Abstract
Pleuroparenchymal fibroelastosis (PPFE) is a rare pulmonary fibrosis that is clinically characterized by upper-lobe predominant fibrosis. PPFE is a slowly progressive disorder and its first symptom is dyspnea or dry cough. Chest pain because of pneumothorax may be the first symptom in some patients. Patients with PPFE are slender with a flat rib cage or abnormally narrowed anterior–posterior thoracic dimension. Decreases in forced vital capacity, total lung capacity, and diffusing capacity are respiratory-function characteristics of PPFE, similar to those seen in idiopathic pulmonary fibrosis (IPF). The most remarkable difference in clinical features between PPFE and IPF is imaging findings, with upper-lobe-predominant lesions in PPFE and lower-lobe-predominant lesions in IPF.
Keywords: Pleuroparenchymal fibroelastosis (PPFE), pulmonary upper lobe fibrosis, pulmonary fibrosis (IPF).
INTRODUCTION
Pleuroparenchymal fibroelastosis (PPFE) was first repor-ted by Frankel et al. [1]. PPFE can occur without any etiology or underlying diseases (idiopathic PPFE), or with underlying diseases or conditions. Idiopathic PPFE has been listed as one of the rare idiopathic interstitial pneumonias (IIPs) in the revised international multidisciplinary classification of the IIPs [2]. One of its characteristics is upper-lobe-dominant progressive pulmonary fibrosis with a peculiar histopathology consisting of visceral pleural thickening with collagenous fibrosis, subpleural elastosis, and intraalveolar collagenous fibrosis [1].
The clinical course of PPFE progresses slowly and is similar to that of chronic fibrosing interstitial pneumonias, such as idiopathic pulmonary fibrosis (IPF) and fibrotic nonspecific interstitial pneumonia (NSIP). Although the fibrosis is rarely encountered in clinical practice, since the appearance of the paper by Frankel et al. [1], an increasing number of studies have examined PPFE. In this chapter, the clinical characteristics of PPFE will be discussed, together with the historical changes in the concept of pulmonary upper-lobe fibrosis.
PULMONARY UPPER-LOBE FIBROSIS: HISTORY OF THE CONCEPT
There is a long history of pulmonary upper-lobe fibrosis with unknown etiology. In 1975, Davies et al. reported five patients with idiopathic progressive pulmonary fibrosis [3]. The fibrosis was confined to the upper parts of the lung and resembled pulmonary lesions in ankylosing spondylitis [4]. Their clinicopathological features, such as progressive dyspnea, marked weight loss, severe restrictive impairment, and upper-lobe-dominant fibrosis, are similar to those observed in PPFE today. Similar cases had already been published in 1962 and 1966 as “chronic idiopathic pneumonia” [5] and “idiopathic cavitation of the lung” [6]. Later, similar cases of upper-lobe fibrosis were reported under the terms “pulmonary upper-lobe fibrocystic changes” [7], “pulmonary apical fibrocystic disease” [8], or “idiopathic progressive pleuropulmonary fibrosis” [9]. The common radiographic feature in these papers was upper-lobe fibrosis and not lower-lobe fibrosis, as seen in IPF.
In 1992, Amitani et al. reported 13 patients with upper-lobe-localized pulmonary fibrosis with unknown etiology [10]. This report, written in Japanese, included nine cases with pathological analysis (open lung biopsy, three cases; autopsy, two cases; transbronchial lung biopsy, four cases), which represented the largest number of patients at that time. In that paper, the authors named the disorder idiopathic pulmonary upper-lobe fibrosis (idiopathic PULF) and presented its clinical and histological characteristics as follows: 1) slender stature with flattened thoracic cage; 2) progressive bilateral subpleural fibrosis in the upper lobes with bullae but without honeycombing; 3) recurrent pneumothorax; 4) no extrathoracic lesions; 5) absence of acid-fast bacilli and lack of effect of antituberculous drugs; 6) aspergillus infection may be superimposed; and 7) slowly progressive and fatal condition with 10–20 years of presentation. The paper by Amitani et al. was followed by several Japanese case reports describing idiopathic PULF [11-16], before the paper on PPFE by Frankel et al. [1] appeared in 2004.
A Japanese review article by Kawabata et al. [17] on the histopathological characteristics of idiopathic PULF was published in 2003, when the fibrosis was well known among the interstitial lung disease community of Japanese pulmonologists, and idiopathic PULF was also called “Amitani disease”, in his honor. Kawabata described the pathological features as follows: subpleural atelectatic induration with the proliferation of elastic fibers and intraluminal organization or intraalveolar fibrosis in the upper lobes, and fibrously thickened pleura. Such pathological features are totally different from those of IPF.
A year after the publication of the paper by Kawabata et al., Frankel et al. reported five patients with upper-lobe-dominant pulmonary fibrosis characterized by intense fibrosis of the visceral pleura and subpleural fibrosis with a mixture of elastic tissue and dense collagen. They termed the fibrosis pleuroparenchymal fibroelastosis [1].
“Subpleural atelectatic induration with the proliferation of elastic fibers and intraluminal organization or intraalveolar fibrosis” described by Kawabata et al. [17] is histologically identical to “subpleural fibrosis with a mixture of elastic tissue and dense collagen” reported by Frankel et al. [1]. The main histological features are almost identical in these two fibroses.
Subsequently, many papers on PULF continued to be published in Japan [18-26]. Recently, however, an increasing number of reports have appeared in English on the fibrosis named PPFE [27-33], and PPFE is now globally accepted as a representative nomenclature for this disorder (Table 1). However, PPFE is a diagnostic term that expresses histological features. The term “upper-lobe fibrosis” is necessary for clinicians, especially when discriminating the disorder from IPF in clinical practice. Therefore, in my opinion, PULF is to be used for a clinical or clinical-radiological-pathological diagnosis, and PPFE is to be used for a histological pattern, as shown in the diagnosis of IIPs in the international multidisciplinary consensus classification of the IIPs [34]. At present, however, surgical lung biopsy is indispensible for the definite diagnosis of this rare pulmonary fibrosis.
Table 1.
Same or Similar Disease Concepts as Pleuroparenchymal Fibroelastosis
| Chronic idiopathic pneumonia [5] 1962 Idiopathic cavitation of lung [6] 1966 Idiopathic progressive pulmonary fibrosis [3] 1975 Pulmonary upper lobe fibrocystic changes [7] 1978 Upper lobe fibrosis and cavitation [44] 1980 pulmonary apical fibrocystic disease [8] 1981 idiopathic progressive pleuropulmonary fibrosis [9] 1984 Apical pulmonary fibrosis [37] 1988 Idiopathic pulmonary upper lobe fibrosis [10] 1992 Marked pulmonary fibrosis in the upper lung field [12] 1999 Marked pulmonary fibrosis in the upper lobe [13] 1999 Upper lobe fibrosis [36, 41] 2003, 2005 Upper lobe-dominat pulmonary fibrosis [20] 2010 |
There is a controversy regarding the extent of the fibrosis: whether the lesions are confined to upper lobes (“pure” PULF or so-called Amitani disease), extend to adjacent lobes, or present as isolated usual interstitial pneumonia (UIP)-like lesions in the lower lobes in addition to the upper-lobe fibrosis [23]. Some pulmonologists in Japan claim that pulmonary “upper-lobe-localized” fibrosis (Amitani disease) should be discriminated from pulmonary “upper-lobe-dominant” fibrosis, because they think that the latter might be a variant of UIP or an upper-lobe manifestation of other fibrosing lung diseases, and Amitani disease is a peculiar form of fibrosis that is confined to the upper lobes, unlike what is observed in other IIPs.
Although the name PULF was originally given to pulmonary fibrosis localized only in the upper lobes, and not extending to other lobes [10, 17], it has been disclosed that the number of patients with fibrosis involving not only the upper lobes, but also other lobes, is much greater than that of patients with upper-lobe-localized fibrosis. Moreover, the boundary between upper-lobe-localized fibrosis and upper-lobe-dominant fibrosis has become ambiguous in clinical practice. In addition, it has also been disclosed that the PPFE pattern as a histological finding is associated with many of the diseases described below; thus, it appears more important today to differentiate idiopathic PPFE from secondary PPFE or PPFE with underlying diseases, rather than to differentiate upper-lobe-localized fibrosis from upper-lobe-dominant fibrosis.
CLINICAL CHARACTERISTICS
In this section, PULF is considered to be the same as PPFE as a clinical entity. Therefore, henceforth, I will use the term PPFE as being representative of both types of pulmonary fibrosis, PPFE and PULF, either upper-lobe-localized or upper-lobe-dominant fibrosis. Moreover, we have to differentiate the use of the term PPFE as a clinical disease from the PPFE histological pattern seen in various diseases.
Normally, clinical characteristics should be separately discussed in idiopathic and secondary forms of PPFE. As seen in patients with ankylosing spondylitis [4], a considerable number of patients with PPFE have underlying diseases or conditions that might be relevant to its occurrence and development (Table 2). In this chapter, the term PPFE will be used for both fibrosis with and without underlying diseases, otherwise specified as idiopathic PPFE or secondary PPFE (or PPFE with underlying diseases), respectively.
Table 2.
Underlying Diseases or Conditions that may be Associated with Pleuroparenchymal Fibroelastosis (PPFE)
| Idiopathic PPFE Radiation Anticancer chemotherapy Bone marrow- or stem cell-transplantation Lung transplantation Occupational dust exposure Asbestos Aluminum Infection Aspergillus Mycobacterium avium-intracellulare Hereditary PPFE ~ PPFE with family history Autoimmune diseases Rheumatoid arthritis Ulcerative colitis Psoriasis Ankylosing spondylitis Hypersensitivity pneumonitis |
Gender and Age
There is no gender preponderance in the incidence described in previous reports. Gender preponderance differed in each report [1, 3, 5-7, 10-33, 35-37] (Table 3).
Table 3.
Clinical Characteristics of Pleuroparenchymal Fibroelastosis
| No gender preponderance Age at onset Wide-ranging, younger than in idiopathic pulmonary fibrosis (IPF) Smoking history Unrelated to the incidence of PPFE Clinical symptoms Exertional dyspnea and dry cough with insidious onset Chest pain due to pneumothorax Loss of body weight Physical findings Slender stature and flattened thoracic cage Crackles sometimes audible Serum biomarkers KL-6 within the normal or around the upper normal limit Elevated Surfactant protein D (SP-D) Autoantibodies such as rheumatoid factor and antinuclear antibody sometimes elevated Prognosis Wide-ranging in each case studies from slowly progressive with 10 - 20 years of presentation to rapidly progressive course Poorer prognosis of secondary PPFE such as transplantation- associated PPFE |
Age at onset is wide-ranging, from young to old age, and is therefore dissimilar to IPF, which affects older individuals. The fact that there are a substantial number of patients with PPFE aged in their third and fourth decade [1, 3-5, 7-13, 18, 26, 29, 30] is quite characteristic, even when transplantation-associated PPFE [24, 27, 28, 32, 33] is excluded. Underlying diseases or hereditary disposition could be responsible for age at onset in some patients with PPFE.
Smoking History
Smoking does not appear to have any effect on the occurrence of PPFE. Previous studies [1, 3, 11-14, 16, 18, 19, 21-24, 26, 27, 29-31, 35, 37] indicate that the smoking rate by current and former smokers was 29% of the total numbers of patients, which is a contrasting difference between PPFE and IPF [38].
Past History and Underlying Diseases or Conditions
There are many patients with PPFE who experienced recurrent pneumothorax. Multiple bullae in upper lung fields that appear in the course of the disease may be torn and be responsible for the recurrence of pneumothorax.
Some patients with PPFE have a past history of radiation therapy and/or anticancer chemotherapy for treating anky-losing spondylitis [4] or malignancy [1, 27]. Postradiation pulmonary fibrosis is usually confined to the irradiated area
of the lung. PPFE might be a special form of radiation injury, extending to nonirradiated areas to form irreversible pulmonary fibrosis. Hamada et al. reported a patient with cyclophosphamide-induced pulmonary fibrosis and elastosis with pleural thickening after treatment for breast cancer [39]. The clinical and pathological features appear similar to those of current PPFE.
Bone marrow or stem cell transplantation [24, 27, 28] and lung transplantation [32, 33] are now known to cause PPFE, as a lung manifestation of chronic graft versus host disease (GVHD) and as a phenotype of chronic rejection of transplanted lungs. The fibrosis of the upper lobes described by Konen et al. [40] and the upper lobe fibrosis reported by Pakhale et al. [41] probably have the same histology as that of PPFE after lung transplantation. Transplantation-associated PPFE highlights the key roles of immune-mediated cells in the development of PPFE.
Occupational exposure to dust [3, 20, 26, 27, 29, 35] such as asbestos [27, 29] or aluminum [26, 35] is another important factor that induces PPFE. In general, pneumoconiosis such as asbestosis, silicosis, and berylliosis may present as upper-lobe fibrosis, although the PPFE pattern has not been histologically demonstrated in all of these pneumoconioses. As the interstitial connective tissue response in asbestosis is fibroelastotic rather than fibrotic [42], with pleural thickening, it is possible that asbestos exposure directly induces the pathology of PPFE.
Pulmonary mycobacterial disease due to the Mycobacte-rium avium-intracellulare complex (MAC) [23] and aspergillus infection [13, 16, 29, 30] have been reported in patients with PPFE. These infectious diseases may coexist, but it is not known currently whether the pathology of PPFE is induced by these infections or whether PPFE provides the circumstances for these infectious agents to grow, as does IPF [43]. Reddy et al. speculated that repeated inflammatory damage in a predisposed individual may lead to the patho-logy of PPFE [30].
Genetic or autoimmune mechanisms may be involved in the pathogenesis of PPFE. Some investigators reported PPFE in siblings [1, 10, 13] and a pair of parents and child [13], and PPFE with a family history of other pulmonary fibrosis [11, 30]. Ankylosing spondylitis [4], ulcerative colitis [36], and psoriasis [37] have also been previously reported as underlying diseases. Pulmonary upper-lobe fibrosis and cavitation in patients with rheumatoid diseases have also been reported [44]. Pleuroparenchymal disease in collagen vascular disease might share common histological features with PPFE.
The fibrotic stage of hypersensitivity pneumonitis, Langerhans cell histiocytosis, and sarcoidosis may also present as upper-lobe fibrosis clinically [45]. Reddy et al. [30] reported that two of 12 PPFE patients were exposed to environmental allergens in the form of mold and birds, and there was one patient whose biopsy specimens showed both a PPFE pattern and bronchocentric chronic inflammation and focal organizing pneumonia with poorly formed nonnecroti-zing granulomas, which are histological features that are consistent with hypersensitivity pneumonitis.
Clinical Symptoms
The main symptoms are nonproductive cough and exertional dyspnea, which are also observed in IPF. Such symptoms appear insidiously. Chest pain due to pneumothorax may be the first symptom in some patients. Many patients complain of weight loss.
Although Amitani et al. pointed out that upper-lobe fibrosis was slowly progressive and fatal, with 10–20 years of presentation [10], some subsequent papers have suggested a poorer prognosis of the disease [23, 30]. There might be a long silent period in PPFE during which patients have no symptoms and the only abnormal finding is a pleural thickening-like appearance in bilateral apical areas of chest radiographs. However, the clinical course may be accelerated once symptoms appear [23].
Physical Findings
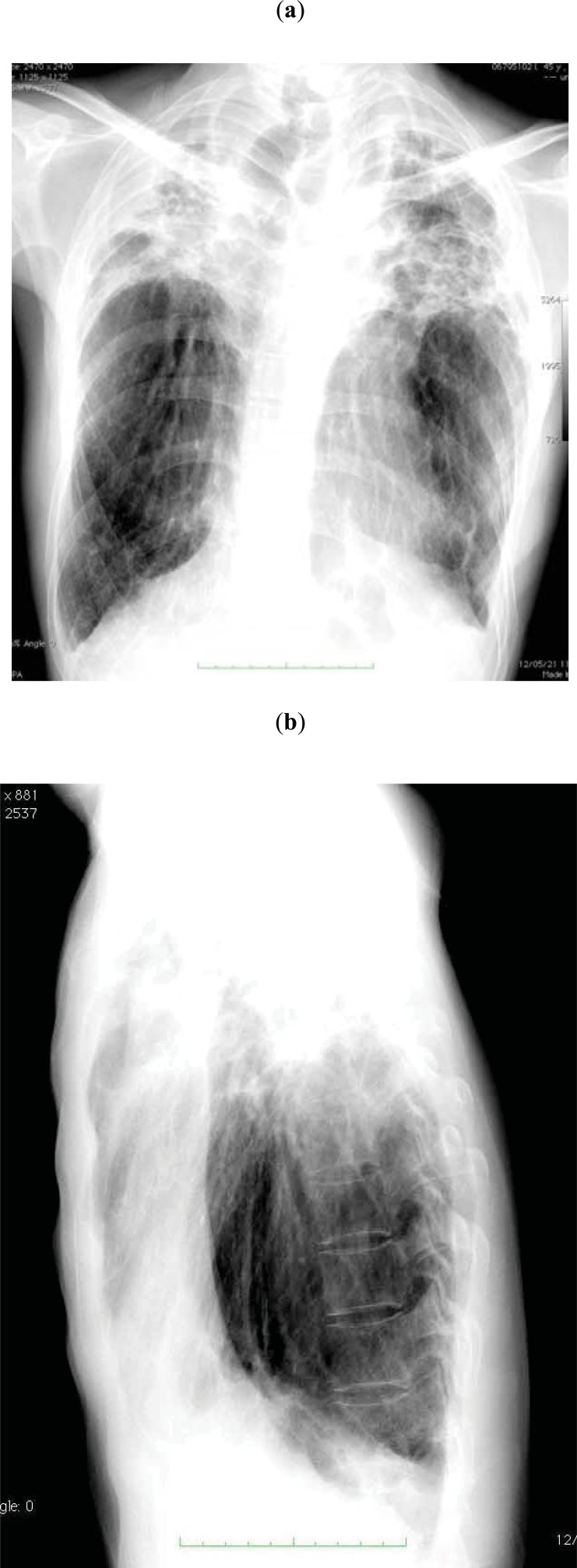
Patients with PPFE are often associated with slender stature [10-13, 15-25, 31]. “Flattened thoracic cage” is another characteristic physical finding in patients with PPFE. Japanese investigators have paid attention to a flattened thoracic cage in idiopathic PPFE [10-12, 14, 18, 21]. In flattened thoracic cages or abnormally narrowed anterior-posterior thoracic dimension, the ratio of the anterior-posterior diameter of the thorax to the transverse diameter of the thorax (APDT/TDT) is lower than that observed in the normal population, which gives an impression of weakness and is shown in the lateral view of chest radiographs (Fig. 1B). A flattened thoracic cage is a similar physical finding to pectus excavatum, but a flattened thoracic cage is not associated with excavation of the sternum.
Fig. (1).
Chest radiographs of a PPFE patient (44-year-old man) showing a thin thoracic cage.
A flattened thoracic cage, which may result from a congenital disposition or may be a secondary change of the thorax as a consequence of the shrinkage of the upper lung lobes through the long-lasting process of fibrosing, may inhibit the distensibility of the lungs and inhibit the constant blood flow in the upper lobes, leading to a ventilation–perfusion imbalance. These pathophysiological conditions may further enhance the progress of the disease. A flattened thoracic cage is observed in patients with not only idiopathic PPFE but also secondary PPFE. Amitani et al. [10] and others [18, 21] have speculated on the importance of a hereditary disposition for the formation of a flattened thoracic cage in the development of idiopathic PPFE. However, Nakasone et al. reported two PPFE patients with a flattened thoracic cage who had undergone bone marrow transplantation [24]. We observed patients with PPFE whose thoracic cage became flattened during the progression of the disease (Fig. 2) [46]. Therefore, a flattened thoracic cage might exist before the onset of overt PPFE, but it is more conspicuous in the course of the disease. Long-term follow-up from an initial stage of PPFE will elucidate the significance of a flattened thoracic cage.
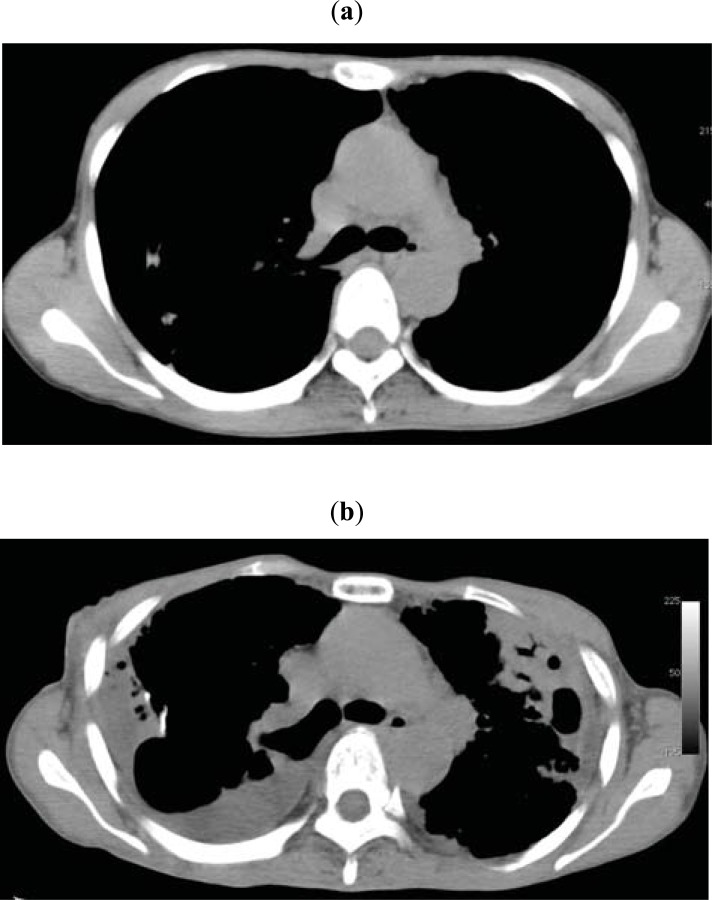
Fig. (2).
(a) Chest CT in a 48-year-old woman with PPFE. (b) Chest CT taken after 4 years. Both CT scans were sliced at the level of the 6th thoracic vertebra. The ratio of the anterior–posterior diameter of the thorax to the transverse diameter of the thorax became lower after 4 years of disease progression.
Clubbed finger, which is often seen in patients with IPF, has been rarely reported in patients with PPFE. In the literature concerning PPFE, only two patients with clubbed fingers were found: a 36-year-old woman with PPFE after autologous stem cell transplantation [24] and a 70-year-old man with aluminum-induced pulmonary upper-lobe fibrosis [35].
A number of case studies have reported that crackles are audible in about half or less than half of patients [1, 3, 6, 11, 12, 15, 16, 19-21, 23, 26, 28, 29, 31, 35], which means that crackles are audible less frequently in PPFE than in IPF. Late-inspiratory crackles are considered to arise from the explosive opening of closed peripheral airways at a time when a critical transmural pressure develops as radial traction increases during lung inflation [47]. As subpleural atelectasis consisting of closely packed elastic fibers and alveoli filled with mature collagen, which no longer open, is an essential histological feature in the lung parenchyma of PPFE, there might be little chance to hear late-inspiratory crackles from such areas. However, fibrotic NSIP-like or UIP-like lesions in the adjacent lobes or lower lobes, which often appear in the advanced stage, may be the source of the crackles.
Laboratory Data
KL-6, a mucin-like glycoprotein, is a reliable serum marker that is used for the diagnosis of interstitial lung diseases [48, 49]. Serum KL-6 is usually within the normal or around the upper normal limit in patients with PPFE [18-21, 23, 26, 30, 31]. However, as the disease progresses, the level tends to become higher [23]. Surfactant protein D (SP-D) may be elevated [23, 24, 31]. Histologically, PPFE is a fibrotic lung disease, but it is not an interstitial pneumonia such as UIP and NSIP, in which KL-6 is highly expressed in the regenerated type II pneumocytes, migrating into the bloodstream through the inflammatory and fibrosing process in the lung parenchyma [49]. Such histological difference could explain the fact that serum levels of KL-6 are normal or around the upper normal limit in the early stage of PPFE. However, in the advanced stage, UIP-like lesions could contribute to the increase in the serum levels of KL-6.
Reddy et al. [30] reported that five of 12 patients with PPFE demonstrated serum auto-antibodies, such as rheumatoid factor (RF), double-stranded DNA, and antinuclear factor, suggesting the role of autoimmune mechanisms in the pathogenesis of the disease in some patients. Frankel et al. [1] and Kusagaya et al. [31] also reported positivity for RF and antinuclear antibody in patients with PPFE (Table 3).
Imaging Findings
Although surgical lung biopsy or autopsy is essential for the definite diagnosis of PPFE, imaging findings are also essential as the first step to the final diagnosis, as in other IIPs.
At the early stage of idiopathic PPFE, bilateral apical pleura appear irregularly thickened on the frontal view of chest radiographs; otherwise, appearance is almost normal, without any subjective symptoms. Such a finding may be incidentally observed in a medical checkup (Fig. 3A). Later, chest radiograph shows the elevation of bilateral hilar opacities. However, a lateral view demonstrates an abnormally narrowed anterior–posterior thoracic dimension. Subsequently, reticular and nodular opacities appear in the bilateral upper lung fields, and hilar opacities are further elevated (Fig. 3B). Chest CT shows subpleural nodular or reticular opacities in the lung parenchyma at the apex. Interlobular septal thickening is associated (Fig. 4A). In contrast to such changes in the upper lobes, changes in the middle and lower lobes are minimal, if any. As the disease progresses, the opacities described above extend to the adjacent lobes.
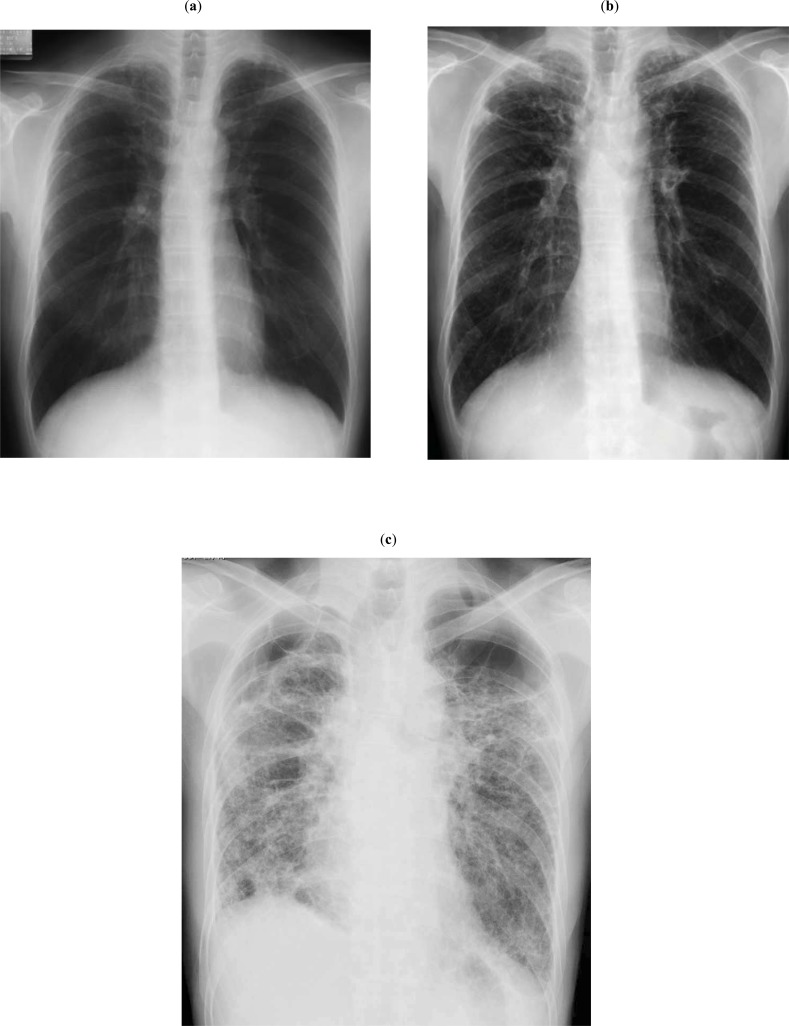
Fig. (3).
(a) Chest radiograph of a 50-year-old man with PPFE. At the early stage, the bilateral apical pleura appeared irregularly thickened; otherwise, they were almost normal. (b) Chest radiograph taken 4.7 years after the image shown in 3a. Reticular and nodular opacities appeared in the bilateral upper lung fields, and hilar opacities were further elevated. (c) Chest radiograph taken 9.7 years after the image shown in 3a. Fibrotic shadows extended to lower lung fields, and the diaphragm was elevated, with the loss of bilateral lung volume. Multiple bullae and large cysts appeared in the upper lung fields.
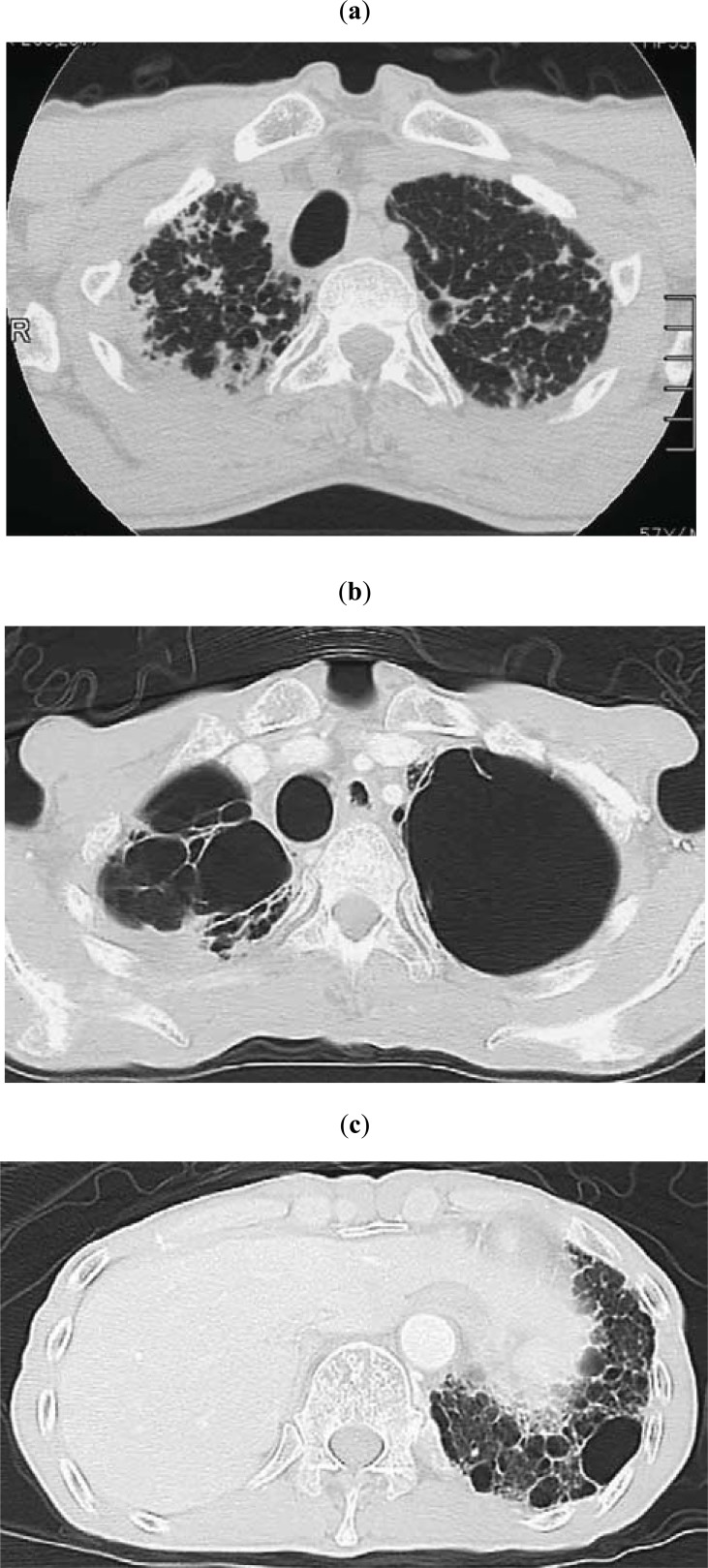
Fig. (4).
(Same patient as in Fig. 3) (a) Chest CT showing subpleural nodular or reticular opacities in the lung parenchyma at the apex. Interlobular septal thickening was associated (taken 7 years after the image shown in Fig. 3a). (b) Chest CT taken 2.8 years after the image shown in 4a. Multiple bullae and large cysts appeared in the upper lung fields. (c) Chest CT taken 2.8 years after the image shown in 4a. Multiple fibrocystic changes appear in the lower lobes.
At the advanced stage, fibrotic shadows extend to lower lung fields, and the diaphragm is elevated with the loss of bilateral lung volume. Multiple bullae and large cysts often appear in the upper lung fields (Figs. 3c, 4b), which allow aspergillus infection. Multiple fibrocystic changes also appear in the lower lobes (Fig. 4C).
Pneumothorax may complicate the course of the disease and recur. Multiple bullae may be responsible for pneumothorax.
Isolated reticular opacities or honeycombing sometimes appear in the subpleural areas of bilateral lower lobes, which raises the possibility of the combination of PPFE with UIP or NSIP (Table 4) [23, 30].
Table 4.
Imaging Characteristics of Pleuroparenchymal Fibroelastosis
|
Chest Radiograph Abnormally narrowed anterior-posterior thoracic dimension (flattened thoracic cage) Elevated hilar opacities Reticular and nodular opacities in the bilateral upper lung fields Fibrocystic opacities in the upper lung fields and occasional reticular opacities in the lower lung fields in the advanced stage Chest CT Initial stage: Subpleural nodular and reticular opacities in the apex, but minimal changes in the middle and lower lobes Advanced stage: Fibrotic opacities with traction bronchiectasis extending to adjacent lobes with multiple bulla and large cysts at the upper lung fields Occasional subpleural reticular opacities in the bilateral lower lobes resembling usual interstitial pneumonia (UIP) |
Respiratory Function
PPFE is histologically characterized by alveolar collapse with subpleural elastosis and intra-alveolar fibrosis, in addition to the thickening of the pleura mainly in upper lobes. It is easy to imagine that such histological changes induce restrictive ventilatory impairment as the main functional abnormality. Forced vital capacity (FVC) and total lung capacity (TLC) are decreased, but the ratio of forced expiratory volume in one second/forced vital capacity (FEV1/FVC) is rather increased. These physiological changes are the same as those of IPF. Fibrotic collapse of upper lobes leads to the compensatory overinflation of lower lobes, resulting in the increase of the ratio of residual volume/TLC (RV/TLC) [31], which is a peculiar functional impairment that is not usually seen in IPF.
Gas exchange impairment also appears as a restrictive impairment. The diffusing capacity of carbon monoxide (DLco) is decreased. However, in many instances, the diffusing capacity is normal or minimally reduced when DLco is divided by alveolar volume (DLco/VA) (Table 5).
Table 5.
Respiratory Function Characteristics in Pleuroparenchymal Fibroelastosis
|
Ventilatory Impairment Decreased FVC Increased FEV1/FVC (%) Decreased TLC Increased RV/TLC (%) Gas Exchange Impairment Decreased DLco Normal or minimally decreased DLco/VA |
We examined the annual decline of FVC in seven patients with idiopathic PULF and found that the yearly decline of FVC was 20.3% [23], which seems larger than that observed in IPF, although the number of patients included in the study was small. Although rapid decline of FVC is related to the prognosis of pulmonary fibrosis [50], the degree of annual decline may depend on the stage of the disease at the start of the examination of the annual change. Further studies are needed.
Treatment
Idiopathic PPFE is usually slowly progressive and refractory to steroids or immunosuppressive agents. Only one paper has reported a beneficial effect of prednisolone, which increased PaO2, although transiently [12]. Therapeutic options for chronic fibrosing interstitial pneumonia, such as IPF and fibrotic NSIP, seem useless. In the advanced stage, home oxygen therapy is necessary if the patient is hypoxemic, and infection control is important, as in IPF. Aspergillus infection will be superimposed, especially in the fibrocystic lesions of the disease [13, 16, 29, 30], as in the advanced stage of IPF [43].
The categorization of the fibrotic process in pulmonary fibrosis into two aspects, collagenosis and elastosis, showed that the predominant process is elastosis in PPFE, whereas collagenosis is predominant in IPF; however, both processes are found in PPFE as well as in IPF [51]. Targeting the inhibition of elastosis instead of collagenosis might represent a novel therapeutic avenue in PPFE.
Prognosis
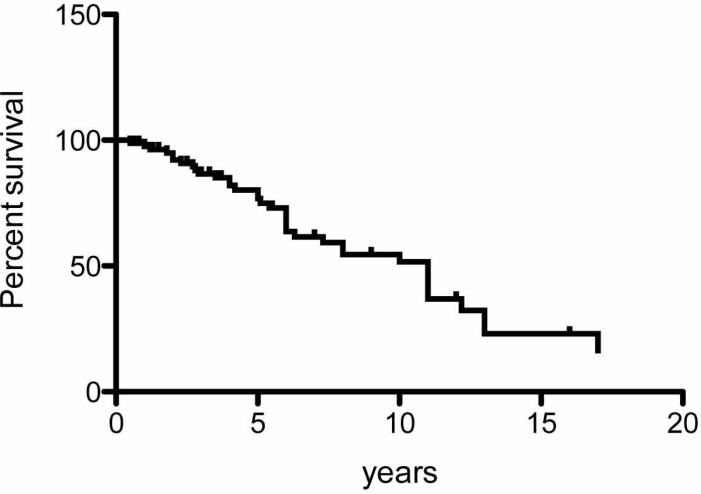
Amitani et al. demonstrated that idiopathic PPFE is slowly progressive with 10-20 years of presentation. A Kaplan–Meier survival curve drawn using 85 patients from studies that included both idiopathic and secondary PPFE [1, 3-6, 10-16, 19-24, 26-31, 33, 35, 37], but not including the patients with lung-transplantation-associated PPFE reported by Ofek et al. [32] (Fig. 5), showed that the median survival of the disease was 11 years. Although the censoring of 49 of the 85 patients was a limitation of the analysis, the survival time of the patients with PPFE was longer than that of IPF patients, which supports the observation of Amitani et al. [10]. However, the prognosis of transplantation-associated PPFE appears to be poorer, as reported by von der Thusen et al. [28] and Ofek et al. [32].
Fig. (5).
A Kaplan-Meier survival curve using 85 patients from previous studies.
We showed poor prognosis in nine patients with idiopathic PPFE [23]. Our result may have been partly derived from the fact that the majority of the patients were in the advanced stage of the disease. The prognosis of PPFE was wide-ranging in each case study.
The definition of the onset time of PPFE is an important determinant for the prognosis of the disease, because idiopathic PPFE may have a long subclinical stage in which lesions are confined at the apex of the lungs, without symptoms. Another important point, which is more essential than the onset of the disease, is the definition of the disease. As stated above, there is a group of patients with PPFE involving the bilateral upper lobes only for a long time, without invasion of adjacent lobes or lower lobes. The majority of these patients may fall under the diagnosis “Amitani disease”. The prognosis of such patients is better than that of patients with multiple lobe involvement, especially lower lobe involvement with UIP-like lesions. Currently, PPFE appears to include patients with heterogeneous etiologies.
Dilemma Between Idiopathic PPFE and Amitani Disease
I started this review article under the assumption that PPFE is the same as PULF, to avoid confusion between the two concepts. However, as described above, Japanese investigators are still debating whether idiopathic PPFE is really the same entity as idiopathic pulmonary upper-lobe-localized fibrosis (Amitani disease) and idiopathic pulmonary upper-lobe-predominant fibrosis. Amitani et al. originally proposed the concept of this disorder, and although they described the clinical characteristics of the disease in detail, its histological description was insufficient [10]. Later, Frankel et al. clearly stated the histological characteristics of PPFE, which were, they thought, considered to be the same as or similar to those of Amitani disease [1], but the description for clinical features was insufficient. Compared with the Japanese literature, the various articles on PPFE from Western countries published to date have not mentioned detailed physical findings, such as slender stature and flattened thoracic cage [27-30].
Although both imaging and histological findings are quite similar, the clinical features of Amitani disease might be different from those of idiopathic PPFE from Western countries.
CONCLUSION
PPFE is a rare pulmonary fibrosis. The number of patients published in case reports or original articles is currently around 100 worldwide. However, the knowledge gathered to date regarding PPFE is totally dependent on case reports or small case series, and not on large-scale studies.
PPFE is tentatively categorized as idiopathic PPFE and secondary PPFE. Idiopathic PPFE is now considered as one of the rare IIPs (2). Because of the rarity of the fibrosis, its clinical characteristics have not been fully elucidated. However, upper-lobe-predominant lesions and pleuroparen-chymal histopatho-logy are quite characteristic of idiopathic PPFE. Subpleural reticular opacities may not be rarely found in the lower lobes of patients with PPFE, which raises the possibility that another IIP coexists with PPFE.
Large-scale international studies are needed to elucidate the natural history and prognosis of this disease, as well as the relationship between idiopathic and secondary PPFE. In addit-ion, such global studies might tell us whether Amitani disease is a different disorder from, or one phenotype of, idiopathic PPFE.
ACKNOWLEDGEMENTS
This work is partly supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labour and Welfare, Japan.
CONFLICT OF INTEREST
The author confirms that this article content has no conflicts of interest.
REFERENCES
- 1.Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis.Description of a novel clinicopathologic Entity. . Chest. 2004;126:2007–13. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 2.An Official American Thoracic Society/European Respiratory Society Statement: Update of the International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Amer J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies D, Crowther JS, MacFarlane A. Idiopathic progressive pulmonary fibrosis. Thorax. 1975;30(3):316–25. doi: 10.1136/thx.30.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies D. Ankylosing spondylitis and lung fibrosis. Q J Med. 1972;41:395–417. [PubMed] [Google Scholar]
- 5.Clinicopathological Conference Undiagnosable lung disease demonstrated at the postgraduate medical school of London. Br Med J. 1962;1:1403–10. [Google Scholar]
- 6.Clinicopathological Conference.A Case of idiopathic cavitation of lung demonstrated at the postgraduate medical school of London. Br Med J. 1966;1(5483):345–8. doi: 10.1136/bmj.1.5483.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kentala E, Repo UK, Lehtipuu AL, Vuornos T. HLA-antigens and pulmonary upper lobe fibrocystic changes with and without ankylosing spondylitis.A report of seven cases. Scand J Respir Dis. 1978;59:8–12. [PubMed] [Google Scholar]
- 8.Repo UK, Kentala E, Koistinen J , et al. Pulmonary apical fibrocystic disease.A serologic study. Eur J Respir Dis. 1981;62:46–55. [PubMed] [Google Scholar]
- 9.Fraisse P, Vandevenne A, Ducolone A, Burghard G. Idiopathic progressive pleuropulmonary fibrosis.Apropos of 2 cases. Rev Pneumol Clin. 1984;40(2):139–43. [PubMed] [Google Scholar]
- 10.Amitani R, Niimi A, Kuze F. Idiopathic pulmonary upper lobe fibrosis. Kokyu. 1992;11:693–9. [Google Scholar]
- 11.Kobayashi Y, Sakurai M, Kushiya M , et al. Idiopathic pulmonary fibrosis of the upper lobe: a case report. Nihon Kokyuki Gakkai Zasshi. 1999;37:812–6. [PubMed] [Google Scholar]
- 12.Jingu K, Kawana A, Furihata K , et al. Two cases of marked pulmonary fibrosis in the upper lung field. Kokyu. 1999;18:318–23. [Google Scholar]
- 13.Shiota S, Shimizu K, Suzuki M , et al. Seven cases of marked pulmonary fibrosis in the upper lobe. Nihon Kokyuki Gakkai Zasshi. 1999;37:87–96. [PubMed] [Google Scholar]
- 14.Kobashi Y, Ohba H, Yoneyama H , et al. A case of so-called idiopathic pulmonary upper lobe fibrosis complicated by both mediastinal emphysema and bilateral pneumothorax at different rimes. Kokyu. 2000;19:292–8. [Google Scholar]
- 15.Iwama N, Maehira N, Takahashi S, Chiba R. A case of idiopathic pulmonary upper lobe fibrosis. Shindan Byori. 2000;17:249–51. [Google Scholar]
- 16.Fuke S, Betsuyaku T, Oizumi S, Nasuhara Y, Saito H, Yamaguchi E, Nishimura M. A case of idiopathic pulmonary upper lobe fibrosis complicated by invasive pulmonary aspergillosis. Nihon Kokyuki Gaskkai Zasshi. 2003;41:196–201. [PubMed] [Google Scholar]
- 17.Kawabata Y, Matsuoka R. Pathology of idiopathic pulmonary upper lobe fibrosis. Nihon Kyobu Rinsho. 0000;62:S161–S202. [Google Scholar]
- 18.Iesato K, Ogasawara T, Masuda A , et al. Idiopathic pulmonary upper lobe fibrosis clinical and pathological features. Rinsho Houshasen. 2005;50:13–25. [Google Scholar]
- 19.Nei T, Kawamoto M, Satoh E , et al. A case of suspected idiopathic pulmonary upper lobe fibrosis (Amitani disease) with acute exacerbation. Nihon Kokyuki Gakkai Zasshi. 2009;47:116–21. [PubMed] [Google Scholar]
- 20.Kaneko Y, Kikuchi N, Ishii Y , et al. Upper lobe-dominant pulmonary fibrosis showing deposits of hard metal component in the fibrotic lesions. Intern Med. 2010;49:2143–5. doi: 10.2169/internalmedicine.49.3801. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto A, Mochizuki Y, Nakahara Y, Kawamura T, Sakaki S, Kobashi Y. A case of idiopathic pulmonary upper lobe fibrosis. Nihon Kokyuki Gakkai Zasshi. 2010;48:944–49. [PubMed] [Google Scholar]
- 22.Satoh S, Kawai S, Usui T, Isogai S, Taura S, Nakaminato S. A case of idiopathic pulmonary upper lobe fibrosis. Rinsho Hoshasen. 2010;55:211–4. [Google Scholar]
- 23.Watanabe K, Nagata N, Kitasato Y , et al. Rapid decrease in vital capacity in patients with idiopathic pulmonary upper lobe fibrosis. Respir Investig. 2012;50:88–97. doi: 10.1016/j.resinv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Nakasone E, Bando M, Nakao T, Yamasawa H, Sugiyama YM. Pleuroparenchymal fibroelastosis in patients with pulmonary disease after bone marrow transplantation. Nihon Kokyuki Gakkai Zasshi. 2012;1:562–6. [Google Scholar]
- 25.Nagashima O, Matsuno K, Tominaga S, Takahashi K. Esophageal diverticulum with idiopathic pulmonary upper lobe fibrosis. Intern Med. 2013;52(1):159. doi: 10.2169/internalmedicine.52.8984. [DOI] [PubMed] [Google Scholar]
- 26.Inuzuka K, Yasui M, Waseda Y, Takato H, Ichikawa Y, Fujimura M. A case of repeated bilateral pneumothorax associated with upper lobe predominat fibrosis in an aluminum processing worker. Nihon Kokyukigakkai Zasshi. 2010;48:492–6. [PubMed] [Google Scholar]
- 27.Becker CD, Gil J, Padilla ML. Idiopthic pleuroparenchymal fibroelstosis: an unrecognized or misdiagnosed entitiy?. Modern Pathol. 2008;21:784–7. doi: 10.1038/modpathol.2008.56. [DOI] [PubMed] [Google Scholar]
- 28.Von der Thusen JH, Hansell DM, Tominaga M , et al. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Modern Pathol. 2011;24:1633–9. doi: 10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 29.Piciucchi S, Tomassetti S, Gasoni G , et al. High resolution CT and histological findings in idiopathic pleuroparenchymal fibroelastosis: Features and differential diagnosis. Respir Res. 2011;12:111. doi: 10.1186/1465-9921-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy TL, Tominaga M, Hansell DM , et al. Pleuroparenchymal fibroelastosis, a spectrum of histopathological and imaging phenotypes. Eur Respir J. 2012;40:377–85. doi: 10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 31.Kusagaya H, Nakamura Y, Kono M , et al. Idiopathic pleuroparenchymal fibroelastosis: consideration of a clinicopathological entity in a series of Japanese patients. BMC Pulmonary Medicine. 2012;12:72. doi: 10.1186/1471-2466-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofek E, Sato M, Saito T , et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Modern Pathol. 2013;26:350–6. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 33.Hirota T, Fujita M, Matsumoto T , et al. Pleuroparenchymal fibroelastosis as a manifestation of chronic lung rejection?. Eur Respir J. 2013;41:243–5. doi: 10.1183/09031936.00103912. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society/European Respiratory Society International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Amer J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Watanabe K, Tamaru N , et al. A case of alminium-induced pulmonary fibrosis. Kokyu. 1989;8:435–9. [Google Scholar]
- 36.Singh R, Sundaram P, Joshi JM. Upper lobe fibrosis in ulcerative colitis. J assoc Physicians India. 2003;51:515–7. [PubMed] [Google Scholar]
- 37.Bourke S, Campbell J, Henderson AF, Stevenson RD. Apical pulmonary fibrosis in psoriasis. Br J Dis Chest. 1988;82:444–6. doi: 10.1016/0007-0971(88)90104-0. [DOI] [PubMed] [Google Scholar]
- 38.Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases, a concise review. Eur Respir J. 2001;17:122–32. doi: 10.1183/09031936.01.17101220. [DOI] [PubMed] [Google Scholar]
- 39.Hamada K, Nagai S, Kitaichi M , et al. Cyclophosphamide-induced late-onset lung disease. Intern Med. 2003;42:82–7. doi: 10.2169/internalmedicine.42.82. [DOI] [PubMed] [Google Scholar]
- 40.Konen Eli, Weisbrod GL, Pakhale S, Chung TB, Paul MS, Hutcheon MA. Fibrosis of the upper lobes: A newly identified late-onset complication after lung transplantation?. Amer J Roentgenol. 2003;181:1539–43. doi: 10.2214/ajr.181.6.1811539. [DOI] [PubMed] [Google Scholar]
- 41.Pakhale SS, Hadjiliadis D, Howell DN , et al. Upper lobe fibrosis: A novel manifestation of chronic allograft dysfunction in lung transplantation. J Heart Lung Transpl. 2005;24:1260–8. doi: 10.1016/j.healun.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Wick MR, Kendall TJ, Ritter JH. Asbestosis: demonstration of distinctive interstitial fibroelastosis.A pilot study. Ann Diag Pathol. 2009;13:297–302. doi: 10.1016/j.anndiagpath.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Calabrese F, Alessandrini L, Loy M , et al. Comparison between referral diagnosis of patients requiring transplantation and pathologic diagnosis of native lungs. J Heart Lung Transpl. 2009;28:1135–40. doi: 10.1016/j.healun.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Petrie GR, Bloomfield P, Grant IW, Crompton GK. Upper lobe fibrosis and cavitation in rheumatoid disease. Br J Dis Chest. 1980;74:263–7. doi: 10.1016/0007-0971(80)90053-4. [DOI] [PubMed] [Google Scholar]
- 45.Parish JM, Muhm JR, Leslie KO. Upper lobe pulmonary fibrosis associated with high-dose chemotherapy containing BCNU for bone marrow transplantation. Mayo Clin Proc. 2003;48:630–4. doi: 10.4065/78.5.630. [DOI] [PubMed] [Google Scholar]
- 46.Harada T, Yoshida Y, Kitasato Y , et al. Thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelstosis. Eur Respir Rev. 2013;in press doi: 10.1183/09059180.00006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax. 1974;29:695–8. doi: 10.1136/thx.29.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6 Chest. 1989;96:68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50:3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Zappala CJ, Latsi PI, Nicholson AG , et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:830–6. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 51.Negir EM, Montes GS, Saldiva PHN, Capelozzi VL. Architectural remodeling in acute and chronic interstitial lung disease, fibrosis or fibroelastosis. Histopathology. 2000;37(5):393–401. doi: 10.1046/j.1365-2559.2000.00992.x. [DOI] [PubMed] [Google Scholar]