Abstract
Background: Acrylamide (ACR) is a well-known industrial toxic chemical that produces neurotoxicity, which is characterized by progressive central and peripheral neuronal degeneration. Chrysin is a natural, biologically active flavonoid compound, which is commonly found in many plants. The antioxidant and neuroprotective properties of chrysin have been demonstrated. Methods: In this study, the possible effect of chrysin on ACR-induced toxicity was evaluated in both in vitro and in vivo experiments. PC12 cells were used as a suitable in vitro model. Cells were exposed to chrysin (0.5-5 µM) for 12 and 24 h, and then ACR in IC50 concentration was added to the cells. Finally, cell viability was determined using (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium assay. For in vivo assay, Wistar rats were treated with ACR (50 mg/kg i.p. for 11 days) alone or in combination with chrysin (12.5, 25, and 50 mg/kg). At the end of treatment, behavioral index was evaluated. Results: ACR decreased cell viability and pre-treatment with chrysin (0.5-5 µM) significantly decreased ACR-induced cytotoxicity in the time- and dose-dependent manner. In Wistar rats, exposure to ACR significantly induced severe gait abnormalities, but treatment with chrysin (50 mg/kg) reduced ACR-induced neurotoxicity in animals. Conclusion: In the current study, chrysin exhibited neuroprotective effect on PC12 cells as an in vitro model and also on Wistar rats. Iran.
Key Words: Acrylamide, Chrysin, Neurotoxicity, Antioxidant
Introduction
Acrylamide (ACR), is an α,β-unsaturated carbonyl compound, which is used extensively to manufacture polyacrylamides. Polymers are used in different industries, including water and wastewater management, soil coagulation, and dye synthesis as well as in laboratories for gel electrophoresis [1, 2]. ACR monomer is a potent neurotoxic in both human and animals, while polymers are not toxic [1]. Besides, occupational exposure (diet) is an important source of environmental ACR in human, because this monomer is formed in carbohydrate- rich foods, when they are prepared in high temperature [3]. Exposure to ACR leads to neurotoxicity, genotoxicity, carcinogenicity, and reproductive toxicity in animals. However, only neurotoxicity has been considered by epidemiological studies on human population [4]. Because of importance of ACR neurotoxicity, many studies have been performed to demonstrate different mechanisms involving in ACR-induced neurotoxicity. Oxidative stress and apoptosis play an important role in ACR-induced toxicity [5-9]. ACR increased lipid peroxidation, declined antioxidant capacity in nervous tissue and sciatic nerve [10] and induced apoptosis in cerebral cortex [9]. Exposure to ACR elevated reactive oxygen spices generation and induced apoptosis in PC12 cells [11]. Exogenous antioxidants exhibited benefit effects for protecting against oxidative damage. Recently, protective effect of crocin as a natural antioxidant has been shown in toxicity induced with ACR or diazinon [11-15]. Therefore, because of both importance of oxidative stress in induction of neurodegenerative diseases and focus on antioxidant activity for ameliorating progressing damage of oxidative stress, we decided to evaluate possible neuroprotective effect of chrysin on ACR-induced neurotoxicity in both in vitro and in vivo experiments.
Chrysin (5,7-dihydroxyflavone) is a natural flavonoid presented in many plant extracts including blue passion flower (Passiflora caerulea) and honey [16, 17]. Similar to other flavonoids, chrysin has many pharmacological effects, including anticancer [17-20], anti-inflammatory [21], antioxidant [22-24], and anti-hypertensive [25] effects. Chrysin interferes with the TNF-α signaling pathway and significantly sensitized human cancer cells to TNF-α-induced apoptosis [17]. Moreover, chrysin (5-25 µM) enhanced doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines via depletion of glutathione [26].
Studies suggested that chrysin could be as a neuroprotective agent in different models. Chrysin markedly decreased the level of MDA as a marker of lipid peroxidation in cortex and hippocampus following chronic cerebral hypoperfusion in rats, while elevated antioxidant enzymes activity [27] and consequently ameliorated brain damage. Another study exhibited that chrysin does-dependently inhibited tunicamycin-induced neuronal cell death in SH-SY5Y cells via inhibition of mitochondrial apoptosis pathway [28]. Regarding to neuroprotective and anti-oxidant activities of chrysin as mentioned above, possible protective effect of this agent was evaluated in current study.
MATRIALS AND METHODS
Chemicals. FBS and RPMI-1640 were purchased from Gibco (USA) and ACR from Merck (Germany). (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) and chrysin were obtained from Sigma (Germany).
Cell culture. PC12 cells were obtained from Pasteur Institute of Iran (Tehran). They were then maintained in a humidified atmosphere (90%) containing 5% CO2 at 37°C. The cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin.
Cell viability. The MTT assay was used to determine cell viability [11]. In this method, PC12 cells were grown in a 96-well microtiter plate at a density of 5000 cell/well. After pretreatment with chrysin (0.5, 0.75, 1, 2.5, and 5 µM) for 12 and 24 h, ACR at final concentration of 5 mM was added to each well. After incubation for 24 h, cells were treated with MTT solution (final concentration 0.5 mg/mL) at 37°C for 3 h. Finally, the insoluble formazan was solubilized in DMSO. The absorbance was measured at 545 nm (630 nm as a reference) in an ELISA reader (Start Fax-2100, UK).
Experimental animals. Male Wistar rats, 230-250 g, were housed in colony rooms with 12/12 h light/dark cycle at 21 ± 2ºC and had free access to food and water. All animal experiments were carried out in accordance with Mashhad University of Medical Sciences, Ethical committee Acts.
Experimental design. ACR at daily dose of 50 mg/kg/day (i.p.) for 11 days was used to induce neurotoxicity in Wistar rats [2, 29]. This daily dose-rate and corresponding route has been well characterized with respect to neuropathological expression and neurological deficits. In our study, the rats were divided at random into 7 groups (n = 6 in each group), and treatment was given as follows:
Group 1, control, normal saline
Group 2) ACR, 50 mg/kg i.p. for 11 days
Group 3) ACR, 50 mg/kg i.p. for 11 days + chrysin 12.5 mg/kg
Group 4) ACR, 50 mg/kg i.p. for 11 days + chrysin 25 mg/ kg
Group 5) ACR, 50 mg/kg i.p. for 11 days + chrysin 50 mg/kg
Group 6) ACR, 50 mg/kg i.p. for 11 days + vitamin E 200 mg/ kg
Group 7) chrysin, 50 mg/kg
The behavioral index (gait scores) examination. At the end of animal treatment, the gait scores were examined according to the methods described by LoPachin [2]. Rats were placed in a clear plexiglass box and observed for 3 min. Following observation, a gait score was assigned from 1 to 4, where 1 = a normal, unaffected gait, 2 = a slightly affected gait (foot splay, slight hind limb weakness, and spread), 3 = a moderately affected gait (foot splay, moderate hind limb weakness, and moderate limb spread during ambulation), and 4 = a severely affected gait (foot splay, severe hind limb weakness, dragging hind limbs, and inability to rear).
Statistical analysis. Results were expressed as mean ± SD. For in vitro assay, IC50 value was calculated using Prism (version 6), and statistical analyses were performed with ANOVA, followed by Tukey-Kramer test to compare the differences between means. For in vivo assay, nonparametric test (Kruskal-Wallis test) was used for statistical analysis. Differences were considered statistically significant when P<0.05.
Results
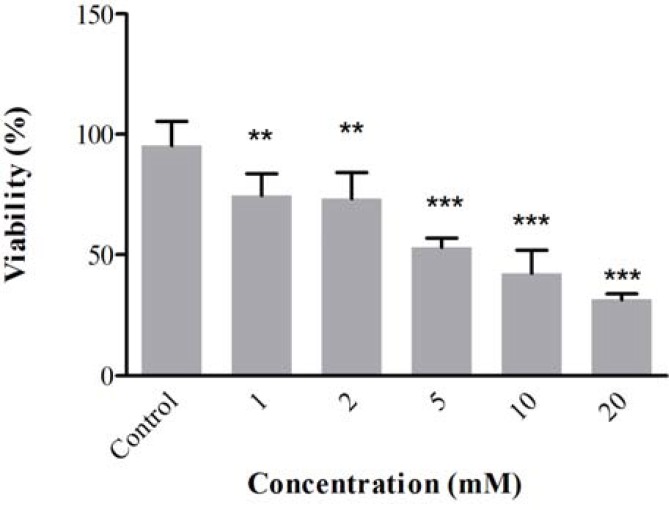
Effect of ACR in PC12 cells. The PC12 cells were treated with different concentrations of ACR for 24 h. After that, cell viability was measured using MTT test. Treatment of cells with ACR decreased cell viability in the dose-dependent manner as shown in Figure 1. The IC50 (50% inhibitory concentration) value for treatment of PC12 cells with ACR for 24 h was 5 mM.
Fig 1.
Cell viability of PC12 cells after exposure to acrylamide for 24 h. Cell viability was determined by MTT test. Data are expressed as mean ± SD of four separate experiments. **P<0.01 and ***P<0.001 vs. control
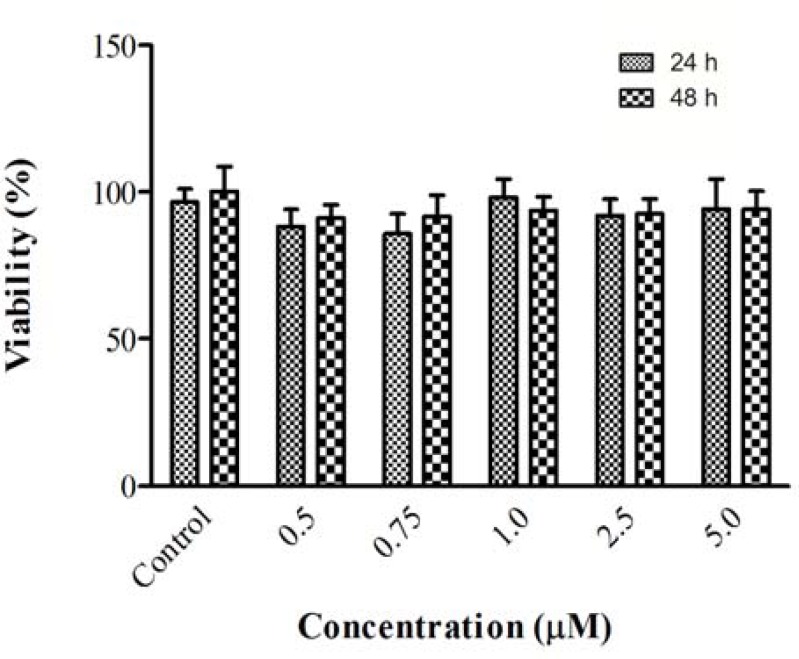
Effect of chrysin on ACR-induced cytotoxicity in PC12 cells. For determination of nontoxic concentration of chrysin in PC12 cells, cells were treated with different concentrations of chrysin for 24 and 48 h. Then, cell viability was determined using MTT assay. Chrysin in higher concentration (more than 10 µM) significantly reduced cell viability (data not shown), while exposure to (0.5, 0.75, 1, 2.5, and 5 µM) for 24 and 48 h did not change cell viability as compared to control (Fig. 2). At the next step to assay protective effect of chrysin, cells were treated with chrysin (0.5, 0.75, 1, 2.5, and 5 µM) for 12 and 24 h.
Fig 2.
Cell viability of PC12 cells after exposure to chrysin for 24 and 48 h. Cell viability was determined by MTT test. Data are expressed as mean ± SD of four separate experiments
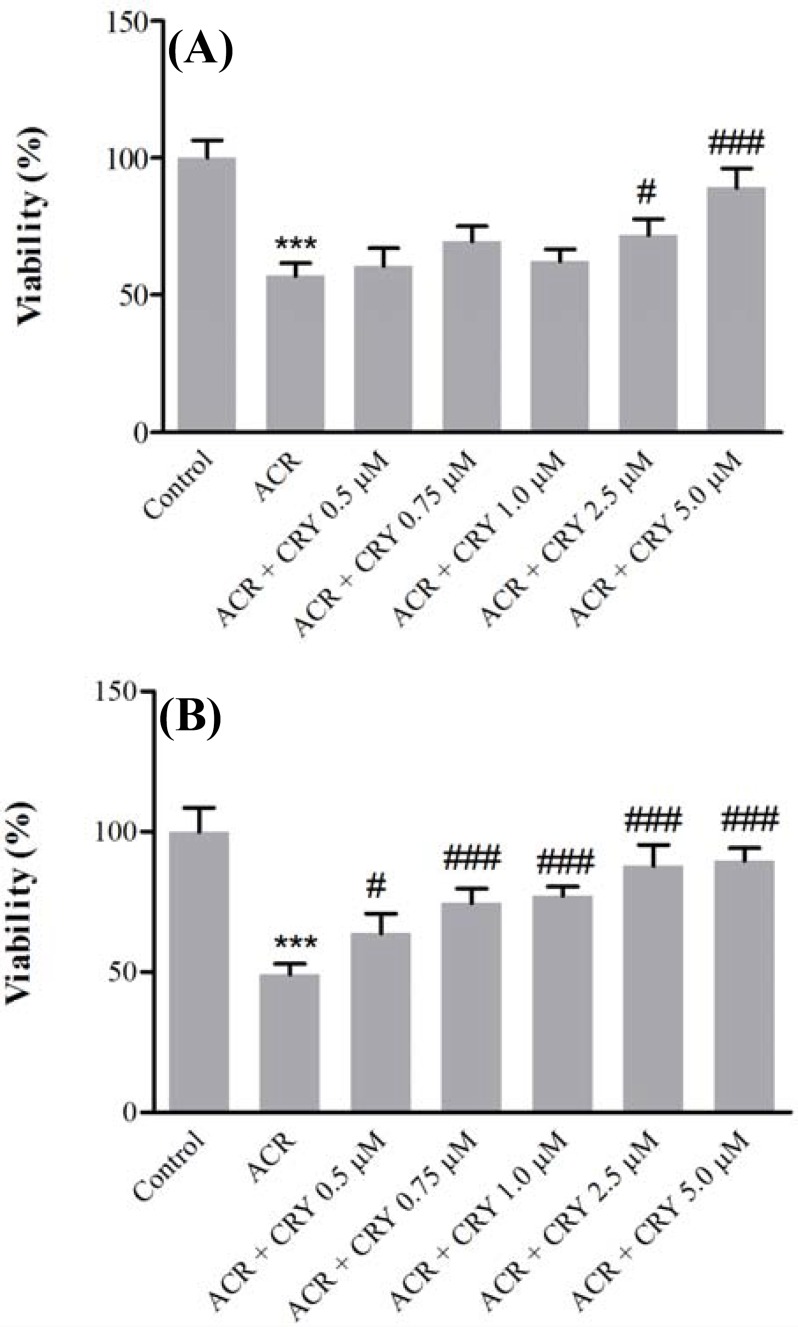
Then, ACR at a final concentration (5 mM) was added to the cells, and after exposure for 24 h, cell viability was evaluated using MTT test. Pretreatment of cells with chrysin (2.5 and 5 µM) for 12 h significantly increased cell viability in comparison with ACR (P<0.05 and P<0.001) (Fig. 3A), while exposure to chrysin (0.5, 0.75, 1, 2.5, and 5 µM) for 24 h significantly decreased the ACR-induced cytotoxicity in comparison to ACR (Fig. 3B).
Fig 3.
Effect of chrysin on acrylamide (ACR)-induced cytotoxicity in PC12 cells. Cells were pretreated with different concentrations of chrysin (0.5, 0.75, 1, 2.5, and 5 µM) for 12 (A) and 24 (B) h before exposure to 5 mM ACR. Data are expressed as the mean ± SD of four separate experiments. ***P<0.001 vs. Control and ###P<0.001, #P<0.05 vs. ACR treated cells
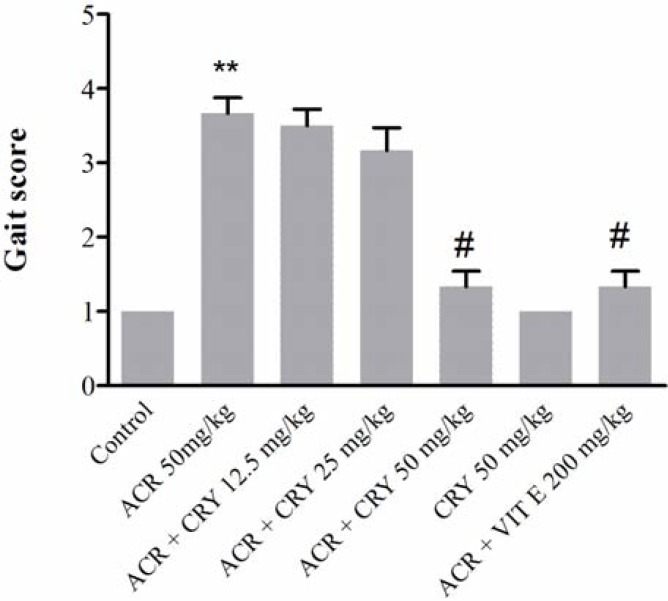
Effect of ACR on the behavioral index (gait scores) in rats and protective effect of chrysin. Exposure to ACR (50 mg/kg, i.p.) for 11 days induced progressive gait abnormalities in rats as shown in Figure 4. Pretreatment of animals with chrysin (50 mg/kg) significantly reduced ACR-induced gait abnormalities (P<0.5).
Fig 4.
Effects of chrysin on behavioral index (gait scores) in rats during the treatment with acrylamide (ACR) (50 mg/ kg, i.p.) for 11 days. Data are expressed as the mean ± SD (n = 6). **P<0.01 vs control, #P<0.05 vs ACR-treated animals
Discussion
In the current study, the neuroprotective effect of chrysin on ACR-induced cytotoxicity in PC12 cells and on ACR-induced neurotoxicity in Wistar rats was determined. According to our results, ACR could decrease cell viability in PC12 cells, and chrysin reduced ACR-induced toxicity in cells in the time- and dose-dependent manner. Also, chrysin significantly suppressed behavioral index changes in Wistar rats during treatment with ACR. Recent studies have clearly exhibited an important role of oxidative stress and apoptosis in ACR-induced toxicity [5, 8, 9]. In neurons and astrocytes, ACR-induced apoptosis in a time- and dose-dependent manner markedly decreased the proliferation of neural progenitor cells, and in high concentrations induced apoptotic and necrotic cell death [30].
In one study, it was shown that administration of ACR to rats elevated lipid peroxidation in nervous tissue and reduced antioxidant capacity [10]. ACR also enhanced levels of lipid peroxidative product, protein carbonyl content, and hydroxyl radical, while it induced apoptosis in cerebral cortex through alteration of Bcl-2 family protein expression [9]. In regards to the important role of lipid peroxidation in ACR-induced neurotoxicity, it has been mentioned that modulation of redox balance to maintain metabolic stage of system and reduction of the apoptosis following exposure to ACR can significantly decrease ACR toxicity.. Researchers evaluated the protective effects of different compounds on ACR-induced toxicity in both in vitro and in vivo models with focus on oxidative stress and apoptosis pathway [6, 8, 9, 11].
In our study, chrysin as an antioxidant and a neuroprotective agent significantly reduced ACR-induced toxicity in PC12 cells and Wistar rats. Results showed that when exposure time to chrysin increased, chrysin, in lower concentrations, markedly could ameliorate toxicity of ACR in PC12 cells. ACR is a potent industrial toxic chemical that produces neurotoxicity by progressive central and peripheral neuronal degeneration. ACR induces ataxia and skeletal muscle weakness in both occupationally exposed humans and experimental animal models [2]. Our results showed that treatment of animals with ACR (50 mg/kg, i.p.) for 11 days caused gait abnormalities and at the end of 11 days, ACR-exposed rats displayed severe abnormal gait scores (3.66 ± 0.5). However, treatment of animals with chrysin (50 mg/kg) significantly reduced gait abnormalities.
The neuroprotective effect of vitamin E is believed is due to its antioxidant activity [31, 32]. Therefore, in the current study, vitamin E was used as a positive control in protection against ACR-induced neuro-toxicity. Our results clearly show that there is no difference between vitamin E and chrysin (50 mg/kg) effect in inhibition of gait abnormalities.
The antioxidant and neuroprotective effects of chrysin have been shown in different studies [24, 27]. Following chronic cerebral hypoperfusion in rats, level of MDA elevated in cortex and hippocampus, while antioxidant enzyme activity decreased [27]. Administ-ration of chrysin ameliorated brain damage through reduction of oxidative stress [27]. Exposure to chrysin decreased neuronal cell death via inhibition of apoptosis [28]. There is a correlation between ACR toxicity and induction of lipid peroxidation. In our study, chrysin showed a neuroprotective effect against ACR-induced toxicity in both PC12 cells and Wistar rats. The results of current study suggest that chrysin has protective effect against ACR toxicity in PC12 cells and Wistar rats.
ACKNOWLEDGMENTS
Authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial support. The results described in this paper are part of a Pharm.D. thesis.
References
- 1.LoPachin RM. The changing view of acrylamide neurotoxicity. NeuroToxicology. 2004 Jun;25(4):617–630. doi: 10.1016/j.neuro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.LoPachin RM. Acrylamide neurotoxicity: Neurological, morhological and molecular endpoints in animal models. Adv Exp Med Biol. 2005;561:21–37. doi: 10.1007/0-387-24980-X_2. [DOI] [PubMed] [Google Scholar]
- 3.Claus A, Carle R, Schieber A. Acrylamide in cereal products: A review. J Cereal Sci. 2008 Mar;47(2):118–133. [Google Scholar]
- 4.Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, et al. Acrylamide: Review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006 Jul-Aug;36(6-7):481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- 5.Sumizawa T, Igisu H. Apoptosis induced by acrylamide in SH-SY5Y cells. Arch Toxicol. 2007 Apr;81(4):279–782. doi: 10.1007/s00204-006-0145-6. [DOI] [PubMed] [Google Scholar]
- 6.Sumizawa T, Igisu H. Suppression of acrylamide toxicity by carboxyfullerene in human neuroblastoma cells in vitro. Archiv Toxicol. 2009 May;:1–8. doi: 10.1007/s00204-009-0438-7. [DOI] [PubMed] [Google Scholar]
- 7.Sumizawa T, Igisu H. Release of heat shock proteins from human neuroblastoma cells exposed to acrylamide. J Toxicol Sci. 2008 Feb;33(1):117–122. doi: 10.2131/jts.33.117. [DOI] [PubMed] [Google Scholar]
- 8.Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, Sener G, Omurtag GZ. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep. 2012 Apr;39(4):4589–96. doi: 10.1007/s11033-011-1249-5. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmi D, Gopinath K, Jayanthy G, Anjum S, Prakash D, Sudhandiran G. Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res. 2012 Sep;37(9):1859–67. doi: 10.1007/s11064-012-0794-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhu YJ, Zeng T, Zhu YB, Yu SF, Wang QS, Zhang LP, et al. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurocheml Res. 2008 Nov;33(11):2310–7. doi: 10.1007/s11064-008-9730-9. [DOI] [PubMed] [Google Scholar]
- 11.Mehri S, Abnous K, Mousavi S, Shariaty V, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012 Mar;32(2):227–35. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013 Jan;16(1):64–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. 2013 May;203(3):547–55. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011 Apr;18(6):499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AT, Moallem SA, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol. 2010 Oct;48(10):2803–8. doi: 10.1016/j.fct.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Williams CA, Harborne JB, Newman M, Greenham J, Eagles J. Chrysin and other leaf exudate flavonoids in the genus Pelargonium. Phytochemistry. 1997 Dec;46(8):1349–53. doi: 10.1016/s0031-9422(97)00514-1. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Huang Q, Ong CN, Yang XF, Shen HM. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010 Jul;293(1):109–16. doi: 10.1016/j.canlet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer Cell Lines. Int J Mol Sci. 2010;11(5):2188–99. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004 Dec;325(4):1215–22. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004 Dec;12(23):6097–105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Yun CW, Park WK, Kong JY, Kim KS, Park Y, et al. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol Res. 2004 Jan;49(1):37–43. doi: 10.1016/s1043-6618(03)00248-2. [DOI] [PubMed] [Google Scholar]
- 22.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005 Jan;69(2):339–45. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol. 2007 Jun;41(1):42–8. doi: 10.1016/j.ijbiomac.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Pushpavalli G, Kalaiarasi P, Veeramani C, Pugalendi KV. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur J Pharmacol. 2010 Apr;631(1-3):636–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Villar IC, Jimenoz R, Galisteo M, Garcio–Saura MF, Zarzuelo A, Luarte J. Effect of chronic chysin treatment in spontaneously hypertensive rats. Planta Med. 2002 Sep;68(9):847–50. doi: 10.1055/s-2002-34400. [DOI] [PubMed] [Google Scholar]
- 26.Brechbuhl HM, Kachadourian R, Min E, Chan D, Day BJ. Chrysin enhances doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines: the role of glutathione. Toxicol Appl Pharmacol. 2012 Jan;258(1):1–9. doi: 10.1016/j.taap.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012 Apr;680(1-3):41–8. doi: 10.1016/j.ejphar.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Izuta H, Shimazawa M, Tazawa S, Araki Y, Mishima S, Hara H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SH-SY5Y cells. J Agric Food Chem. 2008 Oct;56(19):8944–53. doi: 10.1021/jf8014206. [DOI] [PubMed] [Google Scholar]
- 29.Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC. Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res. 2002 May;16(3):256–60. doi: 10.1002/ptr.854. [DOI] [PubMed] [Google Scholar]
- 30.Park HR, Kim M-S, Kim SJ, Park M, Kong KH, Kim HS, et al. Acrylamide induces cell death in neural progenitor cells and impairs hippocampal neurogenesis. Toxicol Lett. 2010 Mar;193(1):86–93. doi: 10.1016/j.toxlet.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Yun JS, Na HK, Park KS, Lee YH, Kim EY, Lee SY, et al. Protective effects of Vitamin E on endocrine disruptors, PCB-induced dopaminergic neurotoxicity. Toxicology. 2005 Dec;216(2-3):140–6. doi: 10.1016/j.tox.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Gumustas K, Guzeyli F, Atukeren P, Sanus GZ, Kemerdere R, Tanriverdi T, et al. The effects of vitamin E on lipid peroxidation, nitric oxide production and superoxide dismutase expression in hyperglycemic rats with ceberal ischemia-reperfusion injury. Turk Neurosurg. 2007 Apr;17(2):78–82. [PubMed] [Google Scholar]