Abstract
Background:
Effective treatments for the behavioral and cognitive deficits in children with fetal alcohol spectrum disorders (FASD) are lacking, and translational approaches using animal models can help develop rational interventions. One such model, binge-like alcohol exposure in neonatal rats during the period of brain development comparable with that of the human third trimester, causes structural and functional damage to the cerebellum and disrupts cerebellar-dependent eyeblink classical conditioning. The eyeblink conditioning deficits first demonstrated in this rat model predicted the similar deficits subsequently demonstrated in children with FASD.
Methods:
The current study extends this translational approach by testing the hypothesis that rehabilitation training involving 20 days of training on traversal of an obstacle course (complex motor learning) would ameliorate the deficits on classical conditioning of eyeblink responses produced by the neonatal alcohol exposure. We have previously shown that this training stimulates cerebellar synaptic plasticity and improves alcohol-induced deficits on motor coordination tasks.
Results:
The current studies found that rehabilitation training significantly attenuated alcohol-induced deficits in acquisition of eyeblink conditioning in females but not in males. These results are consistent with normalization of cerebellar-dependent learning, at least in alcohol-exposed females.
Conclusions:
These findings extend previous studies in this model suggesting that rehabilitation of adolescents with FASD using training with complex motor learning tasks could be effective in ameliorating functional impairments associated with cerebellar damage. Eyeblink classical conditioning deficits are now well documented in children with FASD and could serve as an evaluation measure to continue to develop therapeutic interventions such as complex motor learning.
Keywords: Cerebellum, Ethanol, Eyeblink Conditioning, Fetal Alcohol Spectrum Disorder
FETAL ALCOHOL SPECTRUM disorders (FASD) are preventable by abstinence during pregnancy, but efforts to reduce or eliminate maternal drinking during pregnancy have been largely ineffective in reducing the incidence of FASD (Roach and Anderson, 2008). Consequently, interventions to prevent or treat the structural and functional brain abnormalities and associated behavioral changes are important priorities (Kodituwakku and Kodituwakku, 2011; Warren and Foudin, 2001). Some pharmacological interventions administered during the period of alcohol exposure have shown promise in effectively reducing brain damage in animal models (Ieraci and Herrera, 2006; Spong et al., 2001; Zhou et al., 2004). Interventions during pregnancy have significant limitations, including difficulties in identifying at-risk drinking and in delivering prenatal care (Roach and Anderson, 2008), along with potential risk to the fetus or the mother. In addition, FASD often is not recognized until school age, when the cognitive and behavioral consequences of the prenatal brain damage become apparent (Astley et al., 2002). It is important, therefore, to pursue rehabilitation approaches that can be applied to children and adolescents with FASD to improve outcomes that emerge from the prenatal alcohol-induced brain damage.
One promising approach, developed in animal models of FASD, is to use behavioral procedures known to promote plasticity in brain systems damaged by prenatal alcohol exposure, targeting improved functional outcomes mediated by those systems. Such interventions include complex motor learning (Klintsova et al., 1998, 2002), environmental enrichment (Berman et al., 1996; Mothes et al., 1996; Wainwright et al., 1993), and voluntary exercise (Christie et al., 2005). Training on complex motor learning tasks may be particularly useful for rehabilitating damage to the cerebellum, which is known to have disproportionate volume reductions in cases of FASD (Autti-Ramo et al., 2002; Riley et al., 2004; Roebuck et al., 1998a; Sowell et al., 1996). Training rats for 20 days on a complex motor learning task (traversal of an obstacle course) stimulated significant increases in parallel fiber–Purkinje cell synapses in the cerebellar paramedian lobule (Black et al., 1990), and this synaptic morphological plasticity was retained in rats with cerebellar damage induced by alcohol exposure on postnatal days (PD) 4 to 9 (Klintsova et al., 2002). The training also rehabilitated performance deficits on motor tasks (rope climbing, rotorod, parallel bar traversal) that were not part of the training (Klintsova et al., 1998).
These findings suggest that the neonatal rat model of alcohol-induced brain damage resulting from binge alcohol exposure during the period of brain development comparable with that of the human third trimester (Cudd, 2005; Dobbing and Sands, 1979) is a useful model to extend the analysis of the rehabilitation. Cerebellar structural damage and functional deficits associated with cerebellar pathology have been well documented in FASD (Autti-Ramo and Granstrom, 1991; Coffin et al., 2005; Jacobson et al., 2008, 2011; Kyllerman et al., 1985; Roebuck et al., 1998b). This rat model has proven to have significant predictive validity for cerebellar effects. The neonatal binge alcohol treatment induces permanent, dose-related loss of neurons of the cerebellar cortex and deep nuclei (Bonthius and West, 1991; Goodlett et al., 1998; Green et al., 2002b; Klintsova et al., 2002; Tran et al., 2005). The alcohol-induced cerebellar neuronal loss is associated with enduring deficits in cerebellar-dependent behavioral tasks, including motor coordination tasks (Klintsova et al., 1998; Thomas et al., 1996, 2009); and eyeblink classical conditioning (Green et al., 2002a,b, 2006; Stanton and Goodlett, 1998; Tran et al., 2005, 2007). Notably, the deficits in eyeblink conditioning that were originally observed in this rat model predicted the subsequent demonstration of deficits in eyeblink conditioning in children with FASD (Coffin et al., 2005; Jacobson et al., 2008, 2011).
The current study was conducted to test the hypothesis that complex motor learning would rescue eyeblink conditioning deficits induced by neonatal binge-like alcohol exposure in rats. A key aspect of this study is that it extends the assessment of the effectiveness of this rehabilitation treatment to a cerebellar-dependent associative learning task that is very different from the rehabilitation training itself. Important advantages of using eyeblink classical conditioning are that the behavioral characteristics have been well studied, the essential cerebellar-brain stem neural circuitry involved has been identified in several species, including rats and humans (Christian and Thompson, 2003; Kim and Thompson, 1997; Steinmetz, 2000; Woodruff-Pak and Steinmetz, 2000a,b), and learning-related neuroplasticity within identified sites in the cerebellum is known to mediate normal acquisition and performance of eyeblink conditioning (Christian and Thompson, 2003; Kim and Thompson, 1997; Lavond et al., 1993). Because sex differences in eyeblink conditioning have been reported in which females show faster acquisition and facilitated performance than males (Dalla and Shors, 2009), this study evaluated both sexes.
MATERIALS AND METHODS
Subjects
Timed pregnant Long-Evans dams, purchased from Simonsen Laboratories (Gilroy, CA), arrived in the facility on gestational day (G) 11. The day of birth (G 22) was designated postnatal day (PD) 0. On PD 3, litters were culled to 8 pups, 4 male and 4 female when possible, and given subcutaneous injections of India ink to 1 or more paws for a unique numerical identifier. Experimenters were blind to neonatal treatment condition after the treatment period. Body weights were obtained for all intubated pups on the mornings of treatments (PD 4 to 9) and periodically thereafter. Litters were randomly assigned either to receive intubation treatments (18 litters) or to an undisturbed suckle control (SC) condition (11 litters), in which the litters were left to be reared normally. Within intubated litters, 4 males and 4 females were randomly assigned to one of the following treatments administered on PD 4 to 9: (i) alcohol intubated with 11.9% alcohol in milk formula (AI), or (ii) sham intubated (SI). When more than 1 littermate of the same sex contributed to the data for a given treatment condition (as occurred in 7 SC litters and 1 intubated litter), the data were averaged for those littermates to yield only 1 observation per litter (per sex) for a given treatment and training condition for all analyses.
Neonatal Alcohol Treatment
Alcohol treatment formulas were made in a milk formula and were administered via intragastric intubation as described previously in Goodlett and Johnson (1997). For alcohol infusions (AAPER, Shelbyville, KY), the milk formula contained 11.9%(v/v) alcohol and was delivered in a total volume of 0.02778 ml/g. Each infusion resulted in a 2.625 g/kg dose of alcohol (total daily dose of 5.25 g/kg/d); each intubation was separated by 2 hours. On PD 4, the AI group received an additional 2 intubations of milk formula (without alcohol), each separated by 2 hours; on the remaining treatment days, the AI group was given 1 additional milk-alone intubation, to provide near-normal growth because high-dose alcohol treatment impairs suckling behavior, limiting caloric intake during intoxication. The SI group received sham intubations, in which the tube was inserted into the stomach but no milk formula was infused. On PD 4, 20 μl of blood were collected by tail clip (from all intubated pups) 4 hours after the initial intubation for determination of peak blood alcohol concentrations (BACs) of the alcohol-treated pups. Blood samples were collected in heparinized capillary tubes and centrifuged for plasma separation. BACs were determined using an oximetric procedure with an Analox AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Complex Motor Training
On PD 25, animals were weaned and housed in same sex pairs (typically littermates). Five days later, animals from each treatment group were randomly assigned to be given either rehabilitation (R) using complex motor training or to a training control condition, which consisted of daily handling (H) for a comparable amount of time as animals in the rehabilitation condition were trained. At least 8 males and 8 females from each treatment group (AI-H, AI-R, SI-H, SI-R, SC-H, and SC-R) were used in this study a total of 116 animals in the 6 groups. When possible, same treatment and sex littermates were randomly assigned to alternate training conditions. Rats in the complex motor learning condition received progressively more training trials over the first 3 days until they reached 5 trials per day on day 4. Training trials consisted of traversing 10 elevated obstacles including a horizontal wooden ladder, high steps on a narrow beam, a link chain, a loose rope ladder, ascending and descending stairs, a narrow V-shaped metal bridge, pencil-wide dowels, a drum (with variable rotation speeds), and various other obstacles. Animals were encouraged to traverse all 10 elevated obstacles by gentle prodding of the hindquarters, and the tail was held loosely to prevent falling from the apparatus. The time spent traversing all 10 obstacles was recorded for each trial and averaged for each training day.
Eyelid Surgery
After 20 days of complex motor learning, animals were individually housed with ad libitum access to food and water where they remained until the end of eyeblink training. Surgery was performed 4 to 6 days after complex motor training on PD 53 to 56. Under isoflurane gas anesthesia, animals were subcutaneously implanted with 2 stainless steel electromyographic (EMG) recording electrodes (size 3T; Medwire, Mt. Vernon, NY) through the left orbicularis muscle to record differential activity of the eyelid, and one to serve as a grounding wire (size 10T; Medwire). The headstage also contained a bipolar stimulating electrode (MS303/2; Plastics One, Roanoke, VA) placed just caudal to the same eye, in a V shape, to deliver the electrical stimulation that served as the unconditioned stimulus (US). Headstages were anchored to the skull with 3 screws and secured with cranioplast. Five to 7 days after surgery, the animals were habituated to the conditioning chamber.
Eyeblink Conditioning Apparatus
Animals were allowed to move freely in a test box (30.5 × 24.1 × 29.2 cm; Med-Associates, St. Albans, VT) constructed with aluminum and clear polycarbonate walls. The floor was made of stainless steel rods (4.8 mm) in a polypropylene frame. The test box was contained within a sound-attenuating chamber (BRS-LVE, Laurel, MD). Each chamber was equipped with a fan (55- to 65-dB background noise level), house light (15 W), and 2 piezoelectric speakers (2- to 12-kHz range), 1 of which was used for presentation of the tone conditioned stimulus (CS). Each chamber was fitted with a commutator (AC267-20; Litton Systems, Blacksburg, VA), containing 5 lines of redundancy per channel, connected to peripheral equipment while allowing the rat maximum mobility. The US was delivered by a constant-current, 60-Hz stimulus isolator (A365-R; World Precision Instruments, Sarasota, FL). Two IBM-compatible computers (4 chambers per computer) with custom-developed software controlled the delivery of stimuli and recording of EMG activity (JSA Designs, Raleigh, NC).
Eyeblink Conditioning Procedures
On PD 70 rats began eyeblink classical conditioning using paired CS–US presentations. No unpaired training groups were included because our previous studies have clearly demonstrated that there are no differences between alcohol and control groups during unpaired training (Stanton and Goodlett, 1998; Tran et al., 2005). Conditioning sessions occurred over 3 days, 2 sessions per day separated by a minimum of 5 hours and consisted of 100 trials (90 paired CS–US trials, 10 CS-alone trials per session). Paired trials consisted of a 380-ms tone CS (2.8 kHz, 80 dB) that preceded, overlapped, and coterminated with a periorbital electrical stimulation US (90 Hz, 1.4 mA) that lasted 100 ms, with a 280 ms interstimulus interval between the onset of the CS and onset of the US. The variable intertrial interval averaged 30 seconds. Because the initial US presentations typically produce a strong behavioral startle response, on the first session, the US intensity was increased from 0.2 to 1.4 mA in 0.3 mA increments over the first 10 trials, after which no further adjustments in US intensity were made. Every 10th trial, the tone CS was presented without the US; responses during these trials were used to further assess the acquisition of conditioned responses (CRs) without the response being altered by the 100-ms US delivery.
Eyeblink Data Acquisition
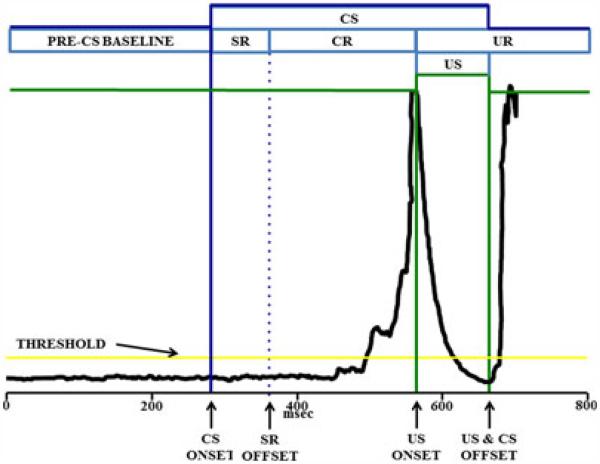
Eyelid EMG activity was amplified (×5,000) and bandpass filtered at 500 to 5,000 Hz by a differential AC amplifier and then rectified and integrated by a DC integrator (×10) before being passed to a computer for storage. Integrated EMG signals were sampled in 2.5 ms bins during the 800 ms trial epoch and then organized into 4 time periods, as shown in Fig. 1: (i) pre-CS period, (ii) startle response (SR) period, (iii) CR period, and (iv) UR period. CRs during probe trials could occur during the typical CR EMG collection period (200 ms), during the typical period of UR EMG collection of paired CS–US trials or both. Using criteria described by Stanton and colleagues (1992), any EMG responses during the SR, CR, or UR periods exceeding 0.4 arbitrary units above the pre-CS baseline mean were registered.
Fig. 1.
Illustration of short-delay eyeblink conditioning procedures and trial performance electromyographic (EMG) tracing examples. The dark line shows the integrated EMG output for the trial epoch; the vertical lines designate onset and offset of stimulus events over the trial epoch. Each trial begins with a 280-ms pre-conditioned stimulus (CS) baseline period, from which the threshold is established at 0.4 standard deviations above mean value of the baseline EMG. EMG responses that surpass threshold are counted as a blink. The tone CS onset is represented by the first vertical bold line, and the 80 ms interval that follows is the startle response (SR) period; blinks during the SR period are considered startle responses to the tone and serve as a measure of sensory reactivity to the tone. Blinks occurring after the end of the SR period (after the first vertical dotted line) are considered conditioned responses (CRs) to the tone in anticipation of the unconditioned stimulus (US). The CR period is the interval between the end of the SR period and the US. The delivery of the electrical stimulation US interferes with the recording of the EMG activity, so the EMG signal is bypassed (i.e., shunted to a value of 0) during the delivery of the US. The EMG tracing shown here for paired short-delay trial is from an animal toward the end of its training, illustrating a well-timed CR.
Statistical Analyses
Because of the known sex differences in acquisition of eyeblink conditioning (Dalla and Shors, 2009), the eyeblink data were analyzed separately for females and males. The frequency (expressed as percent of trials) and amplitude of CRs (eyeblinks occurring during the CR period) were the primary measures of acquisition across the 6 training sessions. The paired trials and the CS-alone trials were analyzed separately using mixed analyses of variance (ANOVAs) (α = 0.05), with neonatal treatment (treatment) and rehabilitation training (rehabilitation) as grouping factors and session as a repeated measure. Where appropriate, follow-up analyses involved 2-way (e.g., treatment × session) ANOVAs. Additional performance measures of CR timing (latency to onset and latency to peak CR) used similar mixed ANOVAs. When appropriate Tukey’s HSD post hoc tests were used to evaluate significant differences between groups for each session (α = 0.05).
RESULTS
Blood Alcohol Concentrations and Growth
Mean (±SEM) peak BACs on PD 4 were 417 ± 9 mg/dl, with no significant differences between males (413 ± 13) and females (420 ± 14). Table 1 shows body weights for SI and AI groups for PD 4 and PD 9 and for all 3 treatment groups on PD 25 and PD 60. A 2 (treatment) × 2 (sex) × 6 (PD4to 9) repeated measures ANOVA on daily body weights confirmed that the AI group had a significant growth lag during the treatment period relative to the SI group, treatment × day: F(5, 340) = 117.6, p < 0.001; main effect of treatment: F(1, 68) = 105.2, p < 0.001, similar to our previous reports (Goodlett et al., 1998; Tran et al., 2005, 2007). There were no significant effects of treatment on body weight either on PD 25 (day of weaning) or on PD 60, indicating that the alcohol-induced growth lag had recovered by weaning.
Table 1.
Blood Alcohol Concentrations (BACs) and Body Weights of the 3 Treatment Groups
| Body weight (g) |
||||||
|---|---|---|---|---|---|---|
| Treatment group | BAC, mg/dl | PD 4 | PD 9 | PD 25 | PD 60 | |
| Females | Suckle control | – | – | – | 63.2 ± 2.1 | 183.6 ± 5.3 |
| Sham intubated PD 4 to 9 | – | 10.3 ± 0.1 | 20.2 ± 0.3 | 63.0 ± 1.8 | 191.2 ± 5.1 | |
| Alcohol intubated PD 4 to 9 | 420 ± 14 | 10.0 ± 0.1 | 16.5 ± 0.3* | 62.5 ± 1.9 | 189.7 ± 5.2 | |
| Males | Suckle control | – | – | – | 69.9 ± 2.0 | 273.1 ± 5.4 |
| Sham intubated PD 4 to 9 | – | 10.6 ± 0.1 | 20.4 ± 0.3 | 68.6 ± 1.8 | 283.1 ± 4.9 | |
| Alcohol intubated PD 4 to 9 | 413 ± 13 | 10.3 ± 1.4 | 16.6 ± 0.3* | 63.2 ± 1.9 | 276.6 ± 5.4 | |
Significantly different from sham intubated control.
There were no significant differences in BACs between the male and female groups given alcohol, and there were no significant body weight differences on or after weaning.
Acquisition of Rehabilitation Training
As shown in Fig. 2, all groups decreased the mean time to traverse the obstacles over the 20 days of rehabilitation training, main effect of day: F(19, 570) = 83.78, p < 0.001; there were no main or interactive effects of neonatal treatment or sex. To assess potential group differences in asymptotic performance, the last 10 days of training were analyzed with a 3 (treatment) × 2 (sex) × 10 (day) mixed ANOVA. There was a main effect of treatment, F(2, 48) = 4.61, p < 0.05, but sex effects failed to reach significance.
Fig. 2.
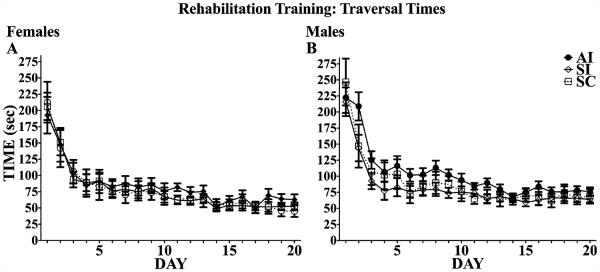
Complex motor skill training performance. Time spent (seconds) traversing all 10 elevated obstacles was recorded for each trial and averaged across the 5 trials for each training day. All animals decreased traversal times over the course of training. Over the last 10 days, females (A) performed significantly better than males (B), and alcohol intubated (AI) males had significantly slower traversal times than both sham intubated (SI) and suckle control (SC) males.
Short-Delay Eyeblink Conditioning
Females
Paired Trials
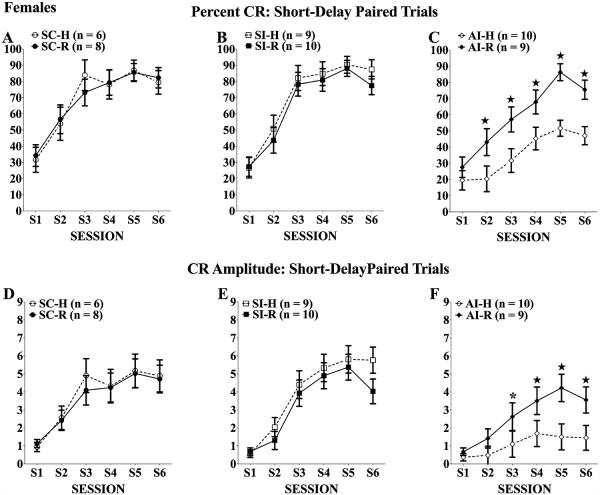
As shown in Fig. 3 for percent CRs (top panels) and CR amplitudes (bottom panels), females given neonatal alcohol treatment and handling (the AI-H group; Panels C and F) showed the expected acquisition deficit in short-delay eyeblink relative to handled controls (SC-H and SI-H; Panels A, B and D, E). The rehabilitation training was sufficient to normalize the short-delay acquisition of the alcohol-exposed females (AI-R group) relative to SI and SC controls and to the AI-H group. For percent CRs, there was a significant main effect of treatment, F(2, 46) = 9.08, p < 0.001, a significant treatment × session interaction, F(10, 230) = 3.61, p < 0.001, and a significant treatment × rehabilitation interaction, F(2, 46) = 3.76, p < 0.05. Follow-up 2-way (rehabilitation × session) mixed ANOVAs comparing AI-H and AI-R groups on percent CRs confirmed that the rehabilitation training significantly improved acquisition of the AI-R group, main effect of rehabilitation: F(1, 17) = 6.20, p < 0.05, Tukey’s post hoc analyses revealed rehabilitation effects emerged during the second session and persisted throughout testing (p < 0.01). Additional follow-up comparisons among just the handled groups (AI-H, SC-H, and SI-H) confirmed the expected significant impairment of the AI-H group, main effect of treatment: F(2, 22) = 11.17, p < 0.001; treatment × session interaction: F(10, 110) = 3.76, p < 0.001. For CR amplitudes, there was a significant main effect of treatment, F(2, 46) = 7.62, p < 0.01, and a significant treatment × session interaction, F(10, 230) = 4.08, p < 0.001, but the interactive effects of rehabilitation did not reach statistical significance (p = 0.099). Nevertheless, follow-up 2-way (rehabilitation × session) mixed ANOVAs on CR amplitudes confirmed that the rehabilitation training significantly improved acquisition of the AI-R group compared with the AI-H group, F(1, 17) = 6.76, p < 0.05. Typical treatment effects were seen, main effect of treatment: F(2, 22) = 8.41, p < 0.01; treatment × session interaction: F(10, 110) = 5.49, p < 0.001, such that the AI-H group was significantly impaired relative to SI-H and SC-H controls (ps < 0.01). In addition, follow-up analyses on both percent CRs and CR amplitudes of just the SC, SI, and AI groups given rehabilitation training indicated that there were no significant group differences, confirming the lack of group differences among the groups given prior rehabilitation training. There also were no significant group differences in the frequency of URs (means >95%) emitted on paired trials, indicating that there were no group differences in US-elicited blink reflex.
Fig. 3.
Percent (A–C)and amplitude (D–F) of conditioned responses (CRs) of females across 6 eyeblink training sessions. All rats increased the frequency of CRs elicited over training; however, neonatal alcohol treatment significantly impaired CR acquisition. Twenty days of complex motor learning significantly increased the percent CRs and the CR amplitudes of alcohol intubated (AI) group compared with the AI handled group (C and F). *Tukey’s post hoc p < 0.05; ★Tukey’s post hoc p < 0.01.
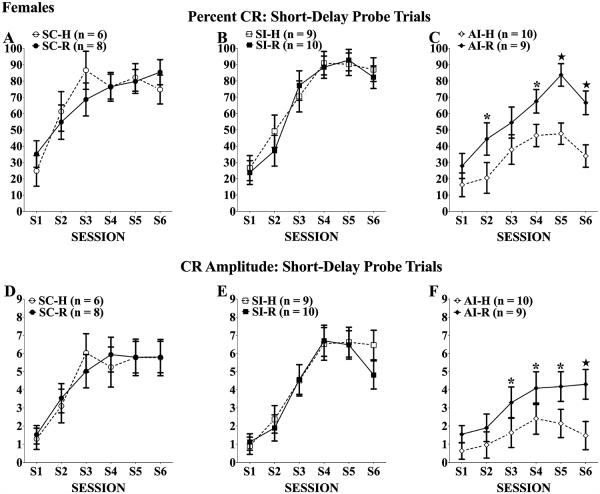
CS-Alone Trials
On the 10 probe trials of each session in which the tone CS was presented without the US, the outcomes were generally similar to paired trials although the effect of rehabilitation was not as strong (Fig. 4). For percent CRs, the neonatal alcohol treatment produced significant deficits, main effect of treatment: F(2, 46) = 8.57, p < 0.01; treatment × session interaction: F(10, 230) = 3.33, p < 0.001, but the treatment × rehabilitation factor did not reach statistical significance (p = 0.071). A follow-up mixed ANOVA comparing the 2 AI groups did indicate that the AI-R group performed significantly better than the AI-H group, main effect of rehabilitation: F(1, 17) = 5.66, p < 0.05. For CR amplitudes, the neonatal alcohol treatment produced significant deficits, main effect of treatment: F(2, 46) = 7.39, p < 0.01; treatment × session interaction: F(10, 230) = 5.01, p < 0.001, but the treatment × rehabilitation factor did not reach statistical significance. The follow-up comparison of the AI-R and AI-H groups did not reach statistical significance (p = 0.061). Analyses of latency to onset and latency to peak CR during probe trials yielded only the expected effect of session, latency to onset: F(5, 230) = 2.50, p < 0.05; latency to peak: F(5, 230) = 3.86, p < 0.01; there were no group differences in these measures of CR timing.
Fig. 4.
Percent (A–C)and amplitude (D–F) of conditioned responses (CRs) of females across 6 eyeblink training sessions. All rats increased the frequency of and amplitude CRs elicited during probe trials over training; however, neonatal alcohol treatment significantly impaired CR acquisition. Twenty days of complex motor learning failed to significantly improve CR frequency and amplitude among alcohol intubated (AI) rats (C and F). *Tukey’s post hoc p < 0.05; ★Tukey’s post hoc p < 0.01.
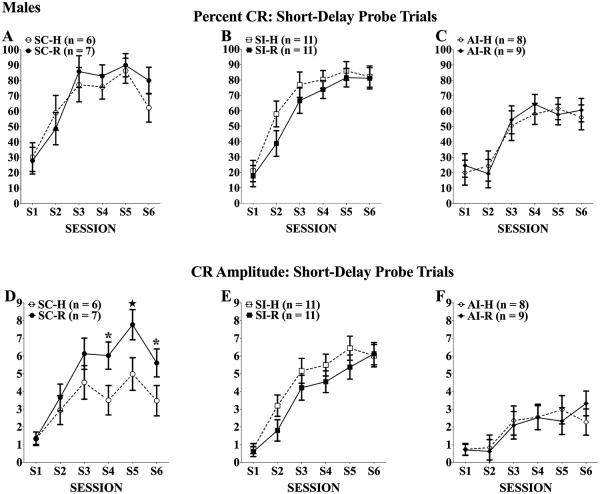
Males
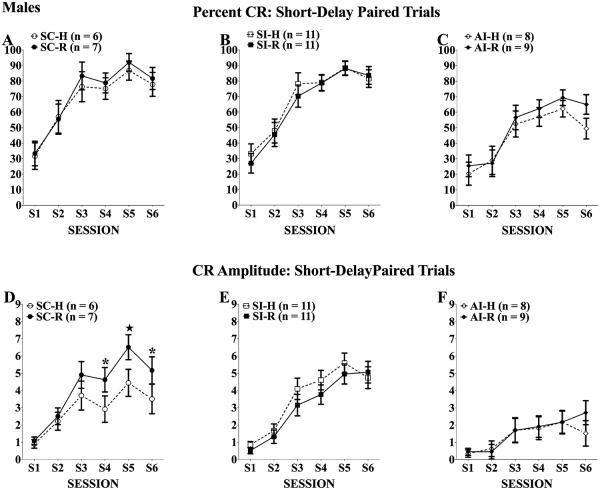
Paired Trials
As shown in Fig. 5 for percent CRs (top panels) and CR amplitudes (bottom panels), the males given neonatal alcohol treatment and handling (AI-H, panels C and F) showed the expected acquisition deficit in short-delay eyeblink relative to handled controls (SC-H and SI-H; Panels A, B and D, E). Unlike females, however, the rehabilitation training did not significantly improve short-delay acquisition of the AI-R group. The mixed ANOVAs yielded significant treatment effects, main effect of treatment percent CRs: F(2, 46) = 8.75, p = 0.01; CR amplitudes: F(2, 46) = 12.20, p = 0.001; treatment × session interaction CR amplitude: F(10, 230) = 5.00, p < 0.001, along with the obvious main effect of session, percent CRs: F(5, 230) = 103.2, p < 0.001; CR amplitudes: F(5, 230) = 70.96, p < 0.001. For both measures, the 2 AI groups were significantly impaired relative to the respective SI and SC control groups (ps < 0.05), and the 2 AI groups did not differ from each other. As with the females, there were no significant group differences in frequency of URs (means >95%) on the paired trials.
Fig. 5.
Percent (A–C) and amplitude (D–F) of conditioned responses (CRs) of males across 6 eyeblink training sessions. All rats increased the frequency and amplitudes of CRs elicited over training; however, neonatal alcohol treatment significantly impaired CR acquisition. Twenty days of complex motor learning failed to significantly improve CR frequency or amplitude in alcohol intubated (AI) rats (C and F). *Tukey’s post hoc p < 0.05; ★Tukey’s post hoc p < 0.01.
CS-Alone Trials
Outcomes for the probe trials were similar to those of the paired trials, such that alcohol-exposed males showed acquisition deficits regardless of rehabilitation condition (Fig. 6). The mixed ANOVAs yielded significant treatment effects, main effect of treatment percent CRs: F(2, 46) = 7.66, p < 0.01; CR amplitude: F(2, 46) = 13.96, p < 0.001; treatment × session interaction percent CR: F(10, 230) = 2.36, p < 0.05; CR amplitude: F(10, 230) = 4.44, p < 0.001, and post hoc tests confirmed the significant impairment of the each alcohol group relative to the respective SI and SC control groups. Analyses of the latency to onset and latency to peak measures for CRs during probe trials yielded only a significant effect of session, latency to onset: F(5, 225) = 5.12, p < 0.001; latency to peak: F(5, 225) = 8.20, p < 0.001.
Fig. 6.
Percent (A–C) and amplitude (D–F) of conditioned responses (CRs) of males across 6 eyeblink training sessions. All rats increased the frequency of and amplitude CRs elicited during probe trials over training; however, neonatal alcohol treatment significantly impaired CR acquisition. Twenty days of complex motor learning failed to significantly improve CR frequency and amplitude among alcohol intubated (AI) rats (C and F). *Tukey’s post hoc p < 0.05; ★Tukey’s post hoc p < 0.01.
DISCUSSION
The significant improvement of acquisition of delay eyeblink conditioning in females given 20 days of training on the complex motor learning task extends our previous findings (Klintsova et al., 1998) that showed similar rehabilitation training improved performance (of both sexes) on several tests of motor performance. The current studies are particularly important because they demonstrate a rehabilitation effect on a cerebellar-dependent learning task that is completely different from the types of actions that are involved in the complex motor learning that is acquired during the 20 days of training on the obstacle course. These findings raise the possibility that there may be rehabilitation-induced synaptic morphological plasticity in parts of the cerebellar cortex implicated in eyeblink conditioning (HVI and anterior lobules), thus extending beyond the previously identified increases in parallel fiber–Purkinje cell synapses shown in the paramedian lobule (Klintsova et al., 2002). If the cerebellar structural plasticity associated with this complex motor learning is generalized beyond the paramedian lobule, it would further support its potential usefulness as a therapeutic intervention.
The sex difference in the effectiveness of the rehabilitation training was unexpected, and there may be several factors that could account for the difference. Sex differences in acquisition of eyeblink conditioning have been reported for normal adult rats in which females can acquire the CR faster than males (Dalla and Shors, 2009), particularly when training occurs during proestrus when estrogen levels are relatively high (Shors et al., 1998). As we did not determine the stage of estrus for any of the females, we cannot rule out potential differences in acquisition between the rehabilitated and the handled AI female rats that may have occurred due to chance differences in the timing of the estrus stages across the 3 days of eyeblink conditioning. For example, if the majority of AI-H females happened to be tested outside of proestrus, then their deficits could reflect an interactive effect related to lower estrogen levels. Given the multiple cohorts used and the within-cohort randomization that was followed, this is unlikely to have happened. We also note that there were no differences in rehabilitation acquisition among the 3 groups of females (AI, SI, and SC); the only impairment was in the handled AI group. If this outcome were due to group differences in estrus cycle, it would involve the unlikely condition that only the AI-H group had a majority of females tested outside the proestrus stage. Another issue is the extent to which there might be sex differences in the ability of the rehabilitation training to produce neuroplasticity in the brain circuitry relevant to eyeblink conditioning. Increased estrogen has been shown to enhance cerebellar plasticity increasing long-term potentiation and parallel fiber–Purkinje cell synapses (Andreescu et al., 2007). It is possible that estrogen in females may have better supported cerebellar synaptogenesis induced by rehabilitation training, contributing to the improved eyeblink performance in the alcohol-treated females. Our previous demonstration that 20 days of rehabilitation training induced a significant increase in the number of parallel fiber–Purkinje cell synapses in the paramedian lobule was conducted only in female rats. The current findings raise the empirical question of whether the training-induced cerebellar synaptic morphological plasticity may be more robust in alcohol-exposed females than males.
Although the extent of cerebellar cell loss and morphological synaptic plasticity of the subjects in this report has not yet been evaluated, the treatment used in this study has previously been shown to produce Purkinje cell and cerebellar deep nuclear cell losses in excess of 40% (Goodlett et al., 1998; Klintsova et al., 2002; Pierce et al., 1993; Tran et al., 2005). In addition, the permanent loss of Purkinje cells (Tran et al., 2005) and cerebellar deep nuclear neurons (Green et al., 2002b) was induced in both males and females by this neonatal alcohol treatment and has been shown to be correlated with significant eyeblink conditioning deficits in both sexes. Consequently, it is reasonable to conclude that the significant effect of the rehabilitation treatment in females was likely associated with improved learning-related neuroplasticity in the surviving (but depleted) cerebellar neuronal populations mediating eyeblink conditioning.
Acquisition of eyeblink classical conditioning requires the functional integrity of a well-defined cerebellar neural circuitry (for review, see Christian and Thompson, 2003). In particular, converging inputs from the lateral pontine nuclei mossy fibers, carrying tone CS-elicited activity, and inputs from the dorsal accessory inferior olivary climbing fibers, carrying US-elicited activity, reach the anterior interpositus nucleus and the anterior and HVI lobules of the cerebellar cortex. These populations are part of the essential circuit for eyeblink conditioning (Kim and Thompson, 1997; Steinmetz, 2000). The neonatal alcohol treatment significantly reduces the number of neurons in the cerebellar deep nuclei and the number of Purkinje cells and granule cells in the cerebellar cortex (Goodlett et al., 1998; Green et al., 2002b, 2006; Tran et al., 2005). It also causes deficits in learning-related changes in neuronal activity of the interpositus nucleus (Green et al., 2002a). One critical question now is whether the rehabilitation training effect in females may reflect a facilitation of eyeblink conditioning due to a rehabilitation-induced increase in functional synapses at either of the 2 primary sites of plasticity involved in eyeblink conditioning (anterior interpositus; lobule HVI of the cortex). In that case, the training-induced cerebellar structural plasticity presumably would then predispose the animal toward facilitation of the learning-related functions typically mediated by those neural circuits. Alternatively, the improved eyeblink conditioning in the AI-R females may not depend on an antecedent rehabilitation-induced structural plasticity, but rather on facilitated molecular signaling associated with learning-related plasticity that becomes engaged at the beginning of eyeblink conditioning and proceeds more efficiently than in the handled group. Additional experiments are needed to identify the neurobiological correlates of the facilitated eyeblink conditioning resulting from the complex motor learning, and why the effectiveness was limited to females.
There are several important translational implications (and caveats) of these findings in this rat model. First, behavioral rehabilitation regimens involving complex motor learning appear to be a viable and potentially effective intervention for FASD to improve motor learning and performance that involve cerebellar function. Studies to date have not fully identified what aspects of the complex motor learning in rats are essential for its effectiveness, although duration of training is one important factor. There are important issues of species differences that must be considered when determining what forms of complex motor learning would be best suited or most effective for humans, for example, whether the regimen should be training on gymnastics or obstacle courses or on other skills such as playing musical instruments or games of motor dexterity. What we can conclude, though, is that complex motor learning activities that engage cerebellar circuits appear to benefit diverse forms of motor learning, not just those that are similar to the rehabilitation training itself, although there appear to be important differences between females and males. This raises the prospect that this form of rehabilitation training in humans may have benefits for cerebellar-mediated cognitive and behavioral functions that extend beyond its traditional motor functions (Strick et al., 2009; Timmann and Daum, 2010). The beneficial effects in this rat model that have now extended to cerebellar-mediated eyeblink conditioning (in females) certainly suggest that a clinical trial of a similar treatment, designed as a therapeutic intervention in children with FASD, would be warranted.
In translating these findings from the rat model to human studies, important issues regarding potential sex differences must be also considered. In rats, sex differences in eyeblink conditioning performance have been reported in the direction of superior acquisition in females (Dalla and Shors, 2009; Shors, 2004). Moreover, prior stress has been found to interact with sex differences in that stress impaired eyeblink conditioning in female rats but facilitated it in male rats (Shors, 2004; Shors et al., 2000). In contrast, human studies generally do not report sex differences in acquisition of delay conditioning, and a recent study by Wolf and colleagues (2009) that explicitly evaluated potential sex differences in humans in the effects of prior stress failed to find sex differences either in the stress groups or in the control groups, although prior stress impaired eyeblink conditioning in both males and females. In addition, to our knowledge, there have been no reports addressing whether sex differences in eyeblink conditioning may exist among children with FASD as the 3 published studies did not report males and females separately. The current findings support additional studies designed to evaluate the relative effectiveness of rehabilitation using complex motor training in females and males with FASD. Perhaps an ideal evaluation measure of effectiveness of rehabilitation in such a clinical trial would be eyeblink classical conditioning.
ACKNOWLEDGMENTS
This study was supported by AA09838 and AA07462.
REFERENCES
- Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MT. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–10839. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr. 2002;141:712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Autti-Ramo I, Granstrom ML. The psychomotor development during the first year of life of infants exposed to intrauterine alcohol of various duration. Fetal alcohol exposure and development. Neuropediatrics. 1991;22:59–64. doi: 10.1055/s-2008-1071418. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH, Sperry MA, Zajac CS. Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol. 1996;13:209–216. doi: 10.1016/0741-8329(95)02049-7. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD, Dillon CJ. The effects of moderate neonatal ethanol exposure on eyeblink conditioning and deep cerebellar nuclei neuron numbers in the rat. Alcohol. 2006;39:135–150. doi: 10.1016/j.alcohol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learn Mem. 2002a;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002b;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006;3:e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Cowell RM, Swain RA, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats. I. Behavioral results. Brain Res. 1998;800:48–61. doi: 10.1016/s0006-8993(98)00495-8. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Scamra C, Hoffman M, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937:83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kodituwakku EL. From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychol Rev. 2011;21:204–223. doi: 10.1007/s11065-011-9170-1. [DOI] [PubMed] [Google Scholar]
- Kyllerman M, Aronson M, Sabel KG, Karlberg E, Sandin B, Olegard R. Children of alcoholic mothers. Growth and motor performance compared to matched controls. Acta Paediatr Scand. 1985;74:20–26. doi: 10.1111/j.1651-2227.1985.tb10915.x. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Mothes HK, Opitz B, Werner R, Clausing P. Effects of prenatal ethanol exposure and early experience on home-cage and open-field activity in mice. Neurotoxicol Teratol. 1996;18:59–65. doi: 10.1016/0892-0362(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Serbus DC, Light KE. Intragastric intubation of alcohol during postnatal development of rats results in selective cell loss in the cerebellum. Alcohol Clin Exp Res. 1993;17:1275–1280. doi: 10.1111/j.1530-0277.1993.tb05241.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127C:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Roach D, Anderson SM. Preventing alcohol, tobacco, and other substance-exposed pregnancies: a community affair. In: Roach D, Anderson SM, editors. Series Preventing Alcohol, Tobacco, and Other Substance-Exposed Pregnancies: A Community Affair, Interagency Coordinating Committee on Fetal Alcohol Syndrome Work Group on Women, Drinking, and Pregnancy. National Institute on Alcohol Abuse and Alcoholism, NIH, American Legacy Foundation; Rockville, MD: 2008. pp. 1–62. [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998a;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998b;22:252–258. [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, Gould E. The modulation of Pavlovian memory. Behav Brain Res. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. NeuroReport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–779. [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22:22–270. [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Timmann D, Daum I. How consistent are cognitive impairments in patients with cerebellar disorders? Behav Neurol. 2010;23:81–100. doi: 10.3233/BEN-2010-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Jackson HD, Horn KH, Goodlett CR. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res. 2005;29:29–117. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Dev Psychobiol. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Levesque S, Krempulec L, Bulman-Fleming B, McCutcheon D. Effects of environmental enrichment on cortical depth and Morris-maze performance in B6D2F2 mice exposed prenatally to ethanol. Neurotoxicol Teratol. 1993;15:11–20. doi: 10.1016/0892-0362(93)90040-u. [DOI] [PubMed] [Google Scholar]
- Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25:153–158. [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Minnebusch D, Daum I. Stress impairs acquisition of delay eyeblink conditioning in men and women. Neurobiol Learn Mem. 2009;91:91–431. doi: 10.1016/j.nlm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning: Volume I: Applications in Humans. Kluwer Academic Publishers; Boston: 2000a. [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning: Volume II: Animal Models. Kluwer Academic Publishers; Boston: 2000b. [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA, Spong CY. A neuroprotective peptide antagonizes fetal alcohol exposure-compromised brain growth. J Mol Neurosci. 2004;24:189–199. doi: 10.1385/JMN:24:2:189. [DOI] [PubMed] [Google Scholar]