Abstract
Fyn kinase is highly expressed in oocytes, with inhibitor and dominant-negative studies suggesting a role in the signal transduction events during egg activation. The purpose of the present investigation was to test the hypothesis that Fyn is required for calcium signaling, meiosis resumption and pronuclear congression using the Fyn-knockout mouse as a model. Accelerated breeding studies revealed that Fyn-null females produced smaller litter sizes at longer intervals and exhibited a rapid decline in pup production with increasing age. Fyn-null females produced a similar number of oocytes, but the frequency of immature oocytes and mature oocytes with spindle chromosome abnormalities was significantly higher than in controls. Fertilized Fyn-null oocytes frequently (24%) failed to undergo pronuclear congression and remained at the one-cell stage. Stimulation with gonadotropins increased the number of oocytes ovulated, but did not overcome the above defects. Fyn-null oocytes overexpressed Yes kinase in an apparent effort to compensate for the loss of Fyn, yet still exhibited an altered pattern of protein tyrosine phosphorylation. In summary, Fyn-null female mice exhibit reduced fertility that appears to result from actin cytoskeletal defects rather than calcium signaling. These defects cause developmental arrest during oocyte maturation and pronuclear congression.
Keywords: actin, cytoskeleton, fertility, mouse, protein kinase, spindle
Introduction
Src family protein tyrosine kinases (PTKs) are cytoplasmic enzymes that can be targeted to the plasma membrane or actin cytoskeleton where they typically act to transduce signals from external stimuli (Bromann et al. 2004). This kinase family has been shown to play a major role during egg activation and early development in species that fertilise externally, such as marine invertebrates, amphibians and fish (Sato et al. 2000; Wu and Kinsey 2000; Runft et al. 2002). In these species, Src family PTKs are activated rapidly after fertilisation and function in triggering the sperm-induced calcium transient that initiates the egg activation process (Giusti et al. 1999; Kinsey and Shen 2000; Kinsey et al. 2003). These kinases also function in later stages of egg activation, such as pronuclear congression and mitosis (Moore and Kinsey 1995; Wright and Schatten 1995), as well as in cell movements involved in epiboly (Sharma et al. 2005; Tsai et al. 2005) and blastopore closure (Vallés et al. 2001). In mammals, there is evidence that Fyn kinase is the most highly expressed member of the Src family in oocytes and that it is important for oocyte maturation and maintenance of the cytoskeleton of the mature oocyte (Luo et al. 2009; McGinnis et al. 2009). The role of Src family PTKs in mammalian fertilisation is clearly different from that in externally fertilising species. For example, in mice, the sperm-induced calcium oscillations are initiated directly by a sperm-borne phospholipase (Carroll 2001; Cox et al. 2002) that does not require PTK regulation (Mehlmann et al. 1998; Kurokawa et al. 2004; Mehlmann and Jaffe 2005). The function of Src family PTKs in later stages of mammalian fertilisation, including the G1/S or S/G2 phase of the first mitotic division, has been demonstrated through the use of chemical inhibitors such as genistein (Besterman and Schultz 1990; Jacquet et al. 1995) and glutathione-S-transferase fusion proteins encoding the SH2 domain of Fyn (Meng et al. 2006). Together, these observations indicate that Src family kinases play an important role in development of the mammalian zygote.
It has proven more difficult to demonstrate which specific Src family kinase plays a critical role during the developmental events described above. Chemical inhibitor and dominant-negative studies suffer from the possibility of non-specific suppression of other Src family members. The availability of Fyn-knockout mice provides a useful alternative to these experimental approaches. Fyn-knockout mice were originally generated by disruption of the fyn gene with a targeting construct that replaced a 3-kb fragment containing the first coding exon with a neo expression cassette (Stein et al. 1992). Mice homozygous for this fyn mutation display no overt phenotype, but defects were reported in the immune, haematopoietic and nervous systems (Stein et al. 1992). Because Fyn-null mice can reproduce and heterozygous crosses result in a normal ratio of Fyn-null pups, it was proposed that fyn is not essential during embryonic development (Stein et al. 1992). The objective of the present study was to evaluate the fertility of Fyn-null females and to characterise the developmental potential of Fyn-null oocytes. The results demonstrate that Fyn-null females are sub-fertile and that oocytes ovulated by these females include a variety of actin cytoskeletal defects that appear to cause failure of spindle chromosome organisation, vesicle transport and defective protein tyrosine phosphorylation. The Fyn-null oocytes were found to overexpress the closely related Yes kinase, suggesting that oocytes possess a mechanism for regulating overall Src family kinase expression. Nevertheless, the results obtained with the Fyn-knockout model clearly demonstrate that Fyn plays a critical role in meiotic maturation and the developmental potential of the oocyte.
Materials and methods
Accelerated breeding study
Breeding pairs of fyn mutant mice (B6;129S7-Fyntm1sor/J strain; Stein et al. 1992) and the suggested wild-type (WT) control mice (B6;129SF2/J strain) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and housed under a14 h light–10 h dark cycle. Housing standards and policies, as well as standard operating procedures, were compatible with the guide recommendations of the Institutional Animal Care and Use Committee (http://www.kumc.edu/lar/procedures.html accessed 12 April 2010). Fifteen Fyn-null and seven WT breeding pairs were set up at 6 weeks of age and maintained for 10 months. Pup delivery was checked daily and pups were weaned at 18–19 days after birth to free the mother for subsequent litters.
Ovulation test
To test ovulation, mature Fyn-null or control females were caged individually with a vasectomised male, then examined for vaginal plugs the next morning. Plugged mice were killed to enable collection of cumulus–oocyte complexes (COCs) at 1200 hours and the number and meiotic status of oocytes were determined by confocal microscopy.
Developmental competency
Control (B6;129SF2/J) or Fyn-null (B6;129S7-Fyntm1Sor/J) females were mated with fertile B6;129SF2/J males and zygotes were recovered by flushing the oviduct at 1400 hours on the day vaginal plugs were detected. The recovered oocytes and zygotes were cultured as described previously (Luo et al. 2009). In other studies, superovulation was achieved by injection of 5 IU pregnant mare’s serum gonadotropin (PMSG; Sigma-Aldrich, St Louis, MO, USA), followed 48 h later by injection of 5 IU of human chorionic gonadotrophin (hCG; Sigma-Aldrich). For in vitro fertilisation, COCs were collected 15–16 h after hCG injection and were fertilised as described by Luo et al. (2009). Developmental success was determined initially by evaluation with a Nikon TE200 microscope equipped with a 20× HMC-ELWP Hoffman modulation contrast objective (Nikon, Melville, NY, USA) 41 h after mating. Oocytes and/or zygotes that failed to divide were fixed, stained with ethidium homodimer-2 (EthD-2) and examined by confocal fluorescence to ascertain whether they were still immature (germinal vesicle (GV) or bivalent chromatids) or were fertilised (sperm head incorporated or two pronuclei). Immature oocytes were not included in the calculation of fertilisation rates. Development to the blastocyst stage was determined after 5 days of culture.
Confocal fluorescence microscopy
Cumulus-free oocytes or fertilised eggs were fixed in 2% paraformaldehyde and 1% picric acid in phosphate-buffered saline (PBS; pH 7.4) for 1 h at room temperature. Fixed oocytes were washed in blocking buffer (PBS containing 3 mg mL−1 bovine serum albumin (BSA), 0.01% Tween 20, 0.1 M glycine). Oocytes were then permeabilised with 0.1% Triton X-100 in PBS containing 3 mg mL−1 BSA for 5 min before being washed with blocking buffer (McGinnis et al. 2007). For detection of cortical granules, oocytes were incubated overnight at 48C with 10 mg mL−1 lectin from Lens culinaris (LCA)–fluorescein iso-thiocyanate (FITC) conjugate (Sigma-Aldrich). Type 1 inositol 1,4,5-trisphosphate (IP3) receptors were localised with rabbit anti-IP3 receptor Type I antibody (1 : 100; EMD Chemicals, Gibbstown, NJ, USA). DNA was detected with 2 mM EthD-2 (Invitrogen, Carlsbad, CA, USA). Bound primary antibody was detected with Alexa 488 goat anti-rabbit IgG (1 : 500; Invitrogen). Microscopy was performed with a Nikon TE2000-U microscope.
Calcium imaging in live oocytes
Cumulus-free MII oocytes were treated in acid Tyrode’s solution (pH 2.5) to remove the zona pellucida, followed by incubation in potassium simplex optimized medium with amino acids (KSOMAA) (Millipore Corporation, Phillipsburg, NJ, USA) containing 1 mg mL−1 BSA (embryo tested; Sigma-Aldrich) and 1 mM fura-2 AM (Invitrogen) premixed with 0.02% pluronic F-127 (Invitrogen) for 30 min at 378C. The Fura-2 loaded eggs (20–25) were bound to coverslips precoated with 0.01% poly-L-lysine (Sigma-Aldrich) in 100 mL KSOMAA, supplemented with the same volume of Hepes-buffered KSOM medium (Millipore Corporation, Phillipsburg, NJ, USA) containing 8 mg mL−1 BSA. Spermatozoa were harvested from WT mouse cauda epididymis and capacitated for over 90 min in modified Tyrode’s solution before insemination. Capacitated spermatozoa were added to a final concentration of 5.0 × 104 mL−1 and the oocytes were monitored at 378C on a Nikon TE2000-U microscope equipped with a Lambda 10-2 Optical Filter Changer (Sutter Instruments, Novato, CA, USA). Fura-2 was excited at 340 and 380 nm and the light emitted over 520 nm was collected at 15-s intervals for 3 h. Typically, 10–12 oocytes were visible in the microscope field of a 10× super fluor objective, of which approximately half were fertilised using this particular concentration of spermatozoa, which was intentionally low to avoid polyspermy. The fluorescence signal was displayed as the ratio of fluorescence intensities for the F340/380 excitation wavelengths, as determined with Metafluor software (Meta Imaging Devices, Downington, PA, USA), and exported to Microsoft Excel for peak integration.
Western blot analysis
Groups of 20 cumulus-free oocytes were washed three times in PBS containing 3 mg mL−1 polyvinylpolypyrrolidone (PVP) 360 (Sigma-Aldrich) before being transferred to 2 mL sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 40 mM phenylarsine oxide and 100 mM sodium orthovanadate as phosphatase inhibitors. Samples were then heated at 908C for 5 min before being resolved by electrophoresis on a 10% SDS gel, transferred to nylon membranes, blocked with Tris-buffered saline containing 0.1% Tween 20, 1% BSA and the aforementioned phosphatase inhibitors. The primary antibodies used included mouse anti-phosphotyrosine (clone 4G10; Millipore, Temecula, CA, USA), rabbit anti-Fyn (Fyn3; 1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-c-Yes (1 : 1000; BD Transduction Laboratories, San Jose, CA, USA), mouse anti-GAPDH (1 : 2000; AbCam, Cambridge, MA, USA) and rat anti-α-tubulin (1 : 2000; AbCam). Bound antibody was localised with a secondary antibody coupled to horseradish peroxidase and detected by chemiluminescence.
Statistical analysis
The significance of results from comparison experiments was determined by t-test or paired t-test, with P ≤ 0.05 considered significant.
Results
Fertility analysis of Fyn-null mice
The B6;129S7-Fyntm1Sor/J strain is self-propagating, allowing continuation of the homozygous knockout line. These Fyn-null females averaged 16.8 g bodyweight between 6 and 12 weeks postpartum and were significantly smaller than control mice (22.0 g; P = 0.001). In addition, the ovaries of the Fyn-null female mice were proportionally smaller than in control mice (7.7 v. 8.8 mg, respectively). In order to quantitate the impact of the Fyn knockout on fertility, an accelerated breeding study was performed comparing litter frequency and size between the control B6;129SF2/J and B6;129S7-Fyntm1Sor/J strains. Mating success was recorded following placement of single females (6–11 weeks of age) with single males. The results show that the Fyn-null mice produced smaller litter sizes (P ≤ 0.05) during the first five parturitions and that the number of viable pups tended to decrease with age (Fig. 1a). In addition, the interval between litters was significantly longer (P ≤ 0.05) and, because fewer pups were delivered after the third pregnancy, the overall reproductive lifespan appeared to be shorter than for control mice (Fig. 1b).
Figure 1.
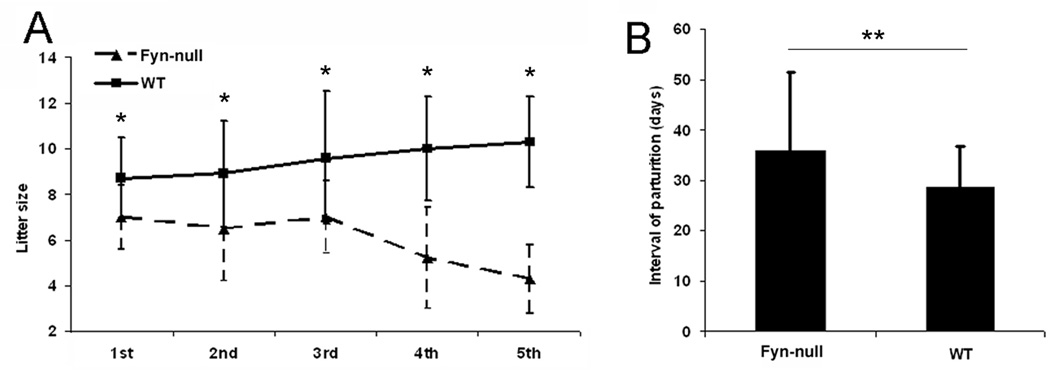
Accelerated breeding analysis of Fyn-null mice. Fifteen Fyn-null and seven wild-type (WT) breeding pairs, at least 6 weeks of age, were set up and maintained for 10 months using an accelerated breeding program wherein mice were bred on the post-parturient oestrus. Pup delivery was checked daily to record the delivery date, numbers and sex. Pups were removed upon weaning at 18–19 days after birth to allow subsequent matings. Values on the vertical axis represent the mean ± s.d. litter size from the different female mice, whereas the x-axis lists the number of pregnancies. (a) Fyn-null female mice produced significantly smaller litters (marked with a single asterisk): 1st, P < 0.022; 2nd, P < 0.043; 3rd, P < 0.028; 4th, P < 0.001; 5th, P < 0.001. (b) The interval of pup delivery was significantly extended in Fyn-null female mice compared with WT mice (marked with a double asterisk; P < 0.025).
Oocyte number and morphology
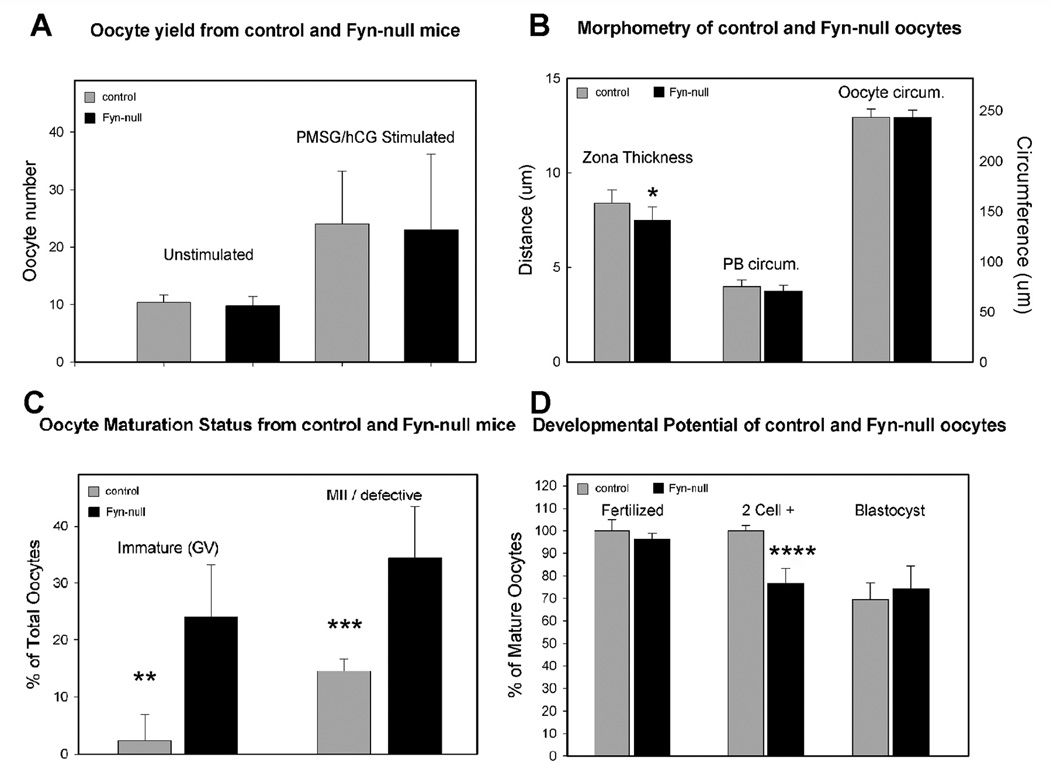
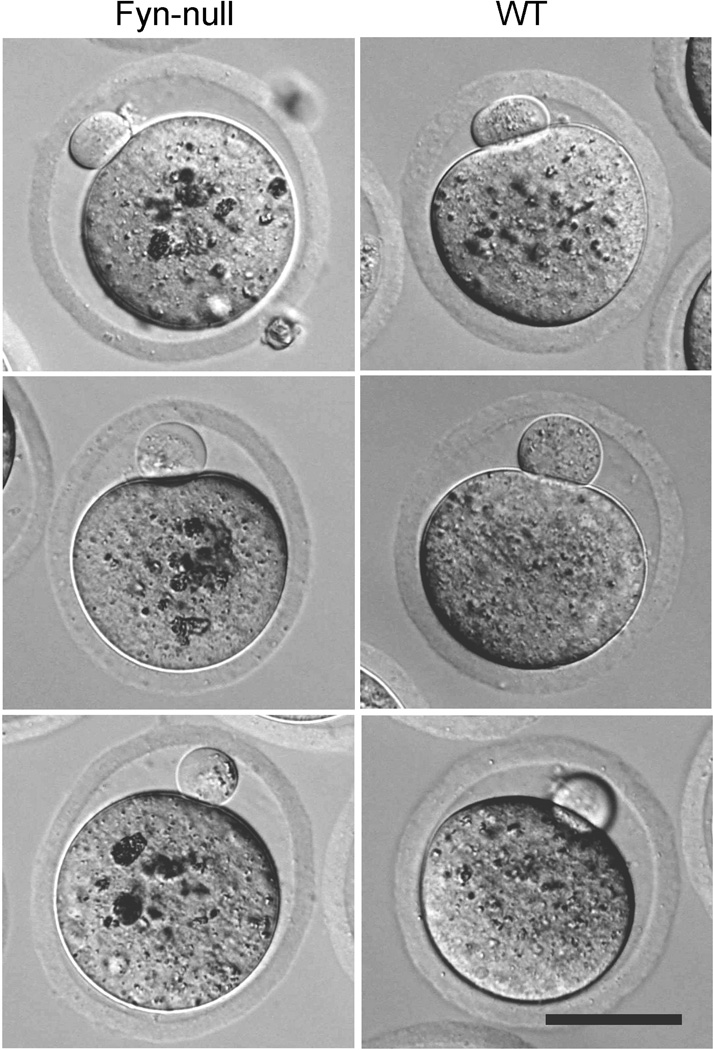
In order to determine whether the reduced fertility observed by accelerated breeding analysis was due to differences in the number and quality of oocytes ovulated, age-matched (6–8-week-old) Fyn-null or WT female mice were mated with vasectomised males and the oocytes were collected the morning on which vaginal plugs were detected. A separate group of female mice was stimulated with PMSG and hCG before mating. Fyn-null females ovulated the same number of oocytes as controls, irrespective of gonadotropin stimulation (Fig. 2a). The Fyn-null oocytes and their first polar body were similar in size to those in control mice, however the zona pellucida of Fyn-null oocytes was significantly thinner than that in controls (Figs 2b, 3). The Fyn-null oocytes also exhibited large, dense cytoplasmic inclusions that were significantly more pronounced than any observed in control oocytes (Fig. 3).
Figure 2.
Oocyte yield, quality and developmental potential. (a) Oocyte yield was determined by mating age-matched Fyn-null or wild-type (WT) female mice with vasectomised WT males. Oocytes were collected the morning on which vaginal plugs were detected. A separate group of female mice was stimulated with pregnant mare’s serum gonadotropin and human chorionic gonadotrophin before mating. (b) Oocyte size and the thickness of the zona pellucida was measured from images made by Hoffman interference microscopy through the equator of oocytes. (c) The maturation status of oocytes recovered from WT and Fyn-null female mice was determined by confocal fluorescence microscopy following fixation and labelling with ethidium homodimer-2 (EthD-2) to reveal chromosome status. The developmental competence of WT and Fyn-null zygotes produced by mating with fertile WT males was determined by recovering oocytes and zygotes the morning on which vaginal plugs were detected and culturing them to allow development under identical conditions. Oocytes that failed to progress to the two-cell stage by 41 h after mating were fixed, stained with EthD-2 and examined by confocal fluorescence microscopy to determine meiotic and fertilisation status. Immature oocytes were excluded from calculation of the percentage rates of fertilisation and development. Significantly different values are indicated by *, **, ***, or **** (P < 0.05).
Figure 3.
Morphology of Fyn-null oocytes. Oocytes recovered from wild-type (WT) B6;129SF2/J and Fyn-null B6;129S7-Fyntm1Sor/J (Fyn−/−) mice were examined by Hoffman modulation contrast. The Fyn-null oocytes contained dense, irregular inclusions. The diameter of the two groups of oocytes did not differ significantly (P = 0.563); however, the thickness of the zona pellucida of Fyn-null oocytes was significantly less than that of WT oocytes (P < 0.001). Scale bar = 50 mm.
Oocyte maturation and developmental competency
Because our previous studies indicated that suppression of Fyn expression impaired maturation of oocytes in vitro (McGinnis et al. 2009), we next evaluated the maturation status of the oocytes ovulated by WT and Fyn-null female mice. Although Fyn-null females ovulated a similar number of oocytes, a significant percentage failed to mature (Fig. 2c). In addition, those Fyn-null oocytes that did progress to MII frequently exhibited chromosome and/or spindle errors, resulting in displaced chromosomes (indicated as MII/defective in Fig. 2c). In order to test the developmental competency of those Fyn-null oocytes that did mature to MII, Fyn-null and WT female mice were mated with fertile B6;129SF2/J males. In order to minimise effects of the Fyn-null reproductive tract, the ovulated oocytes and zygotes were recovered and placed into culture on the afternoon of the day on which vaginal plugs were detected. Oocytes that failed to progress to the two-cell stage by 41 h after mating were fixed and examined by confocal fluorescence microscopy to determine meiotic and fertilisation status. The results shown in Fig. 2d demonstrate that most of the mature oocytes were fertilised; however, a significant proportion (24%) failed to divide. Examination of the failed zygotes revealed that approximately 75% arrested during pronuclear congression and approximately 25% remained at metaphase II with severely displaced chromosomes. The zygotes (now heterozygous Fyn−/+) that did divide developed to the blastocyst stage at a rate similar to that seen for controls.
Effect of ovarian stimulation
In order to determine whether the tendency of Fyn-null oocytes to fail during oocyte maturation may reflect impaired pituitary stimulation, we tested the effect of ovarian stimulation with PMSG and hCG on oocyte maturation and developmental competence. Control and Fyn-null female mice were injected with PMSG and hCG. Oocytes were then recovered, freed of cumulus cells and examined as described above. The mean (± s.d.) oocyte yield of the gonadotropin-stimulated Fyn-null female mice did not differ significantly from that of control B6;129SF2/J female mice (23 ± 13 v. 24 ± 9, respectively). However, as with the unstimulated females, the oocytes recovered from gonadotropin-stimulated Fyn-null females included a high percentage of immature oocytes (19% ± 10%). This fraction of immature oocytes was significantly higher than that ovulated by control female mice (2.7% ± 4.1%; P = 0.007), suggesting that additional gonadotropin stimulation did not overcome the tendency of Fyn-null female mice to ovulate immature oocytes.
Cytoskeletal and cortical organisation
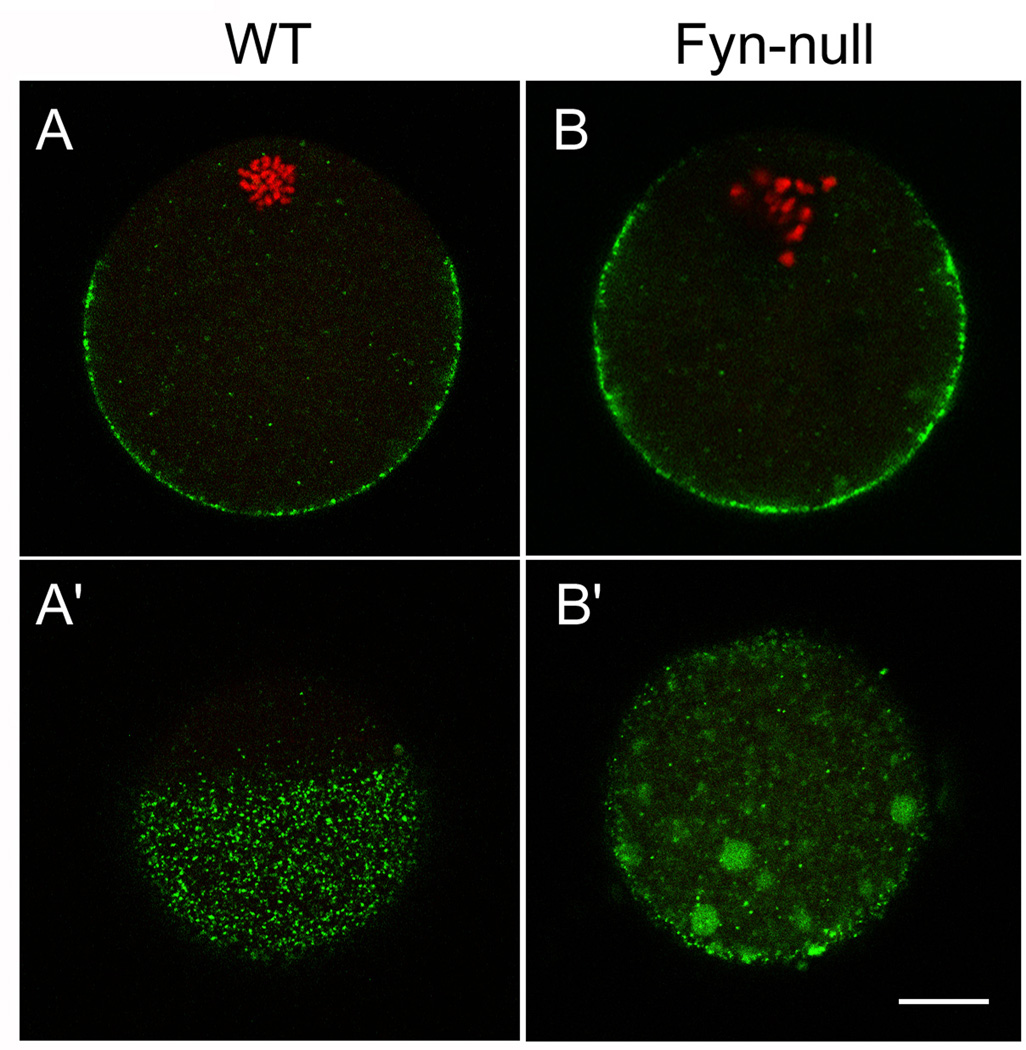
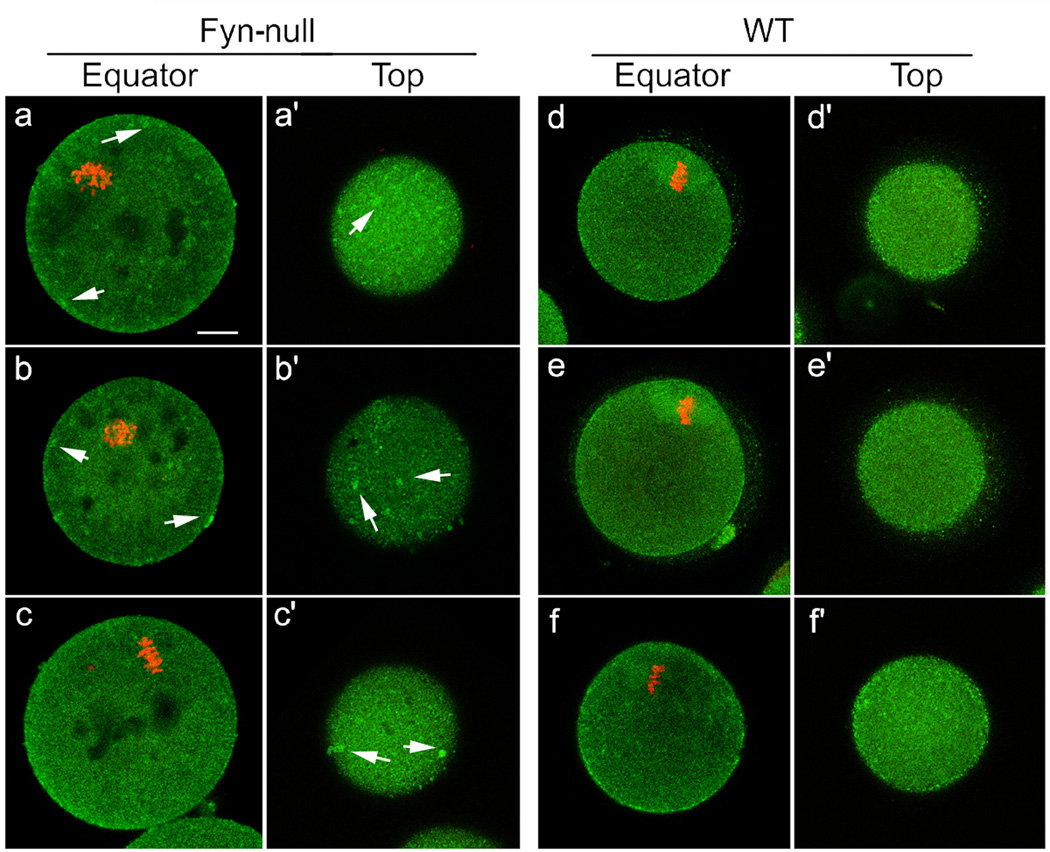
In a previous study, we demonstrated that suppression of Fyn kinase caused defects in the oocyte cortical actin cytoskeleton that resulted in loss of morphological polarity (Luo et al. 2009). One consequence of this is a failure of the cortical secretory granules to migrate to the oocyte cortex or remain tethered at the cortex as the oocyte matures. This defect is shown in Fig. 4, which compares the localisation of cortical granules in mature WT and Fyn-null oocytes. Images taken through the equator of the oocyte (Fig. 4a, b) demonstrate the failure of Fyn-null oocytes to position and maintain the cortical granules at the oocyte cortex. In addition, the Fyn-null oocytes are less effective in positioning the meiotic spindle close to the cortex and, consequently, lack a fully developed cortical granule-free zone over the spindle. Images taken tangentially through the oocyte cortex (Fig. 4a’, b’) demonstrate the irregular distribution of cortical granules in Fyn-null oocytes. This distribution would be likely to alter the effectiveness of the cortical reaction that normally modifies the oocyte surface, as well as the zona pellucida, in response to fertilisation. Because cytoskeletal elements have been shown to influence the distribution of signal transduction systems, such as IP3 receptors (Lange and Gartzke 2006; Bose and Thomas 2009), it was of interest to examine the morphological arrangement of Type 1 IP3 receptors in Fyn-null oocytes. The IP3 receptors in WT oocytes are normally arranged in a gradient of small clusters that appear as fine granularity when observed under confocal immunofluorescence microscopy. The IP3 receptor clusters are most concentrated in the pole opposite the meiotic spindle (Mehlmann et al. 1995; Kline et al. 1999) and appear somewhat concentrated at the egg cortex (Mehlmann et al. 1996; Vanderheyden et al. 2009; Fig. 5d–f). The gradient of IP3 receptors in the Fyn-null oocyte was closely similar to that in control oocytes; however, the clustering was less regular, with numerous dense accumulations of this ion channel (arrows in Fig. 5a–c) that were not typical in control oocytes. Tangential views in which the focal plane passed through the egg cortex revealed these dense accumulations more clearly (Fig. 5a’ –c’).
Figure 4.
Distribution of cortical granules in Fyn-null oocytes. Cortical granules were detected in (a, a’) wild-type (WT) and (b, b’) Fyn-null oocytes by labeling oocytes with lectin from Lens culinaris (LCA)–fluorescein isothiocyanate (FITC), with FITC fluorescence appearing green. The DNA was labeled with ethidium homodimer and appears red in this figure. Photographs taken through the oocyte equator demonstrate the extent of the cortical granule-free zone in (a) WT oocytes (arrows), which was reduced in Fyn-null oocytes (b). Images taken tangentially through the oocyte cortex (a’, b’) demonstrate the distribution pattern of cortical granules within the cortical cytoplasm. Fyn-null oocytes displayed irregular clusters of cortical granules that were rarely seen in control oocytes. Scale bar < 10 mm.
Figure 5.
Distribution of Type 1 inositol 1,4,5-trisphosphate receptor (IP3R1) in Fyn-null oocytes. Immunofluorescence detection of IP3R1 in Fyn-null MII oocytes (a–c, a’ –c’) and wild-type (WT) MII oocytes (d–f, d ‘ –f ‘) that were stained with rabbit anti-IP3R1 antibody, followed by an Alexa fluor 488-labelled secondary antibody (green). Optical sections taken through the equator of the oocyte demonstrate the distribution of IP3 receptors in the oocyte cortex and central cytoplasm (a–f). Tangential optical sections (a’ –f ‘) reveal the distribution in the cortex just below the oocyte surface. DNA was stained by ethidium homodimer-2 (red). Arrows indicate dense accumulation of IP3R1 clusters in Fyn-null oocytes. Scale bar = 10 mm.
Impact of Fyn kinase deficiency on calcium signalling at fertilisation
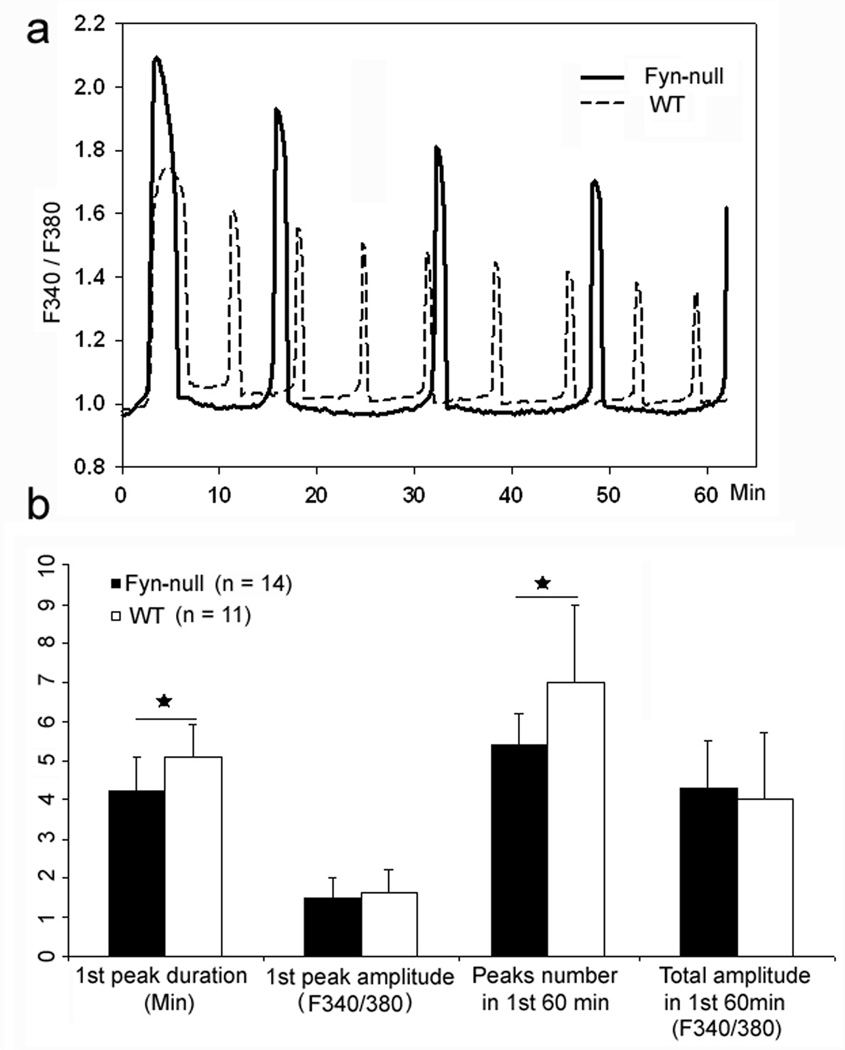
The fact that Fyn-null oocytes exhibited unusual accumulations of IP3 receptor clusters raised the possibility that Fyn-null oocytes may be defective in the fertilisation-induced calcium signalling that is critical to developmental competence. In order to characterize the calcium signalling response to fertilisation, zona-free oocytes from control and Fyn-null female mice were preloaded with Fura 2AM, fertilised in vitro and calcium-induced fluorescence monitored and quantitated. Oocytes from Fyn-null female mice responded to fertilisation with a pattern of calcium oscillations in which the duration of the first peak was significantly shorter, although peak amplitude was higher, than in WT controls (Fig. 6). The subsequent peaks were lower in frequency, but each was of longer duration, with the result that the total integrated calcium input signal (Ozil et al. 2005) during the first 60 min did not differ between the Fyn-null and control groups. Therefore, the irregularities in IP3 receptor distribution in Fyn-null oocytes appeared to have only minor effects on calcium signaling following fertilisation.
Figure 6.
Calcium oscillatory patterns typical of fertilised Fyn-null oocytes. Cumulus- and zona pellucida-free oocytes from nine Fyn-null and five wild-type (WT) female mice were loaded with fura-2, bound to polylysine-coated coverslips and fertilised with WT spermatozoa in vitro so that fertilisation-induced calcium oscillations could be recorded. (a) The typical pattern of calcium oscillations following fertilisation of WT (dashed line) and Fyn-null (solid line) oocytes. (b) The average duration and amplitude of the first calcium oscillation, as well as the total peak number during the first hour after insemination. The total integrated calcium input signal during the first hour after insemination is presented on the far right. The y-axis values show the mean ± s.d.; n values represent the number of recordings analysed from each group. Groups that differ significantly (P < 0.05) are marked with asterisks.
Fyn-null oocytes exhibit altered patterns of protein tyrosine phosphorylation
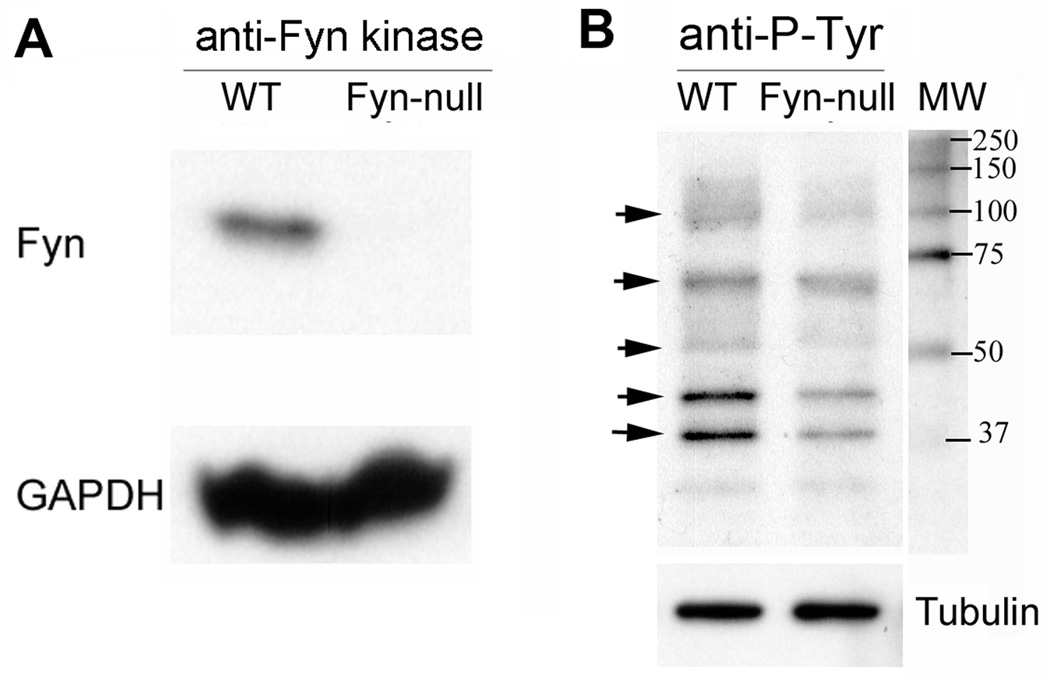
Because Fyn kinase is normally expressed at high levels in oocytes relative to most cell types, the Fyn-null oocyte would be expected to be unable to phosphorylate egg proteins that were unique targets of this Src family PTK. This possibility was tested by comparing the pattern of phosphorylated tyrosine (P-Tyr)-containing proteins in groups of MII oocytes from Fyn-null and WT females using western blot analysis. Initially, whole ovaries from WT and Fyn-null females were probed with an antibody to Fyn protein to confirm that Fyn, which is easily detected in WT ovaries, was not expressed in Fyn-null ovaries (Fig. 7a). The oocyte proteins were analysed in blots prepared from cumulus-free oocytes (Fig. 7b) probed with an anti-P-Tyr antibody, as well as anti-tubulin to control for loading errors. Typical results presented in Fig. 7b revealed that Fyn-null oocytes contained reduced P-Tyr in several protein bands of approximately 120,70, 60, 45 and 39 kDa (Fig. 7). This suggests that several protein targets of Fyn kinase are under-phosphorylated in the Fyn-null oocyte, which may explain the reduced developmental competence resulting from the loss of this kinase. However, because the protein(s) present in each band was not identified, the results may also reflect decreased protein content resulting from a lack of Fyn kinase.
Figure 7.
Protein tyrosine phosphorylation in oocytes from Fyn-null females. (a) Wild-type (WT) and Fyn-null ovaries were probed by western blot using an antibody against Fyn kinase as well as an anti-GADPH antibody as a loading control to confirm that this protein was not present in oocytes or somatic cells. (b) Groups of 20 cumulus-free oocytes from WT and Fyn-null female mice were analysed by western blot using an anti-phosphotyrosine antibody, as well as anti-tubulin as a loading control. Molecular weight standards are presented on the right and arrows indicate the position of phosphorylated tyrosine (P-Tyr)-containing bands that seem to be reduced in Fyn-null oocytes.
Compensation of Fyn kinase loss by Yes kinase expression
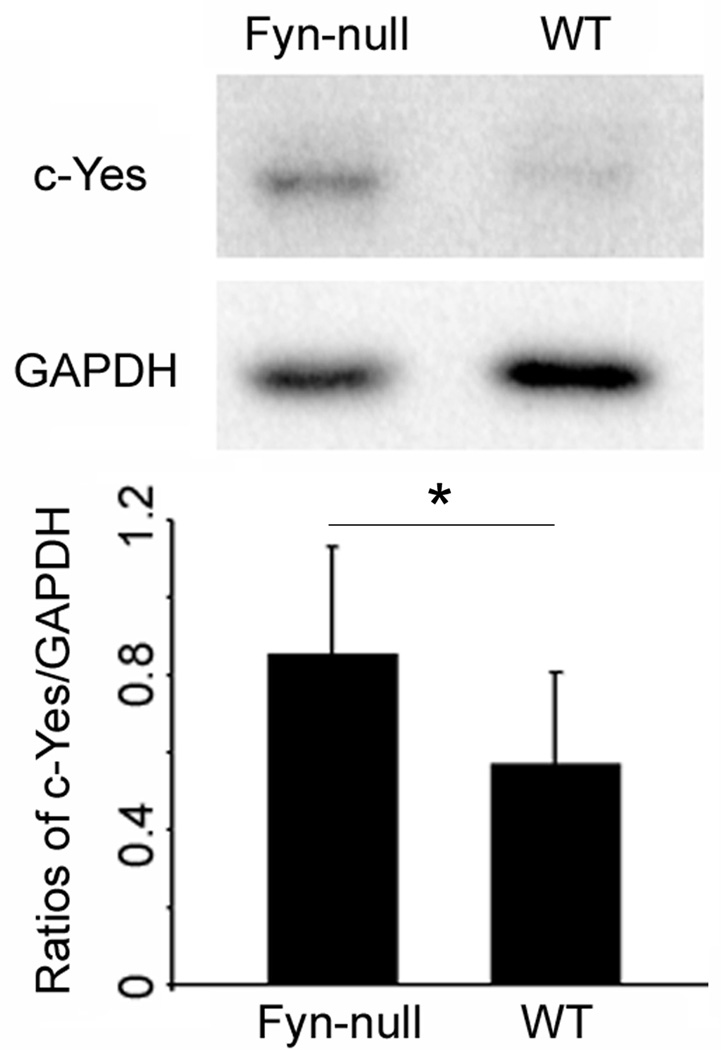
The fact that the defects in the maturation and developmental potential of Fyn-null oocytes were only partial in scope suggests the possibility that some other Src family kinase may compensate for the loss of Fyn. The expression of Yes and Src kinases was quantitated in Fyn-null and WT oocytes in order to test the possibility that Fyn-null oocytes may compensate for reduced Fyn kinase activity by increased expression of other Src family PTKs. Western blot analysis of MII oocytes from Fyn-null and WT female mice was performed with an antibody against Yes and Src kinase, and blots were also probed with an anti-GAPDH antibody to correct for loading errors (Fig. 8). Src kinase was not detectible in oocytes (data not shown), as reported previously (Mehlmann and Jaffe 2005). However, YES protein was clearly detected in WT and Fyn-null oocytes. Quantitation of band intensity by densitometric scanning of multiple samples revealed that Yes expression was significantly higher in Fyn-null oocytes, suggesting that Yes expression had been upregulated to compensate for the loss of Fyn and indicating that the expression of these genes in the oocyte may involve a common feedback control mechanism.
Figure 8.
Expression of c-Yes kinase in Fyn-null oocytes. Expression of c-Yes kinase in wild-type (WT) and Fyn-null oocytes was determined by western blot analysis of groups of 40 cumulus-free MII oocytes. The blots were probed with anti-c-Yes antibody and reprobed with anti-GAPDH. The upper panel shows a typical result. The average intensity of c-Yes and GAPDH bands was measured and the ratio of c-Yes/GAPDH was expressed graphically (lower panel). Values represent the mean ± s.d. from five groups of oocytes. Comparison of the from WT and Fyn-null oocytes by paired t-test indicated that c-Yes expression in Fyn-null oocytes was significantly higher than in controls (*).
Discussion
Experimental evidence from several laboratories has demonstrated that Src family protein kinases are critical to the developmental competence of invertebrate and vertebrate oocytes (Livingston et al. 1998; Runft et al. 2002; Sharma et al. 2005; Tomashov-Matar et al. 2008). However, the fact that Src family kinases share extensive structural similarity has made it difficult to discern which family member is critical for specific signalling events in the oocyte. The Fyn-knockout mouse provides an ideal model in which to test the requirement for Fyn kinase in oocyte maturation and fertilisation. Fyn-knockout female mice have been reported to be fertile based on Mendelian ratios of progeny (Stein et al. 1992, 1994), although triple (Src, Fyn, Yes) mutant embryos exhibit severe developmental defects and die by embryonic Day 9.5 (Klinghoffer et al. 1999). The fact that Fyn-null mice can self-propagate allowed us to study Fyn-null oocytes directly, without possible artefacts inherent in chemical inhibitor and exogenous dominant-negative constructs. Initially, the fertility of Fyn-null female mice was assessed through accelerated breeding studies. The design of the accelerated breeding study used here provided a more exacting test of fertility than that used by earlier studies in which genotypic ratio analysis of embryos resulting from heterozygote matings was the only measure of fertility. The results revealed that Fyn-null females produced fewer pups at longer intervals than did WT controls and that the production of pups deteriorated more rapidly with increasing maternal age. The reduced fertility of Fyn-null female mice was found not to be the result of a reduction in the number of oocytes ovulated, which suggests that the females responded well to pituitary stimulation, but raised the possibility that oocyte quality was adversely effected by deletion of Fyn kinase.
Examination of oocytes ovulated by Fyn-null female mice revealed a high incidence of maturation failure, which would be expected to have a significant impact on litter size. In addition, the developmental competence of those oocytes that did mature was reduced. The mature Fyn-null oocytes were fertilised and formed pronuclei at a rate similar to that in WT controls, but many became arrested at the late pronuclear stage and failed to divide. This result confirms earlier studies in which the chemical inhibitor SKI-606 and dominant-negative fusion proteins were used to suppress Fyn kinase in mouse oocytes (Meng et al. 2006; McGinnis et al. 2007).
Some insight into the mechanisms underlying the above decline in the developmental potential of the oocyte may be obtained from examination of the oocyte structure. The morphology of Fyn-null oocytes was significantly different from that of WT controls, with prominent clusters of cortical granules that failed to migrate to the egg cortex and the under-developed cortical granule-free zone over the spindle. The failure of cortical granule migration seems to indicate that membrane vesicle transport or vesicle–cortex tethering may be impacted by loss of Fyn kinase in the oocyte. We reported earlier that Fyn-null oocytes also fail to position the spindle close to the oocyte cortex, which is required for normal polarisation of the actin cap (Deng et al. 2003) and correlates with the formation of a cortical granule-free zone. Functionally, the proper distribution of cortical granules is critical to the fertilisation-dependent modification of the oocyte surface and zona pellucida. Actin and Rho signalling have been shown to be critical for cortical granule trafficking (Covián-Nares et al. 2004) and the results here indicate that Fyn kinase may also be involved in that process.
Further evidence of defective cytoplasmic organisation was found in our observation that the distribution of Type 1 IP3 receptors was irregular, with multiple large aggregations of receptors in the oocyte cortex. The unusual IP3 receptor distribution was consistent with the atypical calcium oscillation pattern during the response of the Fyn-null oocyte to fertilisation. Although atypical, the fertilised Fyn-null oocyte did produce sufficient calcium oscillatory activity to initiate resumption of meiosis and pronuclear formation, suggesting that Fyn was not absolutely required for fertilisation-induced calcium oscillations. This confirms recent studies using recombinant dominant- negative constructs in the rat oocyte (Tomashov-Matar et al. 2008). The role of actin in maintaining the normal architecture of the endoplasmic reticulum and IP3 receptors, as well as the calcium signaling machinery, has been demonstrated in oocytes as well as other cell types (Lange and Gartzke 2006; Bose and Thomas 2009). The presence of abnormal calcium oscillations in Fyn-null oocytes seems likely to be a result of defects in the actin cytoskeleton typical of Fyn-suppressed oocytes, although isolated reports exist indicating that Fyn can phosphorylate the IP3 receptor in some cell types (Jayaraman et al. 1996; Cui et al. 2004).
The appearance of the above defects in oocyte quality during maturation and fertilisation raises the important question of when and how they first occur during folliculogenesis. Although IVM studies clearly show that Fyn kinase expressed in the oocyte is required for oocyte maturation (McGinnis et al. 2009), it is well known that oocyte growth and maturation relies on, and is controlled by, an intimate interaction with the surrounding granulosa cells. Because the Fyn-null mouse model used here is completely devoid of Fyn expression, it is not easy to resolve defects inherent within the oocyte from those resulting from defective granulosa cell function or granulosa–oocyte communication. FSH control of granulosa cell differentiation and function is known to involve activation of Src, Fyn and Abl kinases, and inhibitor studies have demonstrated that the Src family kinases are required for transient Ras activation that, in turn, leads to extracellular signal-regulated kinase (ERK) 1/2 activation and stimulation of the phosphatidylinositol 3-kinase pathway (Wayne et al. 2007; Fan et al. 2008) in granulosa cells. Activation of ERK within granulosa cells has significant implications for oocyte quality because this pathway is required for normal COC expansion and initiation of oocyte maturation (Fan and Richards 2009; Fan et al. 2009). Disruption of Src family kinase signaling in granulosa cells could have important additional consequences for the oocyte and resolving the contribution of intra-oocyte and granulosa cell Fyn to oocyte quality is clearly an important goal.
In summary, the present study has investigated the fertility of mice in which Fyn kinase has been knocked out in order to establish the specific roles of Fyn kinase in the oocyte without possible artifacts resulting from chemical inhibitors or recombinant protein constructs. The results demonstrate that Fyn-null female mice are subfertile and this is due, in large part, to defects in oocyte quality. The defects observed were primarily involved with cortical cytoskeleton function, meiotic spindle integrity and vesicle transport. The consequences of these defects were apparent as a high failure rate during oocyte maturation and pronuclear congression. The functional basis for these defects could be expected to involve decreased phosphorylation of selected oocyte proteins resulting from the lack of Fyn kinase. The Fyn-null oocytes exhibited reduced levels of phosphotyosine in several protein bands detected in western blots of total egg proteins. Identification of these proteins and the role of Fyn kinase in modulating their function remains an important goal for future studies.
Acknowledgements
The authors are indebted to Lily Zhang for technical assistance. This work was supported by grants from the National Institute of Child Health and Human Development (USA) (14846; to W.H.K.) and the National Center For Research Resources (P20RR024214).
References
- Besterman B, Schultz RM. Regulation of mouse preimplantation development: inhibitory effect of genistein, an inhibitor of tyrosine protein phosphorylation, on cleavage of one-cell embryos. J. Exp. Zool. 1990;256:44–53. doi: 10.1002/jez.1402560107. [DOI] [PubMed] [Google Scholar]
- Bose DD, Thomas DW. The actin cytoskeleton differentially regulates NG115–401L cell ryanodine receptor and inositol 1,4,5-trisphosphate receptor induced calcium signaling pathways. Biochem. Biophys. Res. Commun. 2009;379:594–599. doi: 10.1016/j.bbrc.2008.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Semin. Cell Dev. Biol. 2001;12:37–43. doi: 10.1006/scdb.2000.0215. [DOI] [PubMed] [Google Scholar]
- Covián-Nares F, Martinez-Cadena G, Lopez-Godinez J, Voronina E, Wessel GM, Garcia-Soto J. A Rho-signaling pathway mediates cortical granule translocation in the sea urchin oocyte. Mech. Dev. 2004;121:225–235. doi: 10.1016/j.mod.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Cz from humans and cynomoigus monkeys triggers Ca oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks A. Regulation of Type 1 inositol 1,4,5 trisphosphate receptor by phosphorylation at tyrosine 353. J. Biol. Chem. 2004;279:16311–16316. doi: 10.1074/jbc.M400206200. [DOI] [PubMed] [Google Scholar]
- Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev. Biol. 2003;257:166–176. doi: 10.1016/s0012-1606(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Fan HY, Richards JS. Minireview: physiological and pathological actions of RAS in the ovary. Mol. Endocrinol. 2009;24:286–298. doi: 10.1210/me.2009-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J. Biol. Chem. 1999;274:29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- Jacquet P, Saint-Georges L, Barrio S, Baugnet-Mahieu L. Morphological effects of caffeine, okadaic acid and genistein in one-cell mouse embryos blocked in G2 by X-irradiation. Int. J. Radiat. Biol. 1995;67:347–358. doi: 10.1080/09553009514550401. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Shen SS. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Dev. Biol. 2000;225:253–264. doi: 10.1006/dbio.2000.9830. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Wu W, Macgregor E. Activation of Src-family PTK activity at fertilization: role of the SH2 domain. Dev. Biol. 2003;264:255–262. doi: 10.1016/j.ydbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev. Biol. 1999;215:431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Fissore RA. Mammalian fertilization: from sperm factor to phospholipase Cz. Biol. Cell. 2004;96:37–45. doi: 10.1016/j.biolcel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Lange K, Gartzke J. A critical comparison of the current view of Ca signaling with the novel concept of F-actin-based calcium signaling. Crit. Rev. Eukaryot. Gene Expr. 2006;16:307–365. doi: 10.1615/critreveukargeneexpr.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Livingston BT, VanWinkle CE, Kinsey WH. Protein tyrosine kinase activity following fertilization is required to complete gastrulation, but not for initial differentiation of endoderm and mesoderm in the sea urchin embryo. Dev. Biol. 1998;193:90–99. doi: 10.1006/dbio.1997.8743. [DOI] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol. Reprod. Dev. 2009;76:819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev. Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Kinsey WH, Albertini DF. The functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev. Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev. Biol. 1995;170:607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev. Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cg is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- Meng L, Luo J, Li C, Kinsey WH. Role of SH2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kinsey WH. Effects of protein tyrosine kinase inhibitors on egg activation and fertilization-dependent protein tyrosine kinase activity. Dev. Biol. 1995;168:1–10. doi: 10.1006/dbio.1995.1056. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev. Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev. Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Fukami Y. Fertilization signalling and protein-tyrosine kinases. Comp. Biochem. Physiol. B. 2000;126:129–148. doi: 10.1016/s0305-0491(00)00192-9. [DOI] [PubMed] [Google Scholar]
- Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev. Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- Tomashov-Matar R, Levi M, Shalgi R. The involvement of Src family kinases (SFKs) in the events leading to resumption of meiosis. Mol. Cell. Endocrinol. 2008;282:56–62. doi: 10.1016/j.mce.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Tsai WB, Zhang X, Sharma D, Wu W, Kinsey WH. Role of Yes kinase during early zebrafish development. Dev. Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Vallés AM, Denoyelle M, Boyer B, Lentz D, Thiery JP. Mesoderm-independent regulation of gastrulation movements by Src tyrosine kinase in Xenopus embryo. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- Vanderheyden V, Wakai T, Bultynck G, De SH, Parys JB, Fissore RA. Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation. Cell Calcium. 2009;46:56–64. doi: 10.1016/j.ceca.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil. Cytoskeleton. 1995;30:122–135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- Wu W, Kinsey WH. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int. J. Dev. Biol. 2000;44:837–841. [PubMed] [Google Scholar]