Abstract
Background and Purpose
Loss-of-function mutations of the lipoprotein receptor–related protein-6 (LRP6), a coreceptor in the Wingless-related integration site-β–catenin prosurvival pathway, have been implicated in myocardial ischemia and neurodegeneration. However, it remains to be established whether LRP6 is also involved in ischemic brain injury. We used LRP6+/− mice to examine the role of this receptor in the mechanisms of focal cerebral ischemia.
Methods
Focal cerebral ischemia was induced by transient occlusion of the middle cerebral artery. Motor deficits and infarct volume were assessed 3 days later. Glycogen-synthase-kinase-3β (GSK-3β) phosphorylation was examined by Western blotting with phosphospecific antibodies, and the mitochondrial membrane potential changes induced by Ca2+ were also assessed.
Results
LRP6+/− mice have larger stroke and more severe motor deficits, effects that were independent of intraischemic cerebral blood flow, vascular factors, or cytosolic β-catenin levels. Rather, LRP6 haploinsufficiency increased the activating phosphorylation and decreased the inhibitory phosphorylation of GSK-3β, a kinase involved in proinflammatory signaling and mitochondrial dysfunction. Accordingly, postischemic inflammatory gene expression was enhanced in LRP6+/− mice. Furthermore, the association of mitochondria with activated GSK-3β was increased in LRP6+/− mice, resulting in a reduction in the Ca2+ handling ability of mitochondria. The mitochondrial dysfunction was reversed by pharmacological inhibition of GSK-3β.
Conclusions
LRP6 activates an endogenous neuroprotective pathway that acts independently of β-catenin by controlling GSK-3β activity and preventing its deleterious mitochondrial and proinflammatory effects. The findings raise the possibility that emerging treatment strategies for diseases attributable to LRP6 loss-of-function mutations could also lead to new therapeutic avenues for ischemic stroke.
Keywords: cerebral ischemia, glycogen-synthase-kinase-3, mitochondria, stroke, Wnt signaling pathway
The Wnt pathway plays a central role in the development of the central nervous system during embryogenesis. Binding of wingless-related integration site (Wnt), a family of cysteine-rich proteins, to the membrane receptor Frizzled and low-density lipoprotein receptor–related protein (LRP) coreceptors initiates a signaling cascade that leads to nuclear β-catenin expression and induction of specific transcriptional programs (canonical Wnt pathway), release of intracellular Ca2+ stores (Ca2+ pathway), and cytoskeletal reorganization (planar cell polarity pathway).1 In addition to its role in development, Wnt signaling has been implicated in a variety of pathological conditions, including cancer, myocardial ischemia, and neurodegenerative diseases.1 Binding of Wnt to Frizzled induces the formation of a ternary complex with LRP5/6 and tethering of casein kinase 1 to the complex, resulting in phosphorylation and activation of Dishevelled and subsequent inhibition of glycogen-synthase-kinase-3β (GSK-3β). Reduced GSK-3β activity results in stabilization of β-catenin and activation of the canonical Wnt pathway by allowing β-catenin to escape proteasomal degradation and initiate expression of protective genes after binding to T-cell factor/lymphoid enhancer factor transcription factors.1 Besides β-catenin, GSK-3β has been reported to phosphorylate >40 proteins.2 It seems that in the context of Wnt signaling, ≥2 pools of GSK-3β exist. One pool is associated with the β-catenin destruction complex, whereas the other is independent of β-catenin and regulates mammalian target of rapamycin and transforming growth factor-β/Smad1 signaling,3,4 as well as the mitochondrial permeability transition.5
Maintaining mitochondrial function during ischemic stress is essential for neuronal survival.6 Mitochondrial function is tied to the preservation of the mitochondrial transmembrane potential (ΔΨm), which is essential for oxidative phosphorylation to occur. The collapse of ΔΨm through the opening of the mitochondrial permeability transition pore (mPTP) constitutes a central step in ischemic cell injury and the subsequent induction of apoptosis.7 The mPTP is a multiprotein complex whose activity is controlled by several molecular signals and upstream protein kinases, including GSK-3β. Augmented GSK-3β activity results in enhanced mPTP permeability, potentially leading to the collapse of ΔΨm.
The Wnt pathway has recently been linked to neuronal cell death after cerebral ischemia.8,9 Although modulation of Frizzled has been implicated in cell survival through β-catenin signaling,10 the role of the Wnt coreceptor LRP6, previously implicated in Alzheimer disease11 and in a hereditary form of coronary artery disease,12 remains to be established. Here, we set out to investigate the role of LRP6 in cerebral ischemic injury in mice. We found that LRP6 haploinsufficiency resulted in increased ischemic injury through GSK-3β–mediated impairment of mitochondrial function and upregulation of postischemic inflammatory gene expression. The data identify LRP6 as a critical component of a potent endogenous neuroprotective pathway that acts by suppressing the deleterious effects of GSK-3β, and could be the presumptive target of preventive or therapeutic interventions in stroke.
Material and Methods
A detailed description of the methods is provided in the online-only Data Supplement.
Mice
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Experiments were performed in 2- to 3-month-old male LRP6+/− mice13 (weight, 20–24 g), obtained from in-house colonies. The mice were kept on a C3H/HeJ background. We used heterozygous mice because LRP6−/− mice are not viable.13 Wild-type siblings were used as wild-type controls.
Middle Cerebral Artery Occlusion
Procedures for transient middle cerebral artery (MCA) occlusion were identical to those previously described.14–16 Under isoflurane anesthesia with cerebral blood flow (CBF) monitoring, the MCA was occluded for 40 minutes. Only animals that exhibited a 85% reduction in CBF during MCA occlusion (MCAo) and in which CBF recovered by 80% after 10 minutes of reperfusion were included in the study.15–17 The number of excluded animals was not significantly different in LRP6+/+ and LRP6+/− groups (12% versus 17%; P>0.05), and the survival rate after MCAo was comparable (83% in LRP6+/+ versus 87% in LRP6+/−; P>0.05).
Assessment of Motor Function and Measurement of Infarct Volume
Motor function and edema-corrected infarct volume were assessed by an investigator blinded to the experimental groups on day 3 after reperfusion. Motor function was assessed by the hanging wire test, as described in detail elsewhere.15 Infarct volumes were determined in cresyl violet–stained brain sections as described.15–17
Cerebrovascular Regulation
Procedures for testing cerebrovascular regulation in mice have been described previously.18 After cannulation of the femoral artery, tracheal intubation, and artificial ventilation under isoflurane, anesthesia was maintained with urethane and chloralose. The somatosensory cortex was exposed through a small craniotomy (2×2 mm). The dura was removed, and the site was superfused with a modified Ringer solution. CBF was continuously monitored with a laser-Doppler probe, positioned stereotaxically on the somatosensory cortex, a region supplied by the MCA. CBF changes were expressed as percentage increase relative to the resting level. To study the increase in CBF produced by neural activity (functional hyperemia), the somatosensory cortex was activated by gently stroking the contralateral whiskers with a cotton-tipped applicator. Endothelium-dependent vasodilation was tested by topical superfusion of acetylcholine (10 μmol/L) or bradykinin (50 μmol/L) for 3 to 5 minutes, and the CBF increase was recorded. The CBF response to adenosine (400 μmol/L), an agent that produces vasodilation by acting directly on vascular smooth muscles, was also tested.
Real-Time Polymerase Chain Reaction
The mRNA for proinflammatory genes was examined after ischemia using quantitative real-time polymerase chain reaction as previously described.16,19 A group of genes that have been previously shown to be regulated after MCAo and have been implicated in neuronal cell death and postischemic inflammation were included in the mRNA expression screen. Mice were euthanized 0, 2, 6, 24, and 72 hours after ischemia. Primer details are presented in Table 1 in the online-only Data Supplement.
Double-Label Immunohistochemistry
For identification of the cell types expressing LRP6, paraformaldehyde-fixed brain sections were first processed for LRP6 immunocytochemistry, followed by incubation with antibodies against the neuronal marker NeuN, the endothelial cell marker CD31, the astrocytic marker glial fibrillary acidic protein or the microglial marker ionized calcium–binding adaptor molecule 1. Images of double-labeled neocortex were sequentially acquired using confocal laser-scanning microscope.
Western Blot Analysis
In GSK-3β phosphorylation studies, mice were euthanized 0, 1, 3, 6 hours after ischemia-reperfusion. The cortex of the ischemic hemisphere was sampled, and equal amount of proteins were gel-separated and transferred to polyvinylidene fluoride membranes. The blots were probed with phospho-GSK-3β-Ser9 or phospho-GSK-3β-Y216–specific antibodies and reprobed with pan-GSK-3β antibody or β-actin–specific antibody to verify equal loading. For β-catenin detection, cytosolic protein lysates were prepared from the cortex of right hemisphere at each time point and analyzed by Western blotting with antiβ-catenin antibodies.
Mitochondrial Transmembrane Potential
Crude mitochondria from mouse brain were isolated as previously described.6 The mitochondria were used for experiments within 4 hours. ΔΨm was measured according to the method of Liu et al20 using safranine O as a probe as previously described.6 Changes in ΔΨm were induced by adding 50 nmol aliquots of CaCl2. In some experiments, LRP6+/+ and +/− mice were treated with the GSK-3β inhibitor SB216763 (1 mg/kg; IP)21 for 16 hours. The effectiveness and specificity of the dose of SB216763 has been previously documented.21
Statistical Analysis
Data are presented as mean±SEM. Comparisons between 2 groups were statistically evaluated by the Student t test. Multiple comparisons were evaluated by ANOVA followed by the Newman–Keuls test. Differences were considered significant for P<0.05.
Results
LRP6 Is Localized to Neurons in the Mouse Neocortex
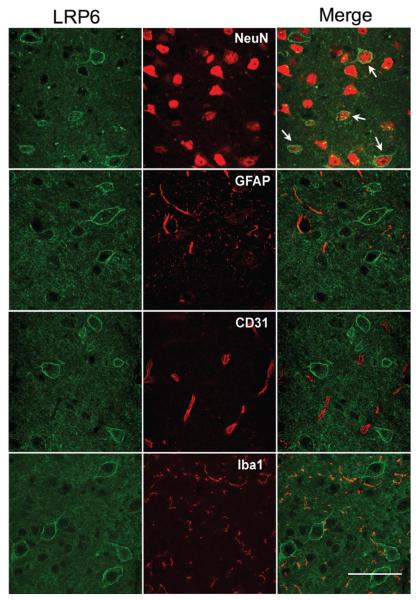
First, we sought to determine the cellular localization of LRP6 in the adult mouse neocortex. LRP6 immunoreactivity was observed throughout the neocortex clearly demarcating the plasma membrane outlining the somata of large cells consistent with a predominantly plasmalemmal localization of the receptor (Figure 1A). We found that LRP6 was colocalized with the neuronal marker NeuN, but not with the astroglial marker glial fibrillary acidic protein or the microglial marker ionized calcium–binding adaptor molecule 1 (Figure 1). Similarly, LRP6 was not observed in CD31-positive endothelial cells (Figure 1). Therefore, in the mouse neocortex, LRP6 is localized to the plasma membrane of neurons.
Figure 1.
Lipoprotein receptor–related protein-6 (LRP6) is localized to neurons in the adult mouse cerebral cortex. Representative micrographs (n=4) of mouse cortex stained with antibodies specific for LRP6, NeuN (neurons), glial fibrillary acidic protein (GFAP; astrocytes), CD31 (endothelium), and ionized calcium–binding adaptor molecule 1 (Iba1; microglia). Scale bar=50 μm.
LRP6+/− Mice Have Larger Infarcts and More Severe Motor Deficits After MCAo
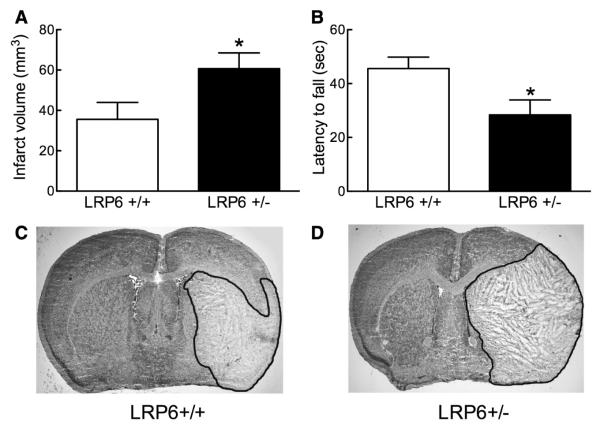
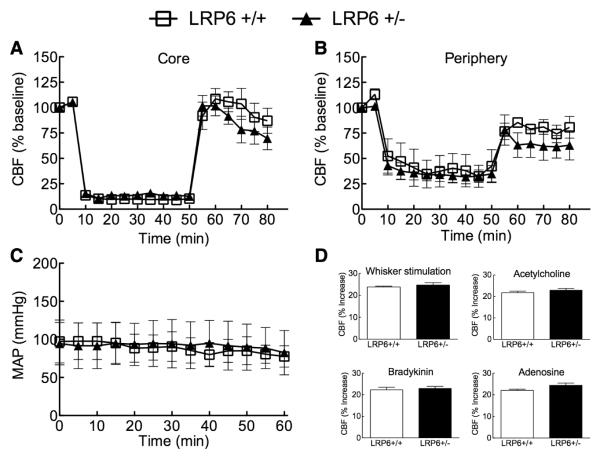
Next, we examined the role of LRP6 in the brain injury 72 hours after transient MCAo. The infarct was larger in LRP6+/− mice, the expansion involving both the neocortex and the basal ganglia (Figure 2A, 2C, and 2D). The motor deficits induced by MCAo, as reflected by the latency to fall at the hanging wire test,22 were more severe in LRP6+/− than in LRP6+/+ mice (Figure 2B). The increase in infarct volume could not be attributed to differences in the degree of ischemia because the reduction in CBF was comparable in the center and periphery of the ischemic territory in LRP6+/+ and LRP6+/− mice (Figure 3A, and 3B). Furthermore, no differences in arterial pressure were observed between LRP6+/+ and LRP6+/− mice during and after MCAo (Figure 3C). Similarly, plasma glucose did not differ between LRP6+/− (204±14 mg/dL) and LRP6+/+ mice (190±35 mg/dL; P>0.05; n=10/group). Next, we examined whether defects in neurovascular regulation could play a role in the increased ischemic injury observed in LRP6+/− mice. In anesthetized mice, the increase in CBF produced by whisker stimulation, by the endothelium-dependent vasodilators acetylcholine and bradykinin or the smooth muscle relaxant adenosine was comparable between LRP6+/+ and LRP6+/− mice (Figure 3D). Therefore, the increased stroke volume in LRP6+/− cannot be attributed to alterations in cerebrovascular regulation.
Figure 2.
Increased infarct volumes and impaired motor function in lipoprotein receptor–related protein-6 (LRP6) +/− mice. A, Edema-corrected infarct volume was determined 72 hours after MCAo (n=8/group); *P<0.05 Student t test. B, The Hanging Wire Test was applied 72 hours after tMCAo (n=9/group); *P<0.05 Student t test. C and D, Representative Nissl-stained brain sections derived from animals analyzed in A.
Figure 3.
Cerebral perfusion and cerebrovascular regulation are not compromised in lipoprotein receptor–related protein-6 (LRP6)+/− mice. Core (A) and penumbral (B) blood flow during and after middle cerebral artery occlusion (MCAo; n=5/group). C, Intraarterial blood pressure recordings in mice undergoing MCAo (n=5/group). D, Cerebral blood flow (CBF) responses in LRP6+/+ or LRP6+/− mice undergoing whisker stimulation or topical application of acetylcholine, bradykinin, or adenosine. Significance was tested by ANOVA followed by the Newman–Keuls test (A–C) or Student t test (D). MAP indicates mean arterial pressure.
GSK-3β Phosphorylation Is Altered in LRP6+/− Mice
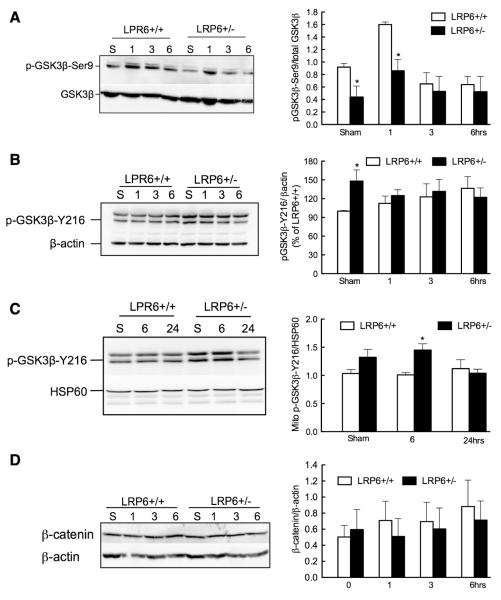
GSK-3β, a kinase regulated by activating (Y216) and inhibitory (Ser9) phosphorylation,23 is a critical component of the LRP6-Wnt signaling pathway. Because GSK-3β activation is deleterious to the brain24,25 and LRP6 inhibits GSK-3β activity,26,27 we examined the phosphorylation state of GSK-3β in LRP6+/− mice. After MCAo in LRP6+/+ mice, GSK-3β activating phosphorylation (pGSK3-Y216) in the ischemic lesion remained stable, whereas the inhibitory phosphorylation (pGSK3-Ser9) increased at 1 hour and returned to normal by 3 hours (Figure 4A and 4B). In sham-operated LRP6+/− mice, pGSK3-Ser9 was reduced, whereas pGSK3-Y216 was increased (Figure 4A and 4B). After MCAo in LRP6+/− mice, the pGSK3-Y216 was not different from LRP6+/+ mice (Figure 4A), whereas pGSK3-Ser9 was suppressed compared with LRP6+/+ mice (Figure 4B). These observations are consistent with the hypothesis that GSK-3β activity is increased in LRP6+/− mice.
Figure 4.
Increased cerebral glycogensynthase-kinase (GSK)-3β activity in lipoprotein receptor–related protein-6 (LRP6)+/− mice. A, Western blot analysis of inhibitory GSK-3β-Ser9 phosphorylation; quantification of p-GSK-3β-Ser9 signals after normalization by total-GSK-3β signals of the same sample (right, n=4). B, Western blot analysis of activating GSK-3β-Y216 phosphorylation; quantification of p-GSK-3β-Y216 signals after normalization by β-actin signals of the same sample (right, n=4). C, Western blot analysis of mitochondrial GSK-3β-Y216 phosphorylation; quantification of p-GSK-3β-Y216 signals after normalization by heat shock protein 60 (HSP60) signals of the same sample (right, n=4). D, Western blot analysis of cytoplasmic β-catenin levels; quantification of β-catenin signals after normalization by β-actin signals of the same sample (right, n=4). *P<0.05 (ANOVA and Newman–Keuls test).
Mitochondrial translocation of GSK-3β is associated with mitochondrial dysfunction and injury.5,28 Therefore, we examined the association of GSK-3β with mitochondria and its phosphorylation state. In sham-operated mice, pGSK3-Y216 associated with mitochondria tended to be more abundant in LRP6+/− mice than in LRP6+/+ mice (Figure 4C). An increase was also observed 6 hours after MCAo, but no differences were seen at 24 hours (Figure 4C).
β-Catenin Levels Are Not Reduced in LRP6+/− Mice
β-catenin is a key signaling molecule in the LRP6-Wnt pathway, which has a protective role in cerebral ischemic injury.9 Phosphorylation of β-catenin by GSK-3β leads to its degradation and loss of its prosurvival effects.23 Therefore, we examined whether the increased injury in LRP6+/− mice is related to suppression of β-catenin expression. β-Catenin protein levels were not significantly reduced in LRP6+/− mice, either before or after ischemia (Figure 4D), indicating that increased degradation of this protein is unlikely to play a role in the increased injury in LRP6+/− mice.
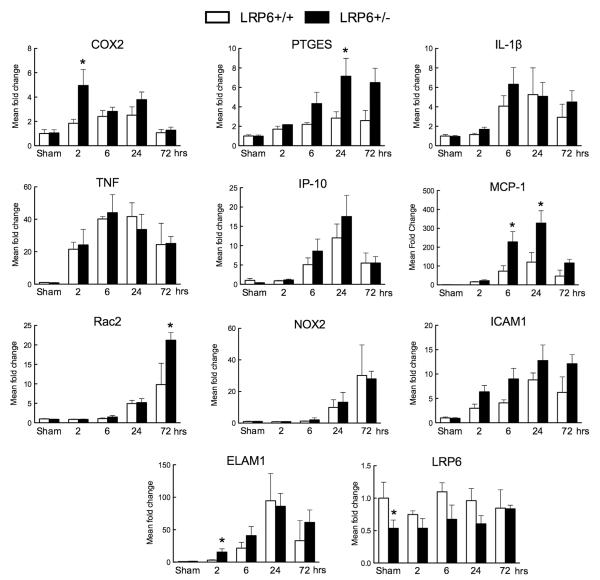
Postischemic Expression of Inflammatory Genes Is Enhanced in LRP6+/− Mice
GSK-3β activation is well known to promote inflammatory signaling.2 Therefore, we examined whether the exacerbation of the ischemic injury in LRP6+/− mice was related to enhancement of postischemic inflammation (Figure 5). Cyclooxygenase-2 and prostaglandin E synthase, which are mainly localized to neurons,29,30 were markedly upregulated in LRP6+/− mice. The upregulation of the chemokine monocyte chemotactic protein-1, and of Rac2, a small guanosine triphosphatase enriched in neutrophils, was increased in LRP6+/− mice (Figure 5). Endothelial-leukocyte adhesion molecule 1 expression was significantly higher in LRP6+/− mice, while intercellular adhesion molecule 1 showed the same trend without reaching significance. In contrast, the upregulation of the cytokine interleukin-1β and tumor necrosis factor, the chemokine IP-10, and of the NOX2 subunit of the superoxide-producing enzyme NADPH oxidase did not differ between LRP6+/+ and LRP6+/− mice (Figure 5). As anticipated, LRP6 mRNA expression was reduced in sham-operated LRP6+/− mice (Figure 5).
Figure 5.
Inflammatory marker genes are increased in the cerebral cortex of lipoprotein receptor–related protein-6 (LRP6)+/− mice after middle cerebral artery occlusion (MCAo). mRNA expression of cyclooxygenase-2 (COX-2), prostaglandin E synthase (PTGES) interleukin (IL)-1β, tumor necrosis factor (TNF), IP-10, monocyte chemotactic protein-1 (MCP-1), Rac2, NOX2, intercellular adhesion molecule 1 (ICAM1), endothelial-leukocyte adhesion molecule 1 (ELAM1), and LRP6 were analyzed by quantitative real-time polymerase chain reaction 2, 6, 24, and 72 hours after reperfusion. Mean fold increase was calculated relative to sham-operated LRP6+/+ animals (n=4–7/group). *P<0.05 from LRP6+/+ mice at the same time point (Student t test).
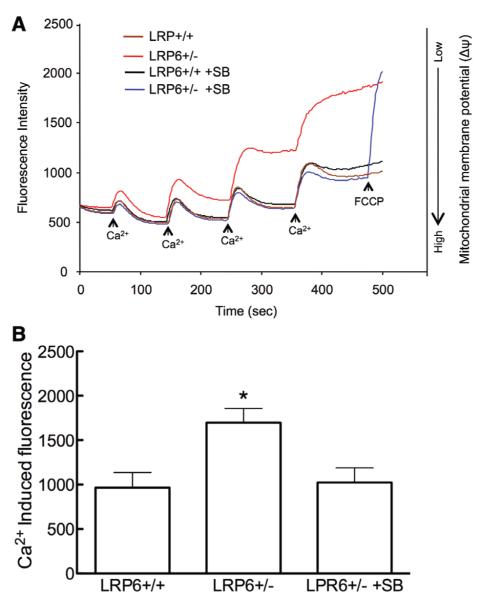
Mitochondria of LRP6+/− Mice Are More Susceptible to Ca2+-Induced Depolarization, an Effect Mediated by GSK-3β
Mitochondrial localization of GSK-3β may increase their susceptibility to injury.31 Therefore, we examined whether the mitochondria of LRP6+/− mice are more vulnerable to the loss of ΔΨm induced by Ca.2+ In isolated mitochondrial preparations of LRP6+/+ mice, administrations of Ca2+ aliquots (CaCl2; 50 nmol) produced transient mitochondrial depolarizations, which fully recovered up to the third or fourth aliquot administered, at which point ΔΨm remained elevated after the Ca2+ challenge (Figure 6A). In contrast, LRP6+/− mitochondria were unable to maintain ΔΨm and, subsequent to the first Ca2+ challenge, they progressively became more depolarized (Figure 6A). Administration of the GSK-3β inhibitor SB216763, before mitochondria isolation, did not affect mitochondrial depolarization in LRP6+/+ mice, but it reversed the increased Ca2+-induced depolarization in LRP6+/– mice (Figure 6B).
Figure 6.
Increased glycogen-synthase-kinase (GSK)-3β activity impairs mitochondrial transmembrane potential in lipoprotein receptor–related protein-6 (LRP6)+/− mice. A, Representative traces of Ca2+-induced mitochondrial membrane depolarization. Mitochondria isolated from the cerebral cortex of LRP6+/+ or LRP6+/− mice treated with vehicle or the GSK-3β inhibitor SB216763 were exposed to increasing Ca2+ concentrations, and safranine O fluorescence was recorded. Addition of the uncoupling agent carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; 0.15 μm) to mitochondria from LRP6+/− mice treated with SB216763 led to complete depolarization attesting to the integrity of the preparation. Traces shown are representative of 3 independent experiments with pooled mitochondria from 3 mice in each group. B, Mean fluorescence was recorded during the time period of 500 sec (n=3); *P<0.05 ANOVA followed by the Newman–Keuls test.
Discussion
We have demonstrated that LRP6 haploinsufficiency exacerbates the brain injury and motor deficits produced by MCAo. The effect was associated with increased phosphorylation of GSK-3β at the activating site (Y206) and reduced phosphorylation at the inhibitory site (Ser9), suggesting increased GSK-3β activity. β-Catenin levels were not reduced in LRP6+/− mice, but MCAo results in an enhancement of the expression of inflammatory genes, well known to contribute to ischemic brain injury.32 In addition, consistent with an increase in p-GSK-3β targeted to mitochondria, resistance to Ca2+-induced depolarization was reduced in LRP6+/− mitochondria, an effect reversed by the GSK-3β inhibitor SB216763. Collectively, the data indicate that LRP6 haploinsufficiency leads to activation of GSK-3β, which exacerbates ischemic injury by promoting postischemic inflammation and reducing mitochondrial resistance to stress. Therefore, neuronal LRP6 may act as an endogenous neuroprotective receptor that seeks to maintain the homeostasis of the postischemic brain by suppressing GSK-3β activity.
The enhancement of injury observed in LRP6+/− mice could not be attributed to an increase in the intensity of the ischemic insult because the reduction in CBF produced by MCAo in the center and periphery of the ischemic territory was comparable in LRP6+/+ and LRP6+/− mice. Similarly, alterations in the ability of cerebral blood vessels to increase CBF in response to vasoactive stimuli are unlikely to play a role in the increased injury because the reduction in CBF produced by MCAo and the increase in CBF produced by functional hyperemia and endothelium-dependent relaxing agents did not differ between LRP6+/+ and LRP6+/− mice.
Given its predominant neuronal localization, we hypothesized that LRP6 haploinsufficiency might contribute to ischemic neuronal injury by decreasing canonical Wnt signaling, resulting in reduced β-catenin activity. However, cytosolic β-catenin levels were not different in LRP6+/− mice before or after cerebral ischemia, although activating GSK-3β-Y216 phosphorylation was increased at the same time points. We, therefore, investigated whether other downstream targets of the GSK-3β pathway could potentially account for the increased ischemic brain injury observed in LRP6+/− mice.
In addition to its role in the Wnt signaling pathway, GSK-3β has been linked with enhanced proinflammatory gene transcription and impaired mitochondrial function.2,5 Analysis of a panel of proinflammatory genes shown to be involved in neuronal injury and postischemic inflammation revealed a gene-specific effect of LRP6 expression. Genes associated with neuronal injury, such as cyclooxygenase-2 and Ptges,29,30 were upregulated in LRP6+/− mice undergoing MCAo. Similarly, we observed increased expression of the chemokine monocyte chemotactic protein-1 and increased presence of Rac2 transcripts, possibly indicating greater leukocyte infiltration and larger infarcts in LRP6+/− mice 72 hours after ischemia-reperfusion. Expression of endothelial adhesion molecules, endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1, was moderately increased, whereas tumor necrosis factor and interleukin-1β mRNA levels were not different in LRP6+/+ and LRP6+/− animals. The apparent selectivity of LRP6-dependent postischemic inflammatory gene expression could be indicative of a divergent role of GSK-3β in regulating gene expression in different inflammatory models, having suppressive or enhancing activities depending on the inflammatory cascades involved.33 To more definitively establish the involvement, the role of neuronal GSK-3β in postischemic inflammation mice with conditional deletion of neuronal GSK-3β would be needed.
Elevated GSK-3β activity has been shown to decrease mitochondrial ΔΨm stability by reducing the threshold for mPTP opening.5 The fact that mitochondria isolated from the cerebral cortex of LRP6+/− mice showed less tolerance for Ca2+-induced ΔΨm alterations is consistent with a decreased mPTP threshold, an effect that was dependent on increased GSK-3β activity as shown by its reversal after treatment with the GSK-3β inhibitor SB216763. Therefore, loss of mitochondrial integrity is also likely to contribute to the enhanced cerebral ischemic injury in LRP6+/− mice. Mitochondria dysfunction could lead to brain damage injury through different mechanisms, including, for example, release of proapoptotic factors, oxidative stress, and energy failure.34 The specific mechanisms through which mitochondrial dysfunction increases the susceptibility to ischemic injury in LRP6+/− mice remain to be established.
LRP6 mutations are involved in several human diseases, including osteoarthritis, premature osteoporosis, coronary artery disease, and Alzheimer disease.35 The present results raise the possibility that LRP6 loss-of-function mutations may also increase the susceptibility to ischemic stroke. Although the clinical association with stroke remains to be established, several approaches to modulate LRP5 and 6 signaling are under development, especially for bone diseases,35 and could also benefit patients at risk for ischemic stroke. A limitation of this approach is that excessive or dysregulated activation of Wnt signaling could also lead to pathology.36 Therefore, approaches to provide tight control of the activation would be needed.37
In summary, LRP6 haploinsufficiency leads to exacerbation of ischemic brain injury. The effect is independent of vascular factors or suppression of β-catenin levels, but is related to increased GSK-3β activating phosphorylation and mitochondrial association of GSK-3β, leading to increased inflammatory gene expression and mitochondrial dysfunction, respectively. The data unveil a novel role of LRP6 in neuronal resistance to injury and raise the possibility that loss-of-function mutations of LRP6, in addition to promoting myocardial ischemia and Alzheimer diseases, may also increase the propensity to ischemic stroke.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants NS34179, NS73666, and HD067244.
Footnotes
Disclosures None.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.113.001320/-/DC1.
References
- 1.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38:148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dugo L, Collin M, Thiemermann C. Glycogen synthase kinase 3beta as a target for the therapy of shock and inflammation. Shock. 2007;27:113–123. doi: 10.1097/01.shk.0000238059.23837.68. [DOI] [PubMed] [Google Scholar]
- 3.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Qian L, Zhou T, Iadecola C. Mitochondria are involved in the neurogenic neuroprotection conferred by stimulation of cerebellar fastigial nucleus. J Neurochem. 2005;95:221–229. doi: 10.1111/j.1471-4159.2005.03358.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch T, Marzo I, Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep. 1997;17:67–76. doi: 10.1023/a:1027339418683. [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastroiacovo F, Busceti CL, Biagioni F, Moyanova SG, Meisler MH, Battaglia G, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 10.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 14.Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunz A, Abe T, Hochrainer K, Shimamura M, Anrather J, Racchumi G, et al. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 18.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, et al. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, et al. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 22.Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- 23.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- 25.Koh SH, Yoo AR, Chang DI, Hwang SJ, Kim SH. Inhibition of GSK-3 reduces infarct volume and improves neurobehavioral functions. Biochem Biophys Res Commun. 2008;371:894–899. doi: 10.1016/j.bbrc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Mi K, Dolan PJ, Johnson GV. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J Biol Chem. 2006;281:4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- 27.Piao S, Lee SH, Kim H, Yum S, Stamos JL, Xu Y, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem. 2009;284:3001–3011. doi: 10.1074/jbc.M808698200. [DOI] [PubMed] [Google Scholar]
- 29.Iadecola C. Cyclooxygenase-2 and stroke: the long and short of it. Ann Neurol. 2003;54:141–142. doi: 10.1002/ana.10668. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:11790–11795. doi: 10.1073/pnas.0604400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortés-Vieyra R, Bravo-Patiño A, Valdez-Alarcón JJ, Juárez MC, Finlay BB, Baizabal-Aguirre VM. Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J Inflamm (Lond) 2012;9:23. doi: 10.1186/1476-9255-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab. 2013;24:31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Singh R, Choi CS, Lee HY, Keramati AR, Samuel VT, et al. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem. 2012;287:7213–7223. doi: 10.1074/jbc.M111.286724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn. 2010;239:102–114. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.