of 4.02 ± 1.64 × 6.04 ± 1.44 with or without distorting the endometrial cavity and for those who had repeated in vitro fertilization-embryo transfer failure over three or more cycles. Three months after myomectomy, only 2 out of 12 women got pregnant. This event demonstrates that 3 months after myoma removal, the endometrium does not recover its normal gene expression pattern. It is predicted that endometrium should not have recovered its normal receptivity.
In agreement with changes in endometrium in some non-optimal conditions, Horcajadas et al. [31] investigated the global gene expression profile of the endometrium during the window of implantation in the same fertile woman in the presence or absence of an intrauterine device after two months and one year. Their results demonstrated that after one year, the majority (80%) of the genes recovered their normal expression profile at mid-secretory phase.
In a study by Daftary et al. [32], expression of HOXA10 was examined during the midluteal phase in endometrium obtained from infertile women with hydrosalpinges before and after salpingectomy. They showed that salpingectomy resulted in a statistically significant, a 15-fold increase in endometrial HOXA10 expression.
In our study, the endometrial biopsies were performed three months after myoma removal in midluteal phase. This strategy has been accepted for the normalization of the endometrium after three cycles of continued treatment in certain disorders such as dysfunctional uterine bleeding [33, 34].
To detect the progressive change in gene expression in endometrium, sequential biopsies should be performed; however, this is not possible due to ethical concerns. Therefore, the gene expression and timing of optimal improvement in endometrial receptivity markers following myomectomy remains undetermined.
ACKNOWLEDGEMENTS
We are most grateful to all patients who kindly participated in the present study. This research, which is a part of a MSc. thesis, has been funded by Vice Chancellor for Research and Technology of Hamadan University of Medical Sciences and Health Services (Iran).
References
- 1.Manyonda I, Sinthamoney E, Belli AM. Controversies and challenges in the modern management of uterine fibroids. BJOG. 2004 Feb;111(2):95–102. doi: 10.1046/j.1471-0528.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 2.Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004 Aug;104(2):393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 3.Olive DL, Lindheim SR, Pritts EA. Non-surgical management of leiomyoma: impact on fertility. Curr Opin Obstet Gynecol. 2004 Jun;16(3):239–43. doi: 10.1097/00001703-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Amir M, Romano S, Goldman S, Shalev E. Plexin-B1, glycodelin and MMP7 expression in the human fallopian tube and in the endometrium. Reprod Biol Endocrinol. 2009 Dec;:7:152. doi: 10.1186/1477-7827-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002 Jun;17(6):1424–30. doi: 10.1093/humrep/17.6.1424. [DOI] [PubMed] [Google Scholar]
- 6.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007 Apr;87(4):725–36. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 7.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008 Apr;198(4):357–66. doi: 10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010 Apr;93(6):2027–34. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003 May;9(5):253–64. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 10.Meola J, Dentillo DB, Rosa e Silva JC, Ferriani RA, Veiga LC, Paro de Paz CC, et al. Glycodelin expression in the endometrium of healthy women and in the eutopic and ectopic endometrium of women with endometriosis. Fertil Steril. 2009 May;91(5):1676–80. doi: 10.1016/j.fertnstert.2008.02.158. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002 Aug;23(4):401–30. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 12.Julkunen M, Koistinen R, Sjöberg J, Rutanen EM, Wahlström T, Seppälä M. Secretory endometrium synthesizes placental protein 14. Endocrinology. 1986 May;118(5):1782–6. doi: 10.1210/endo-118-5-1782. [DOI] [PubMed] [Google Scholar]
- 13.Park JK, Song M, Dominguez CE, Walter MF, Santanam N, Parthasarathy S, et al. Glycodelin mediates the increase in vascular endothelial growth factor in response to oxidative stress in the endometrium. Am J Obstet Gynecol. 2006 Dec;195(6):1772–7. doi: 10.1016/j.ajog.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Vaisse C, Atger M, Potier B, Milgrom E. Human placental protein 14 gene: sequence and characterization of a short duplication. DNA Cell Biol. 1990 Jul-Aug;9(6):401–13. doi: 10.1089/dna.1990.9.401. [DOI] [PubMed] [Google Scholar]
- 15.Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, et al. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9(1):19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- 16.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 17.Beltran-Garcia MJ, Espinosa A, Herrera N, Perez-Zapata AJ, Beltran-Garcia C, Ogura T, et al. Formation of copper oxychloride and reactive oxygen species as causes of uterine injury during copper oxidation of Cu-IUD. Contraception. 2000 Feb;61(2):99–103. doi: 10.1016/s0010-7824(00)00085-8. [DOI] [PubMed] [Google Scholar]
- 18.Kingsley PD, Whitin JC, Cohen HJ, Palis J. Developmental expression of extracellular glutathione peroxidase suggests antioxidant roles in deciduum, visceral yolk sac, and skin. Mol Reprod Dev. 1998 Apr;49(4):343–55. doi: 10.1002/(SICI)1098-2795(199804)49:4<343::AID-MRD1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Horcajadas JA, Goyri E, Higón MA, Martínez-Conejero JA, Gambadauro P, García G, et al. Endometrial receptivity and implantation are not affected by the presence of uterine intramural leiomyomas: a clinical and functional genomics analysis. J Clin Endocrinol Metab. 2008 Sep;93(9):3490–8. doi: 10.1210/jc.2008-0565. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data Using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Makker A, Goel MM. Uterine leiomyomas: effects on architectural, cellular, and molecular determinants of endometrial receptivity. Reprod Sci. 2013 Jun;20(6):631–8. doi: 10.1177/1933719112459221. [DOI] [PubMed] [Google Scholar]
- 22.Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010 Feb;25(2):418–29. doi: 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 23.Li TC, Mortimer R, Cooke ID. Myomectomy: a retrospective study to examine reproductive performance before and after surgery. Hum Reprod. 1999 Jul;14(7):1735–40. doi: 10.1093/humrep/14.7.1735. [DOI] [PubMed] [Google Scholar]
- 24.Horne AW, Critchley HO. The effect of uterine fibroids on embryo implantation. Semin Repord Med. 2007 Nov;25(6):483–9. doi: 10.1055/s-2007-991046. [DOI] [PubMed] [Google Scholar]
- 25.Bernard G, Darai E, Poncelet C, Benifla JL, Madelenat P. Fertility after hysteroscopic myomectomy: effect of intramural myomas associated. Eur J Obstet Gynecol Reprod Biol. 2000 Jun;88(1):85–90. doi: 10.1016/s0301-2115(99)00123-2. [DOI] [PubMed] [Google Scholar]
- 26.Esworthy RS, Doan K, Doroshow JH, Chu FF. Cloning and sequencing of the cDNA encoding a human testis phospholipid hydroperoxide glutathione peroxidase. Gene. 1994 Jul;144(2):317–8. doi: 10.1016/0378-1119(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 27.Waters KM, Safe S, Gaido KW. Differential gene expression in response to methoxychlor and estradiol through ERα, ERβ, and AR in reproductive tissues of female mice. Toxicol Sci. 2001;63(1):47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005 Mar;11(3):195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 29.Alizadeh Z, Shokrzadeh N, Saidijam M, Sanoee MF. Semi-quantitative analysis of HOXA11, leukemia inhibitory factor and basic transcriptional element binding protein 1 mRNA expression in the mid-secretory endometrium of patients with endometriosis. Iran Biomed J. 2011;15(3):66–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Shokrzadeh N, Alizadeh Z. Semi-quantitative analysis of endometrial receptivity marker mRNA expression in the mid-secretory endometrium of patients with uterine fibromas. African J Biotechnol. 2012 Mar;11(23):6220–5. [Google Scholar]
- 31.Horcajadas JA, Sharkey AM, Catalano RD, Sherwin JR, Domínguez F, Burgos LA, et al. Effect of an intrauterine device on the gene expression profile of the endometrium. J Clin Endocrinol Metab. 2006 Aug;91(8):3199–207. doi: 10.1210/jc.2006-0430. [DOI] [PubMed] [Google Scholar]
- 32.Daftary GS, Kayisli U, Seli E, Bukulmez O, Arici A, Taylor HS. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril. 2007 Feb;87(2):367–72. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Seli E, Kayisli UA, Cakmak H, Bukulmez O, Bildirici I, Guzeloglu-Kayisli O, et al. Removal of hydrosalpinges increases endometrial leukaemia inhibitory factor (LIF) expression at the time of the implantation window. Hum Reprod. 2005 Nov;20(11):3012–17. doi: 10.1093/humrep/dei188. [DOI] [PubMed] [Google Scholar]
- 34.Speroff L, Fritz MD. Clinical gynecologic endocrinology and infertility. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]