Abstract
Bacground: Evidence from several lines of investigations suggests that Toll-like receptor 4 (TLR4) is involved in atherosclerosis as a bridge between innate and acquired immunity. Percutaneous coronary intervention (PCI) can trigger inflammation through activation of human TLR4 (hTLR4) on monocytes. Hydrocortisone as an anti-inflammatory and immuno-suppressant agent has multiple mechanisms of action. In this study, we aimed at assessing the effects of hydrocortisone on monocyte expression and activity of hTLR4 in patients underwent PCI. Methods: Blood samples were taken from a total of 71 patients with chronic stable angina who were scheduled for a PCI, before the intervention. Thirty patients received 100 mg hydrocortisone prior to the procedure. Control group was composed of 41 patients underwent PCI without receiving hydrocortisone. Blood collection was repeated 2 and 4 h after PCI. The expression of hTLR4 on the surface of CD14+ monocytes and the serum levels of TNF-α and IL-1β were measured using flowcytometry and Sandwich ELISA. Results: Compared with controls, hydrocortisone significantly reduced monocyte expression of hTLR4 in test group (P<0.01). In addition, it had a significant effect on reduction of serum concentrations of TNF-α and IL-1β in test group in a time-dependent manner (P<0.01). Conclusion: In this study, hydrocortisone was able to reduce the hTLR4/CD14 positive monocytes and its related pro-inflammatory cytokines, thus it can decrease inflammatory responses following PCI.
Key Words: Toll-like receptor 4, Cytokines, Hydrocortisone
Introduction
Inflammation and immune cell activation are well-established processes in ischemic heart diseases. A considerable amount of evidence links the mechanisms underlying the initiation of coronary plaque development and disruption with inflammation [1-3]. A large body of interest has continuously centered on understanding the role of cellular and molecular components of inflammation involved in coronary artery diseases (CAD). Toll-like receptors (TLR), a class of pattern recognition receptors, have emerged as bridges between innate and adaptive immunity [4]. Most mammalian species have between 10 and 15 types of TLR. Ten functional TLR have been identified in human [5]. Among them, human TLR4 (hTLR4) has gained prominence in researches related to atherosclerosis and cardiovascular disorders [6]. hTLR4 can be activated by a number of exogenous and endogenous ligands such as bacterial lipopolysaccharides, fungi [7], heat shock protein 60 and 70 [8], minimally modified LDL and oxidized LDL [9]. hTLR4 has considerable expression on leukocytes including CD14+ monocytes and dendritic cells [10]. Its activationis by ligands is followed by interaction with an adaptor protein, myeloid differentiation factor (MyD88), and translocation of nuclear factor kappa B (NF-κB) to nucleus which eventually leads to production of pro-inflammatory cytokines, such as TNF-α, IL1-β, and IL-6 [11, 12]. Pro-inflammatory cytokines in the myocardium and peripheral tissues have been identified as important markers of myocardial dysfunction [13, 14]. Glucocorticoids are widely recognized as anti-inflammatory agents that are of immense value in suppressing immunity. The immuno-suppressive and anti-inflammatory actions of glucocorticoids are inextricably tied together; perhaps they both involve inhibition of leukocyte functions [15]. Revasculariz-ation therapies such as percutaneous coronary intervention (PCI) are applied for patients with stable angina who are refractory to medical therapy [16]. We previously reported that PCI could trigger inflammatory responses through up-regulation of monocyte expression of hTLR4 and elevation in serum levels of TNF-α and IL1-β [17]. Hydrocortisone is used prior to PCI procedure to prevent allergic reactions due to contrast materials. However, its administration is not conventionally performed in all of the PCI procedures. Previous studies have not adequately assessed the effects of different drugs on expression and activity of inflammatory components in PCI setting [6, 16]. We hypothesized that a part of anti-inflammatory effects of hydrocortisone is mediated through suppression of hTLR4 expression and activity. The present study was designed to investigate the effects of hydrocortisone on the expression of hTLR4 on circulating CD14+ monocytes in patients underwent PCI and also to explore its effect on hTLR4 downstream signaling products.
MATERIALS AND METHODS
Study patients. We studied 30 patients with stable angina, who were scheduled for an elective PCI, in Shahid Madani Heart Hospital, Tabriz, Iran. The exclusion criteria were as follows: previous myocardial infarction within 6 months, autoimmune diseases, inflammatory conditions, advanced hepatic or renal disease, and malignant neoplastic diseases. None of the patients had valvular heart disease. Cardiovascular risk factors, medications, sex, age, and previous medical history were obtained by a questionnaire and by medical records of the patients. All PCI procedures were carried out according to protocols of the hospital. White blood cell count, cholesterol, glucose, prothrombin time, partial thromboplastin time, blood urea nitrogen, creatinine, sodium, and potassium were measured according to routine protocols. Control group was composed of 41 patients with stable angina who underwent PCI without receiving hydrocortisone. The study was approved by the local ethical committee, and an informed consent letter was obtained from all participants.
Blood collection and processing. A total of 6 ml blood was taken via antecubital venipuncture at time of admission (0h). Of 6 ml blood, 2 ml was kept in sterile EDTA bottles for flowcytometry analysis and 4 ml was centrifuged (3000 ×g for 5 min) to obtain serum. Then, the serums were kept in -80oC. Blood collection was repeated 2 and 4 h after termination of PCI procedure.
Stenting procedure. Test and control groups received intravenous heparin (10,000 U) before the stenting procedure. Hydrocortisone succinate (100 mg, Rotexmedica, Trittau, Germany) was intravenously administered into test group 30 min before the procedure. All individuals took aspirin (80 mg) and clopidogrel (75 mg) per day.
Flowcytometry analysis. Briefly, cells were stained at 4°C for 30 min with monoclonal antibodies for human CD14 (Abcam, Cambridge, UK) conjugated with FITC and hTLR4 (Abcam) conjugated with phycoerythrin. FITC and phycoerythrin-conjugated non-specific mouse IgG2a antibodies were used for isotype controls (Abcam). Cells were washed, and cell-associated fluorescence was measured using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA). Data were analyzed by CellQuest software (BD Biosciences).
Measurement of TNF-α and IL-1β serum levels by ELISA. For measurement of TNF-α and IL-1β serum levels, a Sandwich ELISA was performed (Ray Biotech, Norcross, Georgia, US). In short, 100 µl serum was added to microtiter plates. The incubation time was at room temperature for 2.5 h. After that, 100 µl prepared biotinylated antibody and streptavidin solution were added to each well and incubated at room temperature. The intensity of the color was measured at 450 nm by Stat Fax 2600 (Awareness Technology, Palm City, Florida, USA) plate reader. Stat Fax 2100 (Awareness Technology) was used for washing steps.
Statistical analysis. Data are reported as mean ± SD or numbers (percentage). Means were compared by Wilcoxon signed-rank test. Differences at the level of P<0.05 were considered statistically significant. All analyses were performed using SPSS 16.0.
Results
Characteristics of patients . Table 1 shows clinical characteristics of studied patients. No significant difference was observed in gender, age, and use of medications, including aspirin, beta-blockers, statins, angiotensin-converting enzyme inhibitors, nitro-glycerin or blockers of angiotensin receptor 2, white blood cell count or the risk factors among individuals.
Table 1.
Clinical characteristics of the subjects in the study
| Characteristics | Control (n = 41) | Test (n = 30) |
|---|---|---|
| Age, years | 58 ± 9.7 | 54 ± 8 |
| Male | 28 (68.3) | 21 (70) |
| Hypertension | 20 (48.8) | 21 (70) |
| Diabetes | 22 (53) | 24 (80) |
| Hyperlipidemia | 11 (26.8) | 11 (36.6) |
| Familial history | 9 (22) | 10 (33.3) |
| Smoking | 13 (31.7) | 11 (36.6) |
| Medications | ||
| Nitrates | 21 (51.2) | 21 (70) |
| β blockers | 27 (65.9) | 27 (90) |
| ACE inhibitors | 19 (46.3) | 14 (46.6) |
| α2 blockers | 13 (24.07) | 4 (13.3) |
| Calcium channel blockers | 7 (17.1) | 5 (16.6) |
| Statins | 35 (85.4) | 28 (93.3) |
| ASA | 38 (92.7) | 26 (86.6) |
| Laboratory parameters | ||
| C- reactive protein (mg/dl) | 0.4 ± 1.7 | 0.4 ± 1.3 |
| Cholesterol (mg/dl) | 190 ± 40 | 188 ± 37 |
| LDL (mg/dl) | 141 ± 31 | 141 ± 21 |
| HDL (mg/dl) | 42 ± 6 | 41 ± 9 |
| Glucose (mg/dl) | 111 ± 23 | 97 ± 21 |
| White blood cell count (U/L) | 7.8 ± 0.2 | 7.1 ± 0.4 |
Data are shown in number (%) or mean ± SD; ACE, angiotensin converting enzyme; ASA, acetyl salicylic acid; Angiotensin receptor 2 (A2 blockers)
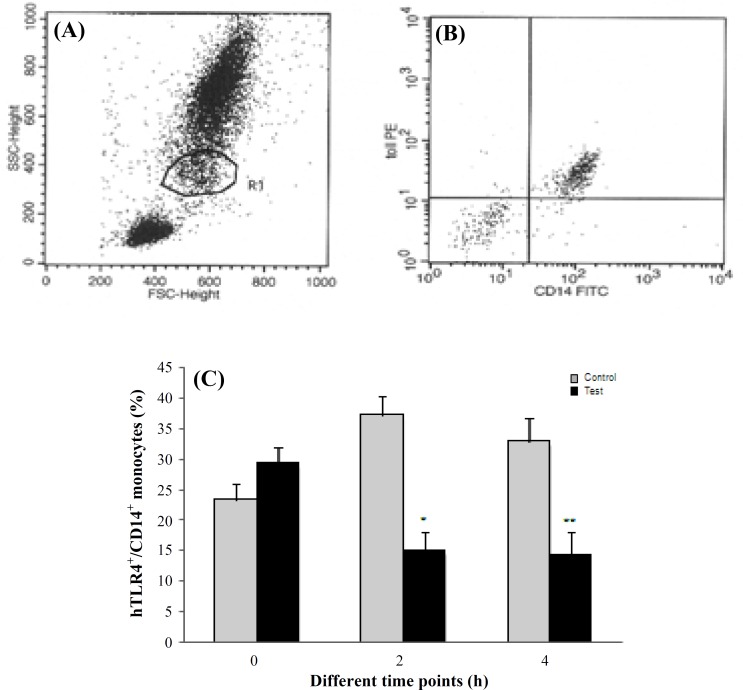
Expression of hTLR4 on CD14 positive monocytes. As assessed by flowcytometry, monocytes showed surface staining for hTLR4 and the monocyte marker CD14 (Fig. 1A and 1B). The percentage of CD14 positive monocytes containing hTLR4 was 23.3 ± 2.7 in control group and 29.3 ± 2.1 in test group at baseline. In controls, hTLR4+/CD14+ monocytes were 37.2 ± 3.7% and 32.9 ± 3.3%, respectively 2 and 4 h after PCI. A significant reduction in hTLR4+/CD14+ monocytes was seen in test group 2 and 4 h after PCI (15.1 ± 3.7% and 14.3 ± 3.3%, respectively, P<0.01, Fig. 1C) as compared to control group,.
Fig. 1.
Monocyte expression of hTLR4 in the study subjects. (A) Gating monocyte population. (B) Representative dot plot showing hTLR4+/CD14 + monocytes. Frequency of hTLR4+/CD14+ on circulating monocytes at different time points (%) (C). Data are shown as mean ± SD. Cells (n = 10,000) were analyzed using flowcytometry in controls and patients before PCI (0h) and 2 hour (2 h) and 4 hour (4 h) after PCI, * P<0.01 vs. control group at 2h; ** P<0.01 vs. control group at 4h.
Serum levels of TNF-α. As presented in Table 2, serum concentration of TNF-α significantly eas reduced significantly to 13.4 ± 2.7 (pg/ml) and 11.7 ± 1.6 (pg/ml) 2 and 4 h after PCI, respectively as compared to controls.
Table 2.
Serum levels of TNF-α (pg/ml) at different time intervals
| Group |
Time intervals (h)
|
||
|---|---|---|---|
| 0 | 2 | 4 | |
| Control (n = 41) | 23.5 ± 3.7 | 35.4 ± 5.1 | 34.2 ± 3.3 |
| Test (n = 30) | 21.5 ± 2.2 | 13.4 ± 2.7* | 11.7 ± 1.6** |
P<0.01 vs. control group at 2 h;
P<0.01 vs. control group at 4 h
Serum levels of IL-1β. Two and 4 h after PCI, a significant reduction of IL-1β was seen in serum (9.1 ± 2.3 and 8.5 ± 1.4 (pg/ml), respectively) (Table 3).
Table 3.
Serum levels of IL-1β (pg/ml) at different time intervals
| Group |
Time intervals (h)
|
||
|---|---|---|---|
| 0 | 2 | 4 | |
| Control (n = 41) | 14.8 ± 1.8 | 30.1 ± 4.0 | 18 ± 2.4 |
| Test (n = 30) | 15.1 ± 3.3 | 9.1 ± 2.3* | 8.5 ± 1.4 |
P<0.01 vs. control group at 2 h
Discussion
In the present study, we showed, for the first time, that hydrocortisone was able to reduce the expression of hTLR4 on CD14+ monocyte and decrease serum levels of TNF-α and IL-1β in patients who underwent PCI compared to those who did not received this drug. Our results suggest that hydrocortisone suppresses inflammatory responses following PCI by inhibiting the monocyte expression of hTLR4+/CD14+. It also causes a considerable reduction in serum levels of pro-inflammatory cytokines. Several mechanisms are involved in the suppression of inflammation by glucocorticoids. Direct physical association between activated glucocorticoid receptors and NF-κB inhibits transcription activity. Theses protein-protein cross talks have been observed between the glucocorticoid receptors and NF-κB, which regulates the expression of many components of the immune system. Glucocorticoids can up-regulate the cytoplasmic inhibitor of NF-κB, I-κB and thus inhibit translocation. In addition, glucocorticoids have inhibitory effects on genes for inducible nitric oxide synthase, cyclooxygenase-2, and inflammatory cytokines [15, 18, 19]. These results highlight the distinguished role of glucocorticoids as anti-inflammatory and immuno-suppressive agents. Immune system activation and inflammation are widely involved in the development and progression of CAD [20]. Therefore, markers of
inflammation are used prognostically for patients with acute coronary syndromes. It is well established that monocytes contribute to all steps of atherosclerosis, from plaque formation to plaque instability and finally thrombosis [21, 22]. In concert with T cells, they secrete multiple cytokines and enzymes that contribute to plaque rupture. Significant data support the expression of TLR4 on the surface of monocytes not only in blood but also in arterial intima during atherogenesis [23, 24]. Fishbein et al. [25] demonstrated that in atherosclerotic lesions TLR4 is mainly expressed on monocytes and up-regulated by oxidized LDL, suggesting a link between lipids, inflammation, and atherosclerosis. A study showed that patients with acute coronary syndromes expressed enhanced TLR4 circulating monocytes compared to the patients with stable angina [6]. In addition, in an experimental study, inhibition of TLR4 caused a remarkable reduction in detrimental effects of myocardial ischemia-reperfusion [26]. During injury, HSP-60 and HSP-70 may be released and trigger inflammatory response through activation of hTLR4 [8]. This finding was supported by Yang et al. [27] that confirmed a time-dependent expansion of hTLR4 monocytes in patients with ST-elevation myocardial infarction who received urokinase as a reperfusion therapy. They also showed that TNF-α serum levels were increased in a time-dependent manner after a successful reperfusion therapy. In contrast, Versteeg et al. [28] provided evidence that PCI negatively downregualted hTLR4+ monocytes in patients with chronic stable angina. However, its contribution to the inflammatory responses is an area of controversy. Inoue et al. [29] findings supported the PCI role in inflammation. Our previous study also proved that PCI positively regulated hTLR+/CD14+ monocytes and increased serum levels of pro-inflammatory cytokines [17], whereas opposite results were found in other studies [28, 30]. It is of note that most of investigations support the critical role of inflammation in restenosis even after implantation of drug-eluting stents. Along with multiple components of inflammation, cytokines play important roles in atherosclerosis. Ridker et al. [31] demonstrated that patients with higher levels of TNF-α were more prone to recurrent coronary events after myocardial infarction suggesting the role of cytokines in pathogenesis of myocardial infarction. On the other hand, IL-1β induces the activation and proliferation of monocytes and stimulates further production of cytokines in atheroegensis [32]. Contrary to expectations, glucocorticoids administration has not been a successful treatment and did not improve clinical outcome. Hoeven et al. [33] reported that treatment with dexamethasone-eluting stents in patients with diabetes mellitus is linked with higher restenosis rate in patients without diabetes mellitus. In contrast, Kakio et al. [34] revealed that administration of hydrocortisone reduced the rate of in-stent restenosis. Taken together, it seems that current results are far from conclusive and are not uniformly consistent. Currently, it is not known with certainty that how hydrocortisone can influence expression patterns of hTLR4+/CD14+ monocytes and related downstream signaling. It should be further investigated whether it can impair protein or mRNA levels of hTLR4. It is noteworthy that statins have been suggested to suppress monocyte expression of hTLR4. However, no significant difference was seen among our studied patients in the use of medications. In conclusion, in the present study, we could demonstrate that hydro-cortisone reduces monocyte expression of hTLR4 and pro-inflammatory cytokines. This finding may be applied in other inflammatory disorders in which hTLR4 are involved. Beneficial effects of gluco-corticoids in reduction of coronary restenosis and possible association between reduction in hTLR4 expression and incidence of stent thrombosis or other coronary events should be adequately assessed. Furthermore, an effective and standard therapy to prevent stent thrombosis should be ascertained. Some limitations of our study should be acknowledged. Due to limited number of patients, our data should be confirmed in larger studies. It is worth noting that only a short-term effect of hydrocortisone on hTLR4 was assessed. Accordingly, more studies are needed to ascertain whether the effect of repeated doses of hydrocortisone on hTLR4 expression persists at long-term follow-up.
ACKNOWLEDGMENTS
This study was supported by a grant from Vice Chancellor for Research of Tabriz University of Medical Sciences. The authors wish to thank the staff of Shahid Madani Heart hospital for all of their contributions.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999 Jan;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association Circulation. 1994 May;89(5):2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 3.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003 Feb;41(4 Suppl S):15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001 Aug;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001 Nov;1(2):135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of Circulating Toll-Like Receptor 4-Positive Monocytes in Patients with Acute Coronary Syndrome. Circulation. 2005 May;111(20):2654–61. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 7.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003 Feb;15(1):5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000 Jan;164(2):558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001 Dec;104(25):3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001 Sep;6(9):733–42. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004 Jul;101(29):10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palsson-McDermott EM, O’Neill LAJ. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004 Oct;113(2):153–62. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IrwinW , Mak S, Mann DL, Qu R, Penninger JM, Yan A, et al. Tissue expression and immunolocalization of tumor necrosis factor-α in post infarction dysfunctional myocardium. Circulation. 1999 Mar;99(11):1492–8. doi: 10.1161/01.cir.99.11.1492. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan;328(2):106–13. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 15.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008 Oct;4(10):525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 16.Rihal CS, Raco DL, Gersh BJ, Yusuf S. Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations. Circulation. 2003 Nov;108(20):2439–45. doi: 10.1161/01.CIR.0000094405.21583.7C. [DOI] [PubMed] [Google Scholar]
- 17.Bagheri B, Sohrabi B, Movassaghpour A, Mashayekhi S, Garjani A, Shokri M, et al. Monocyte expression of toll-like receptor-4 in patients with stable angina undergoing percutanoeus coronary intervention. Iran J Immunol. 2012 Sep;9(3):149–58. [PubMed] [Google Scholar]
- 18.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kB or activator protein-1: Molecular mechanisms for gene repression. Endocr Rev. 2003 Aug;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 19.Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: A family affair. Trends Endocrinol Metab. 2008 Nov;19(9):331–9. doi: 10.1016/j.tem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Nature. 2002 Dec;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Okamoto Y, Rocha V, Folco E. Inflammation in atherosclerosis: Transition from theory to practice. Circ J. 2010 Feb;74(2):213–20. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 22.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 23.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008 Aug;18(6):228–32. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002 Mar;105(10):1158–61. [PubMed] [Google Scholar]
- 25.Xu X, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, et al. Toll-Like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001 Dec;104(25):3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 26.Chong A, Shimamoto A, Hampton C, Takayama H, Spring D, Rothnie C, et al. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004 Aug;128(2):170–9. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Jin LY, Ding JW, Zhou YQ, Yang J, Yang R, et al. Expression of Toll-like receptor 4 on peripheral blood mononuclear cells and its effects on patients with acute myocardial infarction treated with thrombolysis. Arch Med Res. 2010 Aug;41(6):423–9. doi: 10.1016/j.arcmed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Versteeg D, Hoefer IE, Schoneveld AH, de Kleijin DP, Busser E, Strijider C, et al. Monocyte Toll-Like receptor 2 and 4 responses and expression following percutaneous coronary intervention: association with lesion stenosis and fractional flow reserve. Heart. 2008 Jun;94(6):770–6. doi: 10.1136/hrt.2007.117259. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T, Komoda H, Kotooka N, Morooka T, Fujimatsu D, Hikichi Y, et al. Increased circulating platelet-derived microparticles are associated with stent-induced vascular inflammation. Atherosclerosis. 2008 Jan;196(1):469–76. doi: 10.1016/j.atherosclerosis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Tiong AY, Lowe HC, Freedman SB, Brieger DB. Lack of widespread inflammation after contemporary PCI. Int J Cardiol. 2010 Apr;140(1):82–7. doi: 10.1016/j.ijcard.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-a and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000 May;101(18):2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 32.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006 Apr;86(2):515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 33.Van der Hoeven BL, Pires NM, Warda HM, Putter H, Quax PH, Schalij MJ, et al. Dexamethasone-eluting stents for the prevention of in-stent restenosis: evidence for a differential effect in insulin-dependent and non-insulin-dependent diabetic patients. Int J Cardiol. 2008 Feb;124(2):166–71. doi: 10.1016/j.ijcard.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Kakio T, Matsumori A, Ohashi N, Yamada T, Nobuhara M, Saito T, et al. The effect of hydrocortisone on reducing rates of restenosis and target lesion revascularization after coronary stenting less than 3 mm in stent diameter. Intern Med. 2003 Nov;42(11):1084–9. doi: 10.2169/internalmedicine.42.1084. [DOI] [PubMed] [Google Scholar]