AKAP79/150 and AKAP15 exert functionally antagonistic effects on CaV1.2 channels.
Abstract
The CaV1.1 and CaV1.2 voltage-gated calcium channels initiate excitation-contraction coupling in skeletal and cardiac myocytes, excitation-transcription coupling in neurons, and many other cellular processes. Up-regulation of their activity by the β-adrenergic–PKA signaling pathway increases these physiological responses. PKA up-regulation of CaV1.2 activity can be reconstituted in a transfected cell system expressing CaV1.2Δ1800 truncated at the in vivo proteolytic processing site, the distal C-terminal domain (DCT; CaV1.2[1801–2122]), the auxiliary α2δ and β subunits of CaV1.2 channels, and A-kinase anchoring protein-15 (AKAP15), which binds to a site in the DCT. AKAP79/150 binds to the same site in the DCT as AKAP15. Here we report that AKAP79 is ineffective in supporting up-regulation of CaV1.2 channel activity by PKA, even though it binds to the same site in the DCT and inhibits the up-regulation of CaV1.2 channel activity supported by AKAP15. Mutation of the calcineurin-binding site in AKAP79 (AKAP79ΔPIX) allows it to support PKA-dependent up-regulation of CaV1.2 channel activity, suggesting that calcineurin bound to AKAP79 rapidly dephosphorylates CaV1.2 channels, thereby preventing their regulation by PKA. Both AKAP15 and AKAP79ΔPIX exert their regulatory effects on CaV1.2 channels in transfected cells by interaction with the modified leucine zipper motif in the DCT. Our results introduce an unexpected mode of differential regulation by AKAPs, in which binding of different AKAPs at a single site can competitively confer differential regulatory effects on the target protein by their association with different signaling proteins.
INTRODUCTION
Voltage-gated Ca2+ (CaV) channels initiate excitation-contraction coupling in muscle cells, excitation-transcription coupling in neurons, and many other physiological events (Reuter, 1979; Catterall, 1991; Bers, 2002; West et al., 2002). In skeletal and cardiac muscle, up-regulation of the activity of CaV1.1 and CaV1.2 channels increases contractile force in response to activation of the β-adrenergic signaling pathway in the fight or flight response (Reuter, 1983; Tsien et al., 1986; Catterall, 2000). In neurons, activation of the dopamine and β-adrenergic signaling pathways increases CaV1.2 channel activity and modulates gene transcription and synaptic plasticity (Lovinger, 2010; Gerfen and Surmeier, 2011; Qian et al., 2012). β-Adrenergic receptors activate adenylyl cyclase, increase cAMP, activate cAMP-dependent protein kinase (PKA), and phosphorylate CaV1.1 and CaV1.2 channels (Reuter, 1983; Tsien et al., 1986; Catterall, 2000). Targeting PKA to specific subcellular cellular compartments or substrates by binding to A-kinase anchoring proteins (AKAPs) exerts spatiotemporal control over these regulatory processes (Wong and Scott, 2004).
CaV1.1 and CaV1.2 channels form autoinhibitory signaling complexes, which are essential for regulation of their activity by the PKA pathway (Hulme et al., 2004; Catterall, 2010). Their pore-forming α1 subunits are proteolytically processed in vivo near the center of their large intracellular C-terminal domains (De Jongh et al., 1989, 1991, 1996; Hell et al., 1996). The membrane-anchored AKAP15/181 (Gray et al., 1997, 1998; Fraser et al., 1998) binds to the distal C-terminal domain (DCT; CaV1.2[1801–2122]) of these channels via a modified leucine zipper motif (Hulme et al., 2002, 2003). The DCT and AKAP binding are required for regulation of CaV1.1 and CaV1.2 channels by PKA in skeletal and cardiac myocytes (Gray et al., 1998; Hulme et al., 2002, 2003; Ganesan et al., 2006; Fu et al., 2011). Moreover, the proteolytically cleaved DCT binds to the remainder of the CaV1.1 and CaV1.2 channels by interaction with a site in the proximal C-terminal domain (Hulme et al., 2005, 2006) and is a potent autoinhibitor of the activity of CaV1.2 channels when coexpressed in nonmuscle cells (Hulme et al., 2006). Activation of protein phosphorylation by PKA increases ion conductance activity by relieving the autoinhibitory effect of the DCT (Fuller et al., 2010).
Regulation of CaV1.2 channels by PKA has been reconstituted by coexpression of the components of this autoinhibitory signaling complex in transfected cells (Fuller et al., 2010). CaV1.2Δ1800 truncated at the site of in vivo proteolytic processing (Emrick et al., 2010) and the DCT composed of CaV1.2[1801–2122] interact with each other when expressed as separate proteins, and the DCT markedly inhibits CaV1.2 channel activity (Hulme et al., 2006; Fuller et al., 2010). Coexpression of these two components of the α1 subunit as separate proteins together with the auxiliary α2δ and β subunits of CaV1.2 channels and AKAP15 yields an autoinhibited CaV1.2 signaling complex whose activity can be increased three- to fourfold by activation of adenylyl cyclase in transfected cells (Fuller et al., 2010). Normal regulation of basal activity requires phosphorylation of Ser1700 and Thr1704, located at the interface between the DCT and the proximal C-terminal domain, and up-regulation of CaV1.2 channel activity requires PKA phosphorylation of Ser1700 (Fuller et al., 2010). Cardiac myocytes from mice in which these sites are mutated to Ala have reduced basal L-type Ca2+ current and impaired up-regulation by β-adrenergic agonists, confirming the crucial role of this regulatory mechanism in vivo (Fu et al., 2013).
AKAP15 also binds to CaV1.2 channels in brain neurons (Marshall et al., 2011), and AKAP79/1501 binds to CaV1.2 channels in both brain and cardiac muscle (Gao et al., 1997; Hall et al., 2007). Both of these AKAPs are involved in regulation of gene expression in response to activation of CaV1.2 channels in neurons (Oliveria et al., 2007; Marshall et al., 2011). The effects of AKAP79 on gene transcription in neurons are mediated by the Ca2+-regulated phosphoprotein phosphatase calcineurin, which binds directly to AKAP79 (Oliveria et al., 2007). Like AKAP15, AKAP79 binds to CaV1.2 channels via the modified leucine zipper motif in the DCT (Oliveria et al., 2007). Therefore, it is of great interest to explore PKA regulation of CaV1.2 channels mediated via AKAP79 compared with AKAP15 in our reconstituted regulatory system. Here we report strikingly different regulatory properties of these two AKAPs, which depend on binding of the phosphoprotein phosphatase calcineurin by AKAP79. Our results introduce an unexpected mode of differential regulation by AKAPs, in which different AKAPs can compete for binding at a single site and confer differential regulatory effects on the target protein by association with different signaling proteins.
MATERIALS AND METHODS
cDNA constructs
Constructs used in this study include rabbit α1.2a, rat β2b, rabbit α2δ1, AKAP15, AKAP79, PKA-Cα, and PKA-RIIα in pcDNA3 (Fuller et al., 2010). CaV1.2 leucine zipper motif triple mutant (I2073A, F2080A, I2087A) was constructed using PCR overlap extension. Construction of AKAPLZM was previously described (Hulme et al., 2002). AKAP79ΔPIX was provided by J.D. Scott (University of Washington, Seattle, WA). The mutant sequence, orientation, and reading frame of all constructs were confirmed by DNA sequencing.
Cell culture and transfection
Human embryonic kidney tsA-201 cells were cultured in DMEM/Ham’s F12 supplemented with 10% FBS and 100 U/ml penicillin and streptomycin. Cells were grown to ∼70% confluence in 10% CO2 and transiently transfected with cDNAs encoding α1.2a truncated at Ala1800 (CaV1.2Δ1800), β2b, and α2δ1 subunits at a 1:1:1 molar ratio using the FuGENE 6 method (Roche). Wild-type or mutant DCT constructs composed of CaV1.2(1801–2271) were transfected with CaV1.2Δ1800 using a molar ratio of 0.75:1 (DCT/CaV1.2Δ1800). In addition, cDNA encoding eGFP in the pcDNA3 vector was added at a molar ratio of 0.1:1 to each transfection mixture as an indicator of transfection efficiency.
Electrophysiology
24 h after transfection, cells were plated at low density, and recordings were made 38–48 h after transfection using the whole-cell configuration of the patch clamp technique. Patch pipettes (1.5–2 MΩ) were pulled from micropipette glass (VWR Scientific) and fire-polished. Currents were recorded with an Axopatch 200B amplifier (Axon Instruments Inc.) and sampled at 5 kHz after anti-alias filtering at 2 kHz. Data acquisition and command potentials were controlled by either pCLAMP or HEKA Pulse software, and data were stored for later offline analysis. Voltage protocols were delivered at 10-s intervals, and leak and capacitive transients were subtracted using a P/4 protocol. Approximately 80% of series resistance was compensated with the patch clamp circuitry. The extracellular bath solution contained (mM) 150 Tris, 10 glucose, 1 MgCl2, and 10 BaCl2 (adjusted to pH 7.4 with CH3SO3). The intracellular solution contained (mM) 135 CsCl2, 10 EGTA, 1 MgCl2, 4 MgATP, and 10 HEPES (pH 7.3, adjusted with CsOH).
Analysis of electrophysiological recordings of CaV1.2 channels
Current-voltage relationships from peak inward Ba2+ currents were normalized to gating charge (Q) to correct for variation in protein abundance. Gating currents result from the voltage-driven movement of gating charges as conformational changes occur preceding channel opening and are independent of channel unitary conductance and open probability (PO). Gating charge movement was measured as the integral of the gating current transient at the reversal potential of the ionic current (Fig. S1). The reversal potential was determined by applying a series of test pulses at 10-s intervals from the holding potential of −80 mV to potentials between 60 and 80 mV in 2-mV increments. The ionic current that flows upon repolarization (the tail current) gives a functional readout proportional to the number of open channels, the single channel conductance, and channel PO at the end of the depolarizing step. By comparing the ionic and gating currents we determined the efficiency of coupling of the charge movement of the voltage sensors to the subsequent opening of the pore by calculating the ratio of tail current to gating charge (tail current [nA]/integrated gating charge [pC]). Tail currents were recorded after repolarization to −50 mV after each test pulse. All data are expressed as means ± SEM of n cells. Bar graphs are presented for coupling ratio data in the figures, and scatter plots containing all of the individual cell values are presented for representative experiments in Fig. S2. Statistical significance was tested with Student’s t test for pairwise analysis and ANOVA followed by Dunnett’s test for comparison of multiple conditions.
Online supplemental material
Fig. S1 illustrates the approach used for precise determination of the reversal potential and measurement of gating charge at that potential. Fig. S2 illustrates the coupling ratio data for individual cells that contribute to the means for representative experiments. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201311075/DC1.
RESULTS
Differential regulation of CaV1.2 channels by association with AKAP15 versus AKAP79
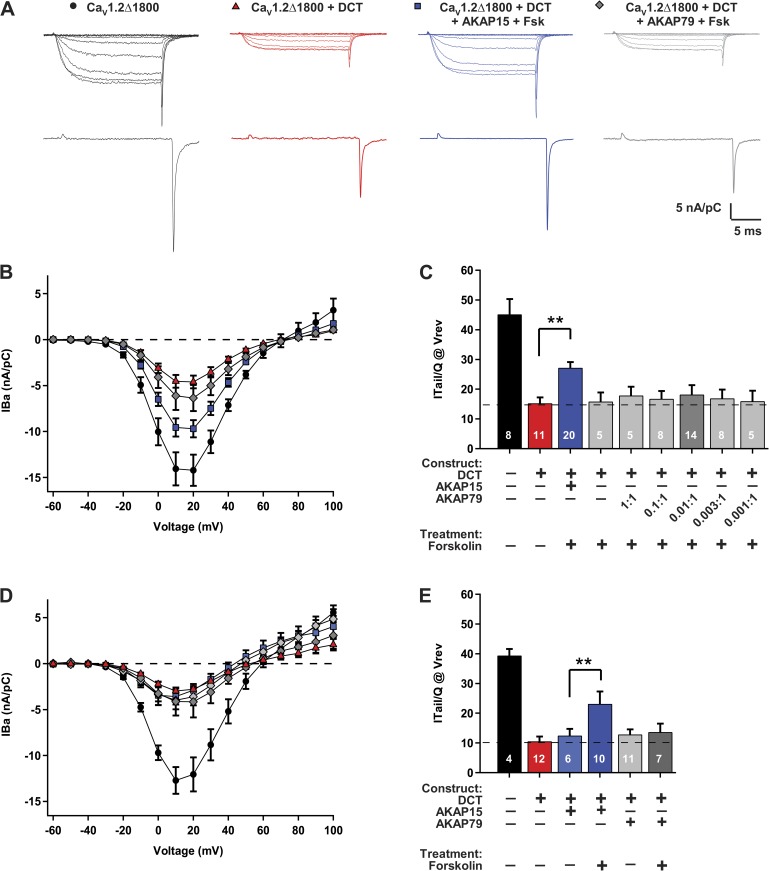
Neurons, myocytes, and other excitable cells express a broad array of AKAPs, which are involved in many aspects of cell signaling (Logue and Scott, 2010). AKAP15 and AKAP79 are both expressed in nerve and muscle cells and interact with a common regulatory site on CaV1.2 channels in those cell types (Gray et al., 1997, 1998; Hulme et al., 2003, 2006; Hall et al., 2007; Oliveria et al., 2007; Marshall et al., 2011). We used reconstitution of CaV1.2 regulation in transfected cells to compare the effects of these two AKAPs on regulation of CaV1.2 channels via the PKA pathway, and we recorded barium currents (IBa) conducted by CaV1.2 channels to minimize activation of Ca2+-dependent regulatory processes. Human embryonic kidney tsA-201 cells were cotransfected with CaV1.2Δ1800, DCT, and Ca2+ channel auxiliary subunits, plus either AKAP15 or AKAP79 (Fig. 1). Cells transfected with CaV1.2Δ1800 without DCT conducted high levels of IBa upon depolarization in whole-cell voltage clamp (Fig. 1, A [top] and B). In contrast, cells transfected with CaV1.2Δ1800 + DCT had much lower levels of IBa, reflecting the autoinhibitory effect of the DCT (Fig. 1, A [top] and B). The autoinhibitory effect of the DCT was also observed in measurements of the coupling ratio of ion channel opening to gating charge movement, which was calculated from measurements of gating charge movement at the reversal potential and tail currents immediately after repolarization (Fig. 1, A [bottom] and C). This measurement is unaffected by the efficiency of transfection and expression of the CaV1.2 channels because it is a ratio of ionic current to gating charge for channels at the cell surface. The coupling ratio (ITail/QVrev) was ∼45 nA/pC for CaV1.2Δ1800 alone and 15 nA/pC for CaV1.2Δ1800 + DCT (Fig. 1 C), as we have observed in previous work (Fuller et al., 2010).
Figure 1.
Differential PKA-dependent regulation of CaV1.2Δ1800 channels via AKAP15 and AKAP79. (A) Representative IBa through CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with cDNA ratios of either 0.003:1 AKAP15 or 0.01:1 AKAP79 in tsA-201 cells in the absence or presence of 5 µM forskolin (Fsk) elicited by test pulses ranging from −60 to 10 mV (top) or test pulses to Vrev (bottom) from a holding potential of −80 mV. (B) Mean current-voltage relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with 0.003:1 AKAP15 or 0.01:1 AKAP79 and 5 µM forskolin. (C) Coupling ratio (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with AKAP15 or AKAP79 at the indicated ratios, without and with 5 µM forskolin. Dashed line indicates mean current for unstimulated CaV1.2Δ1800 + DCT. n values and ±SEM are indicated. **, P < 0.01 versus control. Significance was determined by ANOVA followed by Dunnett’s post-test. (D) Mean current-voltage relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with 0.003:1 AKAP15 or 0.01:1 AKAP79 and 5 µM forskolin in the presence of PKA expressed at 1:1 cDNA ratio. (B and D) Error bars are SEM. (E) Coupling ratio (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with AKAP15 or AKAP79, without and with 5 µM forskolin, in the presence of PKA expressed at 1:1 cDNA ratio. Dashed line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCT. n values and ±SEM are indicated. **, P < 0.01 versus control. Significance was determined by Student’s t test. The reversal potentials determined in these experiments did not vary significantly between groups (P > 0.7).
We used 5 µM forskolin to activate adenylyl cyclase and the PKA signaling pathway. Activation of adenylyl cyclase with forskolin gave a substantial increase in IBa and coupling ratio for CaV1.2Δ1800 + DCT coexpressed with an optimal level of AKAP15 (Fig. 1, A–C). We have shown previously that this increase results from phosphorylation of Ser1700 in CaV1.2Δ1800 by PKA (Fuller et al., 2010). In contrast, forskolin treatment of CaV1.2Δ1800 + DCT coexpressed with AKAP79 over a wide range of cDNA ratios did not result in an increase of either IBa or coupling ratio (Fig. 1, A–C), even though AKAP79 is known to bind to the AKAP-binding site in the DCT of CaV1.2 channels (Oliveria et al., 2007). To assure that PKA was not limiting in these experiments, we overexpressed PKA as described previously (Fuller et al., 2010) and conducted a similar series of experiments (Fig. 1, D and E). Forskolin had no effect in the absence of any AKAP or in the presence of AKAP79, in contrast to the substantial increase in IBa and coupling ratio in the presence of AKAP15. These results reveal striking differential regulation of CaV1.2 channels via the PKA pathway dependent on their association with AKAP15 versus AKAP79.
Functional competition of AKAP15 and AKAP79 in regulation of CaV1.2 channels
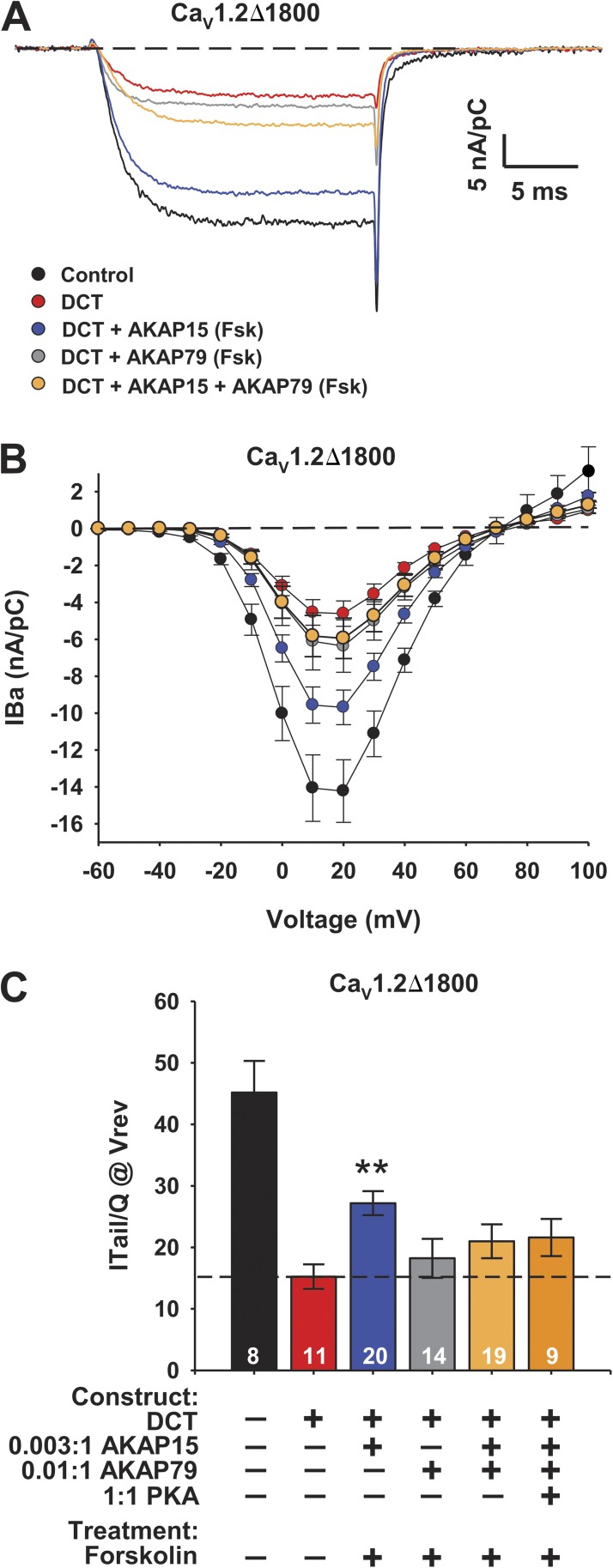
If AKAP15 and AKAP79 both interact with the AKAP-binding domain in the DCT of CaV1.2 channels, they should compete with each other for binding to that regulatory site and PKA regulation via AKAP15 should be inhibited by coexpression of AKAP79. To examine this point, we expressed CaV1.2Δ1800 + DCT with AKAP15 + AKAP79 and measured regulation by activation of adenylyl cyclase with forskolin (Fig. 2). The results show that AKAP79 does indeed inhibit PKA regulation of CaV1.2Δ1800 + DCT coexpressed with AKAP15, as measured by the amplitude of IBa and the coupling ratio (Fig. 2). One potential mechanism of competitive interaction between AKAP15 and AKAP79 would be competitive binding of PKA by AKAP79, which could potentially deplete the cellular pool of PKA. To rule out this possibility, we overexpressed PKA in the presence of the two AKAPs (Fig. 2 C). Even under these conditions, expression of AKAP79 substantially reduced PKA regulation of CaV1.2Δ1800 + DCT via AKAP15 (Fig. 2 C). Together, these results demonstrate competitive regulation of CaV1.2 channels by AKAP15 and AKAP79, dependent on their binding to the AKAP-binding domain in the DCT.
Figure 2.
AKAP15 and AKAP79 compete for binding to the DCT of CaV1.2 channels. (A) Representative IBa through CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with cDNA ratios of 0.003:1 WT AKAP15 and/or 0.01:1 AKAP79 in the presence of 5 µM forskolin (Fsk) elicited by a test pulse to 10 mV from a holding potential of −80 mV. (B) Mean current-voltage relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with 0.003:1 WT AKAP15 and/or 0.01:1 AKAP79 and 5 µM forskolin. Error bars are SEM. (C) Coupling efficiency (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with 0.003:1 WT AKAP15 and/or 0.01:1 AKAP79 and 5 µM forskolin. Dashed line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCT. n values and mean ± SEM are indicated. **, P < 0.01 versus control. Significance was determined by ANOVA.
Differential regulation requires calcineurin association with AKAP79
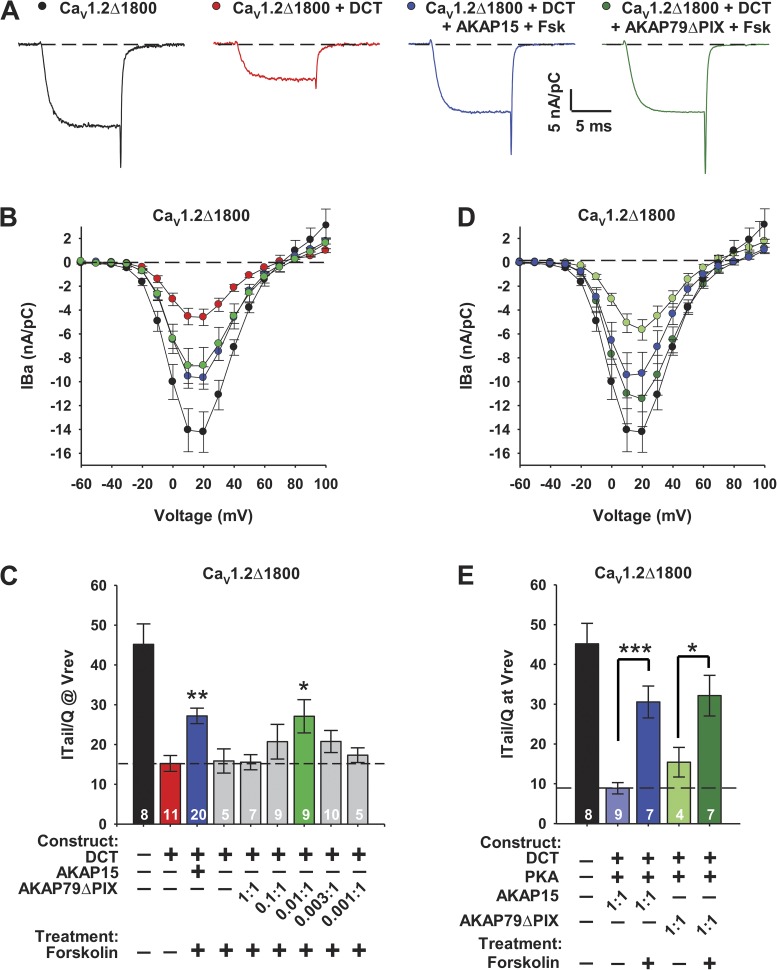
One potential mechanism that could contribute to differential PKA regulation via AKAP15 and AKAP79 is the ability of AKAP79 to bind other signaling molecules and bring them into close association with CaV1.2 channels (Logue and Scott, 2010). In particular, AKAP79 binds the Ca2+-regulated phosphoprotein phosphatase calcineurin (Coghlan et al., 1995; Oliveria et al., 2007), which could dephosphorylate CaV1.2 channels and reduce their up-regulation by PKA. Calcineurin binds to the PXIXIT motif on AKAP79 (Dell’Acqua et al., 2002; Oliveria et al., 2007). Therefore, we examined regulation of CaV1.2Δ1800 + DCT coexpressed with AKAP79 with the PXIXIT site deleted (AKAP79ΔPIX). Under these conditions, forskolin treatment increased the activity of CaV1.2Δ1800 + DCT coexpressed with AKAP79ΔPIX as effectively as CaV1.2Δ1800 + DCT coexpressed with AKAP15, when measured as the amplitude of IBa or the coupling ratio (Fig. 3, A–C). Overexpression of PKA further increased IBa and coupling ratio for CaV1.2Δ1800 + DCT coexpressed with either AKAP79ΔPIX or AKAP15 (Fig. 3, D and E). Under experimental conditions similar to ours, Oliveria et al. (2007) found that competing peptide inhibitors of the binding of calcineurin to CaV1.2 channels gave similar results as the ΔPIX mutation, indicating that the effects of this mutation are caused by inhibition of calcineurin binding and not by a more global conformational change in AKAP79. Moreover, our experiments show a gain of function effect of AKAP79ΔPIX, fully restoring its ability to support PKA regulation at the same level as AKAP15 (Fig. 3 E). Complete restoration of the activity of AKAP79 to the equivalent of AKAP15 would not be expected for a mutation-induced, nonspecific conformational change in AKAP79. Therefore, our results implicate binding of calcineurin to AKAP79 as the primary reason for its differential regulation of CaV1.2 channels via the PKA pathway.
Figure 3.
PKA-dependent regulation of CaV1.2Δ1800 channels via AKAP15 and AKAP79ΔPIX. (A) Representative IBa through CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with cDNA ratios of either 0.003:1 AKAP15 or 0.01:1 AKAP79ΔPIX in tsA-201 cells in the absence or presence of 5 µM forskolin elicited by a test pulse to 10 mV from a holding potential of −80 mV. (B) Mean current-voltage relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with 0.003:1 AKAP15 or 0.01:1 AKAP79ΔPIX and 5 µM forskolin (Fsk). Dashed black line indicates zero current level. (C) Coupling efficiency (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with AKAP15 or AKAP79ΔPIX and 5 µM forskolin. Dashed black line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCT. n values and ±SEM are indicated. **, P < 0.01; and *, P < 0.05 versus control. Significance was determined by ANOVA followed by Dunnett’s post-test. (D) Mean current-voltage relationships determined as in B. Fits to current-voltage relationships showed that there was no significant difference in the voltage dependence of activation (P > 0.7). (B and D) Error bars are SEM. (E) Coupling ratio (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with PKA Cα catalytic subunit plus PKA RIIα regulatory subunit, AKAP15, AKAP79ΔPIX, and 5 µM forskolin. Dashed black line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCT. ***, P < 0.001; and *, P < 0.05 versus controls without forskolin. Significance was determined by Student’s t test. The control results for AKAP15 + DCT are values from the dataset published in Fuller et al. (2010). The experiments presented here overlapped in time with those previously published experiments.
Although calcineurin is strongly Ca2+ regulated, it has a significant basal activity (Stewart et al., 1982; Perrino et al., 1992; Stemmer and Klee, 1994). In our experiments, we measure Ba2+ currents in low extracellular Ca2+ and we chelate intracellular Ca2+ with EGTA; therefore, it is likely that the basal activity of calcineurin in the presence of entering Ba2+ is sufficient to oppose PKA regulation in this experimental system. Consistent with this conclusion, we found that treatment with 5 µM cyclosporin A, which blocks up-regulation of calcineurin activity by Ca2+ without affecting basal activity (Fruman et al., 1992), did not significantly increase Ba2+ currents (P = 0.33). The mechanism of this effect of basal calcineurin activity on PKA regulation of CaV1.2 channels is considered further in the Discussion.
AKAP15 and AKAP79ΔPIX require the modified leucine zipper motif in the DCT
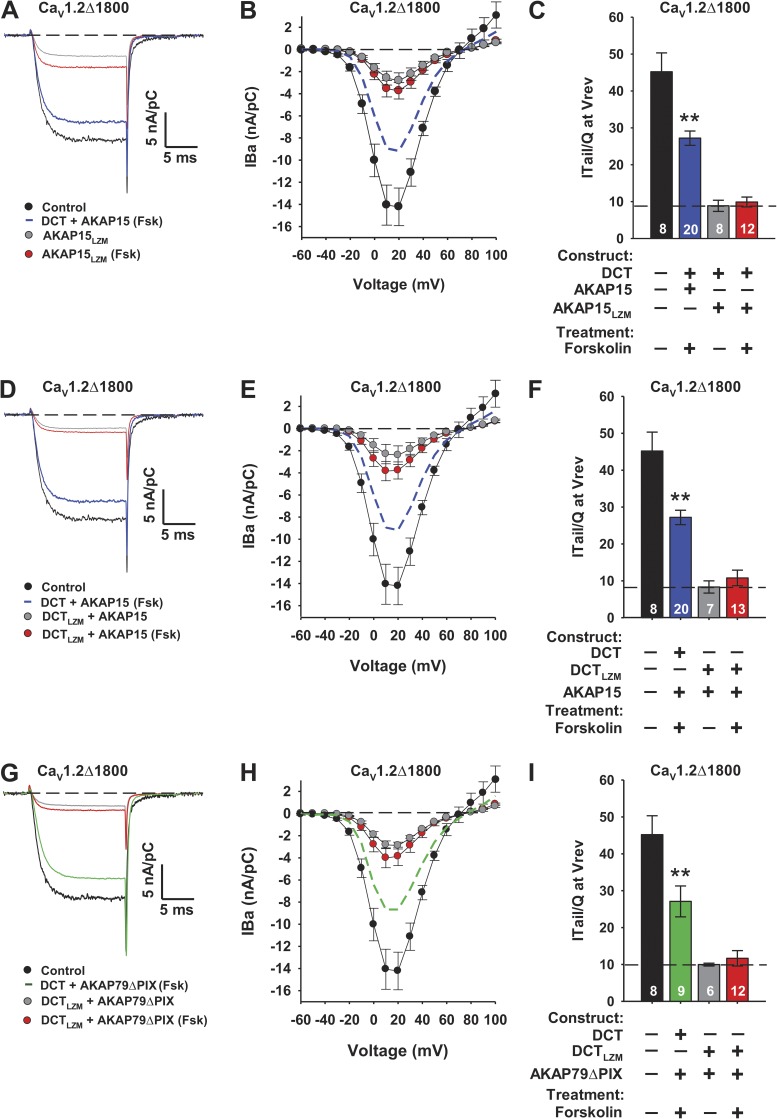
AKAP15 binds to skeletal muscle CaV1.1 channels and cardiac CaV1.2 channels via a modified leucine zipper interaction between a heptad repeat of hydrophobic residues in the AKAP-binding domain in the DCT and a similar heptad repeat of two Leu residues in AKAP15, and this modified leucine zipper interaction is required for PKA regulation of CaV1.2 channels in skeletal and cardiac myocytes (Hulme et al., 2002, 2003). To confirm that this leucine zipper interaction is also required for PKA regulation of CaV1.2Δ1800 + DCT in our reconstituted regulatory system in transfected tsA-201 cells, we tested the regulatory effects of AKAP15LZM, in which the two Leu residues in heptad repeat in AKAP15 are mutated to Ala (Fig. 4, A–C). Our results show that AKAP15LZM is completely ineffective in supporting PKA regulation of CaV1.2Δ1800 + DCT, as measured by increased IBa or increased coupling ratio (Fig. 4, A–C). In complementary experiments, we substituted Ala for the three hydrophobic residues in the heptad repeat that forms the AKAP-binding domain in the DCT of CaV1.2 channels to create the triple mutant DCTLZM. Coexpression of CaV1.2Δ1800 + DCTLZM with AKAP15 also resulted in loss of regulation via the PKA pathway (Fig. 4, D–F). These results confirm that PKA regulation via AKAP15 in our reconstituted system requires interaction of the leucine zipper motif in AKAP15 with the complementary modified leucine zipper motif in the DCT of CaV1.2 channels.
Figure 4.
AKAP15 and AKAP79ΔPIX regulate CaV1.2 channels through a modified leucine zipper motif located in the DCT. (A) Representative IBa through CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with cDNA ratios of 0.003:1 WT AKAP15 or AKAP15LZM in the absence or presence of 5 µM forskolin (Fsk) elicited by a test pulse to 10 mV from a holding potential of −80 mV. (B) Mean current-voltage relationships for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels coexpressed with cDNA ratios of 0.003:1 WT AKAP15 or AKAP15LZM and 5 µM forskolin. (C) Coupling ratio (nA/pC) for CaV1.2Δ1800 and CaV1.2Δ1800 + DCT channels with WT AKAP15 or AKAP15LZM, without and with 5 µM forskolin. Dashed black line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCT with AKAP15LZM. **, P < 0.01 versus control. (D) Representative IBa through CaV1.2Δ1800 channels coexpressed with WT DCT or DCTLZM and a cDNA ratio of 0.003:1 WT AKAP15 in the absence or presence of 5 µM forskolin. (E) Mean current-voltage relationships for CaV1.2Δ1800 channels coexpressed with either WT DCT or DCTLZM and a cDNA ratio of 0.003:1 WT AKAP15 without or with 5 µM forskolin. (F) Coupling efficiency (nA/pC) for CaV1.2Δ1800 channels in E. Dashed line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCTLZM with AKAP15. **, P < 0.01 versus control. (G) Representative IBa through CaV1.2Δ1800 channels coexpressed with WT DCT or DCTLZM and 0.01:1 AKAP79ΔPIX in the absence or presence of 5 µM forskolin. (H) Mean current-voltage relationships for CaV1.2Δ1800 channels with either WT DCT or DCTLZM, a cDNA ratio of 0.01:1 AKAP79ΔPIX, and 5 µM forskolin. (B, C, E, F, and H) Error bars are SEM. (I) Coupling ratio (nA/pC) for CaV1.2Δ1800 channels in H. Dashed line indicates mean coupling ratio for unstimulated CaV1.2Δ1800 + DCTLZM with AKAP79ΔPIX. n values and means ± SEM are indicated. **, P < 0.01 versus control. Significance was determined by ANOVA.
Although AKAP79 is known to interact with CaV1.2 channels via the modified leucine zipper motif in the DCT (Hall et al., 2007; Oliveria et al., 2007), the role of this interaction in up-regulation of ion conductance activity of CaV1.2 channels has not been directly tested. Our ability to reconstitute PKA regulation of CaV1.2 channels by coexpression with AKAP79ΔPIX in transfected cells now allows a direct test of its site of interaction using the DCTLZM mutant. We found that CaV1.2Δ1800 + DCTLZM coexpressed with AKAP79ΔPIX was not up-regulated by activation of adenylyl cyclase with forskolin (Fig. 4, G and H). These results show directly that the regulatory effects of AKAP79ΔPIX, as well as those of AKAP15, require interaction with the modified leucine zipper motif in the DCT of CaV1.2 channels.
DISCUSSION
Reconstitution of regulation of CaV1.2 channels by the PKA signaling pathway
The results presented here further establish the relevance of our reconstitution system for studies of regulation of CaV1.2 channels by the PKA signaling pathway. Coexpression of different members of the autoinhibitory signaling complex formed by CaV1.2 channels allows their differential regulatory properties to be directly determined and compared with the properties of CaV1.2 channels expressed with AKAP15. Using this approach, we have found that AKAP79 confers strikingly different regulation from AKAP15. These results imply that differential expression and localization of AKAP15 and AKAP79 can lead to differential regulation of CaV1.2 channels in different cell types and subcellular compartments. Our results highlight the importance of binding of the Ca2+-regulated phosphoprotein phosphatase calcineurin in determining the regulatory properties of AKAP79. A previous study has demonstrated its role in regulation of basal activity of CaV1.2 channels and in regulation of gene expression via entry of Ca2+ through CaV1.2 channels (Oliveria et al., 2007). Our results provide direct evidence for an important role for calcineurin bound to AKAP79 in opposing up-regulation of CaV1.2 channel function by the PKA pathway. A recent study shows that AKAP79 also binds phosphoprotein phosphatase-1 (Le et al., 2011), which may contribute additional modes of differential regulation of CaV1.2 channels and other PKA signaling targets.
Differential regulation of CaV1.2 channels by PKA bound to different AKAPs
Most cells express several different AKAPs, which have been shown to participate in many different cell signaling pathways (Logue and Scott, 2010). Thus, differential regulation of distinct signaling pathways by different AKAPs is well established. Our results with CaV1.2 channels add a new perspective on the potential molecular mechanisms for differential regulation by AKAPs by revealing that multiple AKAPs can bind at a single regulatory site on their common target protein and have differential effects on the same cell signaling pathway that are mediated by their associations with different regulatory proteins. AKAP15 and AKAP79 have previously been shown to interact with the same, short modified leucine zipper motif, which is required for their binding and support of PKA regulation (Hulme et al., 2002, 2003; Oliveria et al., 2007). However, it was unknown whether interaction with this common site would cause functional competition between the two proteins. We found that AKAP15 and AKAP79 do indeed compete functionally when coexpressed with CaV1.2 channels in the autoinhibitory signaling complex. Moreover, this functional competition is caused by the ability of AKAP79 to bind calcineurin. Because different AKAPs bind many different kinases, phosphoprotein phosphatases, and other signaling proteins (Logue and Scott, 2010), this form of functional competition and differential regulation of target proteins by different AKAPs acting at a common binding site would provide a broad range of regulatory options controlled by expression and localization of AKAPs. These findings add an additional layer of flexibility and complexity to cell signaling pathways in which AKAPs organize multiple regulatory proteins.
Our results demonstrating functional competition among AKAPs interacting with the same site on CaV1.2 channels take on additional significance in light of recent mouse genetic studies of AKAP regulation of the heart (Jones et al., 2012). Deletion of both AKAP15 and AKAP79 in mice is not sufficient to prevent β-adrenergic up-regulation of CaV1.2 channel activity by isoproterenol in ventricular myocytes (Jones et al., 2012). Because β-adrenergic stimulation in ventricular myocytes requires AKAP anchoring at the AKAP-binding domain on CaV1.2 channels (Hulme et al., 2003), these mouse genetic results imply that one or more additional AKAPs besides AKAP15 and AKAP79 can mediate β-adrenergic stimulation of CaV1.2 channels in ventricular myocytes through interaction with the same site. Thus, functional competition for regulation of CaV1.2 channels by AKAPs likely extends to at least one more, yet-unidentified AKAP. The levels of expression and affinity and the different regulatory properties of these AKAPs will determine which one is dominant in regulating CaV1.2 channels and therefore will define the overall pattern of regulation of channel activity. Changes in expression of these AKAPs in different physiological and/or pathophysiological states may be important determinants of the activity of CaV1.2 channels.
Regulation of CaV1.2 channels by calcineurin bound to AKAP79
Differential regulation of CaV1.2 channels by AKAPs might reflect altered interactions between the channel and AKAP or differential interactions of the bound AKAP with other signaling proteins. Our results with AKAP79 show that its binding of calcineurin is responsible for its inability to support PKA regulation of CaV1.2 channels. Surprisingly, the basal phosphatase activity of calcineurin is sufficient for this regulatory effect. Our measurements are made using Ba2+ as the permeant ion, and intracellular Ca2+ is strongly buffered with EGTA in the recording pipette. Therefore, it is unlikely that calcineurin is substantially up-regulated by Ca2+ binding in our experiments. Calcineurin has a significant basal activity, which is ∼0.25–1% of its maximal activity when fully activated by Ca2+ and calmodulin in biochemical assays in solution (Stewart et al., 1982; Perrino et al., 1992; Stemmer and Klee, 1994). The rate of dephosphorylation of phosphoprotein substrates depends on their local concentration in the vicinity of the phosphatase, and tethering of calcineurin directly to the DCT of CaV1.2 channels would increase the local concentration of its substrate site at Ser1700-P by hundreds or thousands of fold. Evidently, the effect of proximity afforded by binding to AKAP79 allows effective dephosphorylation of the CaV1.2 channel by the basal activity of calcineurin at a rate that is comparable with or greater than the rate of phosphorylation by PKA, preventing accumulation of phosphorylated CaV1.2 channels and resulting in functional competition between AKAP15 and AKAP79 at their common binding site. In a cardiac myocyte, increases of cAMP near CaV1.2 channels may be faster and larger because of localized signaling; therefore, activation of calcineurin by Ca2+ entering through CaV1.2 channels may be required to return the activity of these channels to the basal level in vivo. These considerations further emphasize the importance of a signaling complex for regulation of CaV1.2 channels in vivo.
Regulation of CaV1.2 channels in different tissues
CaV1.2 channels conduct L-type Ca2+ currents in several different cell types. In skeletal and cardiac myocytes, up-regulation of CaV1.2 channel activity in response to activation of the β-adrenergic signaling pathway contributes to the increase in contractile force during the fight or flight response (Reuter, 1983; Tsien et al., 1986; Catterall, 1991). In brain neurons, CaV1.2 channels are involved in synaptic plasticity on the postsynaptic side of the synapse, and up-regulation of their activity by the β-adrenergic and dopaminergic signaling pathways enhances synaptic transmission (Davare et al., 2001; Young and Yang, 2004; Hall et al., 2007). In endocrine cells, CaV1.2 channels mediate Ca2+ entry that triggers secretion of hormones, and regulation by the PKA signaling pathway is an important regulator of hormone release (Baldelli et al., 2004; Yang and Berggren, 2006). AKAP15 and AKAP79 can regulate CaV1.2 channels in skeletal and cardiac myocytes (Hulme et al., 2002, 2003) and brain neurons (Hall et al., 2007; Marshall et al., 2011), and AKAPs are also implicated in control of hormone secretion (Lester et al., 2001; Yang and Berggren, 2006). Our results presented here imply that competitive binding of AKAP15, AKAP79, and potentially other AKAPs at the AKAP-binding domain on CaV1.2 channels can transform the regulatory responses of CaV1.2 channels to the PKA signaling pathway and potentially to other intracellular signaling pathways and thereby can fine-tune the regulation of muscle contraction, synaptic transmission, and hormone secretion.
Supplementary Material
Acknowledgments
This work was supported by Research Grant R01 HL085372 from the National Institutes of Health to W.A. Catterall and a postdoctoral fellowship from the American Heart Association to M.D. Fuller.
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- AKAP
- A-kinase anchoring protein
- DCT
- distal C-terminal domain
References
- Baldelli P., Hernández-Guijo J.M., Carabelli V., Novara M., Cesetti T., Andrés-Mateos E., Montiel C., Carbone E. 2004. Direct and remote modulation of L-channels in chromaffin cells: Distinct actions on α1C and α1D subunits? Mol. Neurobiol. 29:73–96 10.1385/MN:29:1:73 [DOI] [PubMed] [Google Scholar]
- Bers D.M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Catterall W.A. 1991. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell. 64:871–874 10.1016/0092-8674(91)90309-M [DOI] [PubMed] [Google Scholar]
- Catterall W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- Catterall W.A. 2010. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci. Lett. 486:107–116 10.1016/j.neulet.2010.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan V.M., Perrino B.A., Howard M., Langeberg L.K., Hicks J.B., Gallatin W.M., Scott J.D. 1995. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 267:108–111 10.1126/science.7528941 [DOI] [PubMed] [Google Scholar]
- Davare M.A., Avdonin V., Hall D.D., Peden E.M., Burette A., Weinberg R.J., Horne M.C., Hoshi T., Hell J.W. 2001. A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 293:98–101 10.1126/science.293.5527.98 [DOI] [PubMed] [Google Scholar]
- De Jongh K.S., Merrick D.K., Catterall W.A. 1989. Subunits of purified calcium channels: A 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc. Natl. Acad. Sci. USA. 86:8585–8589 10.1073/pnas.86.21.8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K.S., Warner C., Colvin A.A., Catterall W.A. 1991. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc. Natl. Acad. Sci. USA. 88:10778–10782 10.1073/pnas.88.23.10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K.S., Murphy B.J., Colvin A.A., Hell J.W., Takahashi M., Catterall W.A. 1996. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 35:10392–10402 10.1021/bi953023c [DOI] [PubMed] [Google Scholar]
- Dell’Acqua M.L., Dodge K.L., Tavalin S.J., Scott J.D. 2002. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J. Biol. Chem. 277:48796–48802 10.1074/jbc.M207833200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrick M.A., Sadilek M., Konoki K., Catterall W.A. 2010. β-adrenergic-regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight-or-flight response. Proc. Natl. Acad. Sci. USA. 107:18712–18717 10.1073/pnas.1012384107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser I.D.C., Tavalin S.J., Lester L.B., Langeberg L.K., Westphal A.M., Dean R.A., Marrion N.V., Scott J.D. 1998. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J. 17:2261–2272 10.1093/emboj/17.8.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D.A., Klee C.B., Bierer B.E., Burakoff S.J. 1992. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc. Natl. Acad. Sci. USA. 89:3686–3690 10.1073/pnas.89.9.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Westenbroek R.E., Yu F.H., Clark J.P., III, Marshall M.R., Scheuer T., Catterall W.A. 2011. Deletion of the distal C terminus of CaV1.2 channels leads to loss of β-adrenergic regulation and heart failure in vivo. J. Biol. Chem. 286:12617–12626 10.1074/jbc.M110.175307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Westenbroek R.E., Scheuer T., Catterall W.A. 2013. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc. Natl. Acad. Sci. USA. 110:19621–19626 10.1073/pnas.1319421110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M.D., Emrick M.A., Sadilek M., Scheuer T., Catterall W.A. 2010. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci. Signal. 3:ra70 10.1126/scisignal.2001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A.N., Maack C., Johns D.C., Sidor A., O’Rourke B. 2006. β-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of α1C but not serine 1928. Circ. Res. 98:e11–e18 10.1161/01.RES.0000202692.23001.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Yatani A., Dell’Acqua M.L., Sako H., Green S.A., Dascal N., Scott J.D., Hosey M.M. 1997. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 19:185–196 10.1016/S0896-6273(00)80358-X [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Surmeier D.J. 2011. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34:441–466 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P.C., Tibbs V.C., Catterall W.A., Murphy B.J. 1997. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J. Biol. Chem. 272:6297–6302 10.1074/jbc.272.10.6297 [DOI] [PubMed] [Google Scholar]
- Gray P.C., Johnson B.D., Westenbroek R.E., Hays L.G., Yates J.R., III, Scheuer T., Catterall W.A., Murphy B.J. 1998. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 20:1017–1026 10.1016/S0896-6273(00)80482-1 [DOI] [PubMed] [Google Scholar]
- Hall D.D., Davare M.A., Shi M., Allen M.L., Weisenhaus M., McKnight G.S., Hell J.W. 2007. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 46:1635–1646 10.1021/bi062217x [DOI] [PubMed] [Google Scholar]
- Hell J.W., Westenbroek R.E., Breeze L.J., Wang K.K., Chavkin C., Catterall W.A. 1996. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 93:3362–3367 10.1073/pnas.93.8.3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme J.T., Ahn M., Hauschka S.D., Scheuer T., Catterall W.A. 2002. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J. Biol. Chem. 277:4079–4087 10.1074/jbc.M109814200 [DOI] [PubMed] [Google Scholar]
- Hulme J.T., Lin T.W., Westenbroek R.E., Scheuer T., Catterall W.A. 2003. β-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc. Natl. Acad. Sci. USA. 100:13093–13098 10.1073/pnas.2135335100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme J.T., Scheuer T., Catterall W.A. 2004. Regulation of cardiac ion channels by signaling complexes: role of modified leucine zipper motifs. J. Mol. Cell. Cardiol. 37:625–631 10.1016/j.yjmcc.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Hulme J.T., Konoki K., Lin T.W., Gritsenko M.A., Camp D.G., II, Bigelow D.J., Catterall W.A. 2005. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc. Natl. Acad. Sci. USA. 102:5274–5279 10.1073/pnas.0409885102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme J.T., Yarov-Yarovoy V., Lin T.W.-C., Scheuer T., Catterall W.A. 2006. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J. Physiol. 576:87–102 10.1113/jphysiol.2006.111799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.W., Brunet S., Gilbert M.L., Nichols C.B., Su T., Westenbroek R.E., Scott J.D., Catterall W.A., McKnight G.S. 2012. Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proc. Natl. Acad. Sci. USA. 109:17099–17104 10.1073/pnas.1215219109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A.V., Tavalin S.J., Dodge-Kafka K.L. 2011. Identification of AKAP79 as a protein phosphatase 1 catalytic binding protein. Biochemistry. 50:5279–5291 10.1021/bi200089z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester L.B., Faux M.C., Nauert J.B., Scott J.D. 2001. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 142:1218–1227 [DOI] [PubMed] [Google Scholar]
- Logue J.S., Scott J.D. 2010. Organizing signal transduction through A-kinase anchoring proteins (AKAPs). FEBS J. 277:4370–4375 10.1111/j.1742-4658.2010.07866.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D.M. 2010. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 58:951–961 10.1016/j.neuropharm.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M.R., Clark J.P., III, Westenbroek R., Yu F.H., Scheuer T., Catterall W.A. 2011. Functional roles of a C-terminal signaling complex of CaV1 channels and A-kinase anchoring protein 15 in brain neurons. J. Biol. Chem. 286:12627–12639 10.1074/jbc.M110.175257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria S.F., Dell’Acqua M.L., Sather W.A. 2007. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 55:261–275 10.1016/j.neuron.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino B.A., Fong Y.L., Brickey D.A., Saitoh Y., Ushio Y., Fukunaga K., Miyamoto E., Soderling T.R. 1992. Characterization of the phosphatase activity of a baculovirus-expressed calcineurin A isoform. J. Biol. Chem. 267:15965–15969 [PubMed] [Google Scholar]
- Qian H., Matt L., Zhang M., Nguyen M., Patriarchi T., Koval O.M., Anderson M.E., He K., Lee H.K., Hell J.W. 2012. β2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J. Neurophysiol. 107:2703–2712 10.1152/jn.00374.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. 1979. Properties of two inward membrane currents in the heart. Annu. Rev. Physiol. 41:413–424 10.1146/annurev.ph.41.030179.002213 [DOI] [PubMed] [Google Scholar]
- Reuter H. 1983. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 301:569–574 10.1038/301569a0 [DOI] [PubMed] [Google Scholar]
- Stemmer P.M., Klee C.B. 1994. Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B. Biochemistry. 33:6859–6866 10.1021/bi00188a015 [DOI] [PubMed] [Google Scholar]
- Stewart A.A., Ingebritsen T.S., Manalan A., Klee C.B., Cohen P. 1982. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: Probable identity with calcineurin (CaM-BP80). FEBS Lett. 137:80–84 10.1016/0014-5793(82)80319-0 [DOI] [PubMed] [Google Scholar]
- Tsien R.W., Bean B.P., Hess P., Lansman J.B., Nilius B., Nowycky M.C. 1986. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. J. Mol. Cell. Cardiol. 18:691–710 10.1016/S0022-2828(86)80941-5 [DOI] [PubMed] [Google Scholar]
- West A.E., Griffith E.C., Greenberg M.E. 2002. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3:921–931 10.1038/nrn987 [DOI] [PubMed] [Google Scholar]
- Wong W., Scott J.D. 2004. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 5:959–970 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- Yang S.N., Berggren P.O. 2006. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr. Rev. 27:621–676 10.1210/er.2005-0888 [DOI] [PubMed] [Google Scholar]
- Young C.E., Yang C.R. 2004. Dopamine D1/D5 receptor modulates state-dependent switching of soma-dendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons. J. Neurosci. 24:8–23 10.1523/JNEUROSCI.1650-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.