Abstract
Ischemic stroke contributes to the majority of brain injuries and remains to be a leading cause of death and long-term disability. Despite the devastating pathology and high incidence of disease, there remain only few treatment options (tPA and endovascular procedures), which may be hampered by time dependent administration among a variety of other factors. Promising research of glutamate receptor antagonists has been unsuccessful in clinical trial. But, the mechanism by which glutamate receptors initiate injury by excessive calcium overload has spurred investigation of new and potentially successful candidates for stroke therapy. Acid sensing ion channels (ASICs) may contribute to poor stroke prognosis due to localized drop in brain pH, resulting in excessive calcium overload, independent of glutamate activation. Accumulating studies targeting ASICs have underscored the importance of understanding inhibition, regulation, desensitization and trafficking of this channel and its role in disease. This review will discuss potential directions in translational ASIC research for future stroke therapies.
Keywords: Acid Sensing Ion Channels (ASICs), Ischemic Stroke, Ion Channels, Neuronal Injury, Ca2+ Overload
Introduction
Advances in neuroscience research over the last several decades have increased our understanding of the ischemic brain injury, by elucidating complex vascular, cellular and molecular interactions which occur during stroke. Neuroprotection and identification of new therapeutic options have been a main focus of strategies for curbing brain injury. It has long been established that ion channels play a prominent role in brain injury during stroke. Particularly, the cause of neuronal injury by excessive postsynaptic activation of glutamate receptors, termed excitotoxcity, has been speculated to be a major cause of neuronal injury. The failure of clinical trials with the antagonists of these receptors, however, has indicated the glutamate-independent mechanism(s) of neuronal injury [16]. In this regard, acid sensing ion channels (ASICs) have been the subject of increasing interest due to their direct relationship with acidosis related neuronal injury and are considered a promising new target for therapeutic intervention of stroke.
To achieve clinical success when translating basic science research to the bedside, selection of a clinically relevant therapeutic target is necessary. Recent translational research and clinical trials involving glutamate antagonists and other agents have thus far been disappointing owing to inadequate margins of safety or failure to show efficacy, etc. [16]. However, ASICs have emerged as an exciting new target. In addition to developing new and specific small molecule inhibitors, ASIC translational studies may focus on key areas pertaining to its physiology such as structure-function relationships, kinetics and gating, and systemic investigation of ASIC modulators. Here we will discuss the potential directions in translational ASIC research covering ASIC inhibition, regulation, desensitization and trafficking.
Acid Sensing Ion Channels - ASICs
ASICs are proton (H+) gated channels that belong to the Degenerin/Epithelial Sodium Channel superfamily. There are four genes (ASIC1–4) in mammals that encode six distinct subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4 [1, 6, 8, 13, 20, 28, 33, 36, 49, 61, 78, 79]. Recently resolved crystal structure from chicken, Gallus galllus, shows that ASIC subunits assemble as trimers to form functional channels which can be homomeric, consisting of identical subunits, or heteromeric, consisting of different subunits [32, 37]. ASIC1a homomers, which are ubiquitously expressed in the nervous system, respond to low pH by mediating a fast and transient inward current with a threshold pH of ~7.0, and the pH for half maximal activation (pH0.5) at ~6.2 [79]. During ischemic stroke, hypoxia induces increased anaerobic glycolysis which leads to lactic acid accumulation thereby decreasing tissue pH. A pH of 6.5 can be achieved during normoglycemic conditions while hyperglycemic conditions decrease pH even further to 6.0 [3, 4, 29, 31]. These pH deviations are sufficient to activate homomeric ASIC1a as well as other ASIC1a containing channels expressed in the brain. ASICs, specifically ASIC1a, have been implicated in pathological conditions such as ischemic brain where activation of ASICs causes acidosis-mediated glutamate-independent neuronal injury [27, 57, 84, 85]. Further, during oxygen glucose deprivation (OGD) several striking functional ASIC1a changes occur: a moderate increase in current amplitude is observed and a decrease in ASIC desensitization [84]. These effects, in combination, potentiate toxic Ca2+ loading and suggest the possibility of chronic activation during ischemic conditions. Likewise concurrent activation of other molecules, such as Ca2+/ calmodulin kinase II, coupled with activation by N-methyl-D-aspartate (NMDA) receptors, subsequently enhances ASIC1a mediated neuronal death [27]. Thus, ASICs represent novel therapeutic targets for stroke intervention. Current translational stroke research may focus on approaches that limit the activity and expression of these channels.
ASIC Desensitization
The detailed molecular mechanisms that promote opening and closing events of ASICs are not well defined. However, we do know some basic properties of ASICs. At neutral pH, ASICs are closed and do not permeate ions. From this resting state they transition to an open, conducting, state when pH decreases below the threshold of activation. While open, their apparent residency time is dependent on proton affinity and subunit composition of the channel complex [9, 40]. As for ASIC1a, after an initial drop in pH activating the channel, the channel becomes desensitized and unresponsive to extracellular protons. In addition to rapid desensitization, one interesting phenomenon associated with ASIC1a is the display of significant rundown, or tachyphylaxis, where ever-smaller current amplitudes, even though the time interval between individual activation is sufficient for recovery, are observed with repeated doses of acid [15]. Another phenomenon, steady-state desensitization of ASICs, is induced when the channels are exposed to mild decreases in pH that are not sufficient for channel activation [5]. Agents that cause or promote desensitization of ASICs could possibly be an area of focus for future translational studies.
Knowledge of ASIC kinetics have been advanced by combining crystal structure information with molecular techniques [32]. So far there has been several studies which describe the crystallized protein visualized in the desensitized state, the low pH state, and with the inhibitor PcTX1 bound to ASIC1a [19, 32, 37]. However, there is not yet available structural information outlining the closed stated of the protein. With discovery of the crystal structure, it was found that three Cl− ions bind the extracellular loop. Cl− ion-exchange experiments have also proven impactful in elucidating ASIC kinetics. Replacing Cl− ions with impermeable anion, methanesulfonate (MeSO3−), has no overwhelming effect but causes ASIC1a to desensitize faster [44]. Increasing concentrations of Cl− are able to modulate the desensitization kinetics of ASIC1a in a concentration dependent manner [44].
ASIC1a and ASIC2 overlap in brain expression [2, 7, 66]. Desensitization kinetics is altered when ASIC1a subunits are combined with other subunits such as ASIC2b. When ASIC1a/2b heteromers are formed, the pH dependence of steady-state desensitization shifts to more alkaline pH (7.28 for ASIC1a/2b versus 7.18 for ASIC1a homomers) [66]. This shift in pH of desensitization results in a decrease of ASIC activity following only slight decreases in baseline pH; this decrease may be neuroprotective in neurons or regions expressing a higher amount of ASIC1a/2b heteromers. Importantly, activation of heteromeric ASIC1a/2b may also lead to intracellular Ca2+ overload and cell death [66], which was previously thought to be mediated only by homomeric ASIC1a. Recently, two desensitization states have been discovered in human ASIC1a channels, a short- and a long-lasting state [5, 48]. One form of desensitization is prevented by high frequency stimulation somewhat modeling usage dependent stimulation [48]. The duration of stimulation also determines desensitization properties. However, long term and repetitive stimulation induces gradual and irreversible loss of channel activity [48]. Human ASIC1a, having only eight residues different from chicken, displays many of the desensitization properties of mouse, rat and chicken ASIC1a [48]. The replacement of transmembrane domain 1 (TM1) of hASIC1a had the largest effect on the desensitization of currents in comparison to individual amino acid mutations [48].
Conversely, there are endogenous agents which can limit ASIC desensitization. Recently found, RFamide-related neuropeptides potentiate ASIC1a activity by preventing steady-state desensitization, an effect in opposition to the action of PcTx1 [67]. RF-amides represent a small family of neuropeptides that contain a C-terminal arginine-phenylalanine-amide consensus. The synthetic peptide FRRFamide, along with dynorphin opioid peptides, also prevents steady-state desensitization of mouse ASIC1a [68]. Dynorphins are among the most basic neuropeptides and are abundantly expressed in the central nervous system, including locations with high levels of ASIC1a implicated in regions affected by ischemic stroke [34]. Localized accumulation of dynorphin in regions affected by ischemic stroke is suggested to be the cause of increased neuronal damage due to increased ASIC activation and subsequent Ca2+ accumulation [68]. There remains a possibility that other endogenous peptides may exist which promote ASIC1a steady-state desensitization. New pharmacological agents that promote the desensitization of ASIC1a and ASIC1a/2b channels are thus expected to have significant therapeutic potential.
ASIC Inhibition
Non-specific Inhibitors- Amiloride
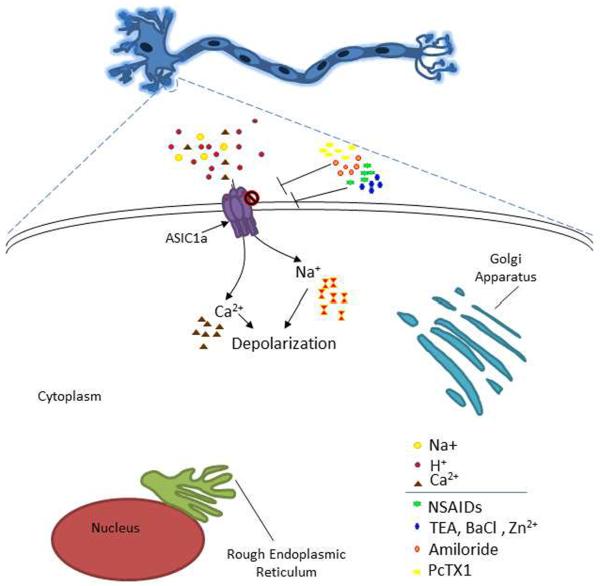
ASIC inhibition has been extensively studied in regard to neuroprotection (Figure 1). ASICs are inhibited by amiloride, an important pharmacological tool for studying all known members of the epithelial sodium channel/degenerin family of ion channels. It inhibits all subunit combinations of ASICs (with the exception of the sustained portion of ASIC3 current). As such, it is classified as a non-specific inhibitor of ASICs. Amiloride is effective at micromolar concentrations and inhibits ASICs in a concentration-dependent manner. Pharmacologic studies have found that ASIC1a has an IC50 for amiloride inhibition around 10 – 20 μM. When used in conjunction with other pharmacological or molecular biological tools, identification of physiologic and pathologic functions of ASICs can be assessed. One potential area of amiloride use is that of neuroprotection in stroke treatment. Intracerebroventricular injection of amiloride in mice before transient middle cerebral artery occlusion significantly decreases ischemic damage, presumably, due to the wide spread inhibition of ASIC channels [84].
Figure 1. ASIC1a as a target for stroke translational research.
Neuron with enlargement of soma displaying ASICs. When ASICs are activated by protons they conduct sodium and calcium into the cell. Activation of ASICs contributes to neuronal injury by increasing the intracellular concentration of calcium. In the presence of these inhibitors ASIC activity is diminished, attenuating ASIC mediated neurotoxicity. Amiloride is a non- specific inhibitor of ASIC subunits and other ion channels. NSAIDs are non-specific drugs, however Ibuprofen and Flurbiprofen are also inhibitors of ASIC. Please see section “ASIC Inhibitors”.
Nonsteroid anti-inflammatory drugs
Like amiloride, nonsteroid anti-inflammatory drugs (NSAIDs) are non-specific inhibitors of ASICs [75]. NSAIDs block some H+-gated channels [76]. Aspirin, in combination with Clopidogrel, is used as a preventative therapy for stroke [73]. The effect is likely due to the inhibition of clot formation in blood, however, a recent study has suggested that high dose of aspirin inhibits acid-induced neuronal injury mediated by ASICs [80]. Other NSAIDs such as Ibuprofen and flurbiprofen are active against ASIC1a channels [23, 76]. Another drug, Diclofenac, also inhibits ASIC1a homomers and ASIC1a containing heteromeric channels. A key consideration for the use of anti-inflammatory drugs in neuroprotection and stroke is that inflammation increases ASIC expression [76]. Perhaps the use of NSAIDs or other anti-inflammatory drugs will limit ASIC1a expression and thereby decrease ASIC mediated-neuronal injury.
Tetraethylammonium and Barium
Other non-specific molecules, the nonselective potassium channel blockers tetraethylammonium (TEA) and BaCl2 (barium), also inhibit ASICs. Recently, these agents were used to distinguish between ASIC1a homomers and ASIC1a/2b heteromeric channels [66]. Importantly, this study determined that ASIC1a/2b heteromeric channels, like homomeric ASIC1a, are also permeable to Ca2+ thus implicating this channel in acidosis-mediated neuronal injury and neuronal death [66]. It was found that ASIC1a was unaffected by 10 mM TEA or barium but ASIC1a/2b heteromeric channels were inhibited by TEA; barium caused more appreciable decrease in the activity of ASIC1a/2b heteromeric channels [66]. TEA has not yet been evaluated for neuroprotective or therapeutic benefit in ischemic stroke. Barium is toxic and therefore is not suitable for animal or human studies.
Zinc
Abnormal zinc homeostasis has been shown to be involved in pathological conditions [10, 65]. Insults that result from traumatic brain injury, brain ischemia and epilepsy, for example, contribute to excessive Zn2+ levels [10, 41, 72]. While others [53] have found that there are significantly lower levels of serum zinc in the blood of stroke patients and that the low level of extracellular zinc may be a risk factor for stroke [53]. Zinc inhibits homomeric ASIC1a and heteromeric ASIC1a/2a with nanomolar affinity [18]. Therefore, high levels of zinc released during pathological conditions may limit ASIC activation. Animal models have shown that zinc injection attenuates hyperalgesia and reduced neuronal overexcitation [45]. Perhaps therapeutics aimed at limiting zinc reuptake from synaptic terminals, while inhibiting intracellular zinc toxic pathways [72], may enhance neuroprotection during ischemic stroke.
Subunit-Specific Inhibitors - Psalmotoxin 1
Specific inhibition of ASICs can be achieved by the use of subunit specific antagonist. Psalmotoxin 1 (PcTx1), isolated from the venom of South American tarantulas (Psalmopoeus cambridgei), is a specific inhibitor for ASIC1a homomeric channels. It has been recently suggested that this compound also inhibits ASIC1a/2b configuration [66]. PcTX1 is a potent inhibitor of ASIC1a with an IC50 of approximately 0.9 nM. Though not a “classical” inhibitor, it functions as a gating modifier inhibiting ASIC1a and ASIC1a/2b channels by shifting the pH dependence of the steady-state desensitization to more alkaline pHs such that the channels desensitize at basal pH 7.4 [14]. In neurons, PcTX1 prevents calcium overload initiated by ASIC1a channels and also limits neuronal firing. Translational animal studies have shown that intracerebroventricular injection of PcTX1, up to five hours post ischemia, significantly reduces MCAO mediated brain injury [57]. However, intracerebroventricular delivery of the peptide is not within clinical guidelines and the efficacy of intravenous injections needs to be rigorously evaluated in animals. Interestingly, intranasal injections have proven to be successful. Combination therapy with N-methyl-D-aspartate receptor agonists, like memantine, enhances the neuroprotective effect of PcTX1 [57].
ASIC Regulation
There may be other translational strategies by which targeting ASICs may lead to the attenuation of brain injury. The novel targets below attest that regulatory mechanisms may contribute significantly to ASIC down regulation and would be advantageous for therapeutic development (Figure 2). The readers may find additional information on ASIC regulators and/or modulators elsewhere [17].
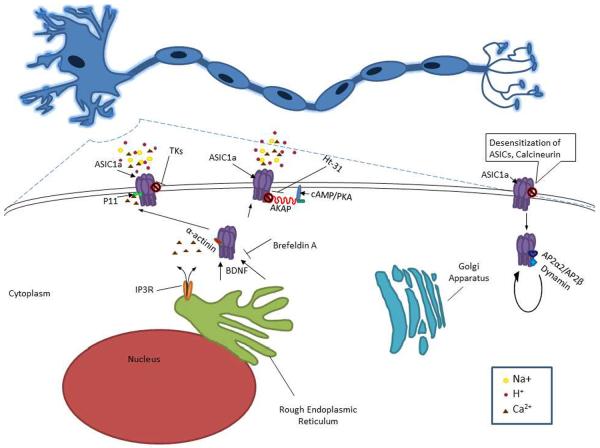
Figure 2. Main pathways/proteins involved in the regulation and/or trafficking of ASICs.
Tissue Kallikreins (TKs) decrease ASIC1a activity through channel cleavage. P11 interacts with ASIC1a and is reported to have a role in trafficking and alterations of current density. α-Actinins assemble membrane proteins and signaling molecules into macromolecule complexes and attach membrane proteins to the actin cytoskeleton. ASIC1a interaction with α-actinin influences its sensitivity to extracellular protons. A-Kinase Anchoring Proteins (AKAPs) are a family of intracellular scaffolding proteins that tether cAMP-dependent protein kinase A (PKA) to cellular membranes and organelles orchestrating signaling and other second-messenger mediated processes. When AKAP79/150 is disrupted with Ht-31 inhibitor peptide it significantly limits ASIC current conductance. Please see section “ASIC Regulation”.
Calcineurin
Calcineurin is a ubiquitous enzyme that is present at high levels in the brain [42]. Calcineurin has been shown to interact and regulate the activity of ASIC1a and ASIC2a [12]. Inhibition of calcineurin with Cyclosporin A, for example, causes an increase in ASIC current suggesting that calcineurin-dependent dephosphorylation is involved in deactivating ASICs [12]. Thus, calcinurin and perhaps other molecules involved in phosphorylation/dephosphorylation might be future targets for stroke research.
Tissue Kallikrein
Tissue Kallikreins (TK) are serine proteases, which free bradykinin (BK) and kallidin from kininogens by activating Bradykinin B2 receptors[35]. Previous studies have demonstrated extracellular and intracellular proteases' abilities to either activate or inactivate ENaC [30, 46, 74]. Similarly, serine proteases such as trypsin, chymotrypsin and proteinase K have been shown to modulate the activity of ASIC1a [58], through channel cleavage [77]. A recent study has shown that TK activity can be inducible during cerebral ischemia and protects cortical neurons from acidosis-related neuronal injury [50]. This neuroprotection may be mediated by ASICs. In ASIC1a-transfected cells, high concentrations of TK (3 μM) show a significant decrease in acidosis-induced LDH release and improvements in cell viability [71]. Aprotinin, a protease inhibitor, can eliminate the effects of TK on LDH release in ASIC1a-transfected-cells, indicating that proteolytic activity of TK is prerequisite for the regulation of ASIC1a [71]. It would be interesting to know whether exogenous administration of serine proteases such as TK could have neuroprotective effects during cerebral ischemia in whole animals.
p11
p11, is a member of the S100 calcium binding protein family and it exhibits two EF-hand Ca2+ binding sites [22]. S100 are involved in many cellular processes such as calcium homeostasis, phosphorylation, transcription factor regulation, inflammatory response, cAMP signaling, cytoskeletal dynamics, and cell proliferation and differentiation [86]. p11 is expressed in nerve tissues expressing ASIC1a. It has no enzymatic activities [86] and does not require calcium-dependent dimerization to become active [62]. Two mutations in the EF-hands makes p11 active [62]. Yeast two-hybrid assay indicates an interaction between p11 and ASIC1a, which is specific to this subunit [22]. p11 is reported to have a role in the trafficking of ion channels [22]. For example, p11 interacts with Nav1.8, TASK-1, TRPV5, and TRPV6 and regulate their plasma membrane expression. Similarly, p11 promotes plasma membrane expression of ASIC1a. In the presence of p11, the intensity of plasma membrane fluorescence corresponding with ASIC1a increases as well as ASIC1a-FLAG protein located at the cell surface [22]. p11 alters ASIC1a current density while activation and desensitization remain unchanged [22]. The interaction between p11 and ASIC1a could be a potential therapeutic target for cerebral ischemia.
α-Actinins
α-Actinins assemble membrane proteins and signaling molecules into macromolecules complexes and attach membrane proteins to the actin cytoskeleton [54]. α-Actinins are encoded by four genes: α-actinin-1, α-actinin-2,α-actinin-3, and α-actinin-4 isoforms [54]. α-Actinins- 1, -2, and -4 are located in dendritic spines, primarily at the postsynaptic membrane [83]. α–Actinins can regulate membrane trafficking and the functions of cation channels such as L-type calcium channels, potassium channels and TRP channels [47, 52, 63]. In neuronal synapses, α-actinin plays a role in the localization and inactivation of N-methyl-D-aspartate receptor for glutamate [54].
A recent study has shown that α-actinin also binds to ASIC1a and modulates the channel function [64]. α-actinin binding motif is located in the C-terminus of ASIC1a and lies in the cytosolic portion of the subunit, allowing accessibility to other cytoskeleton proteins [64]. α-actinin binding sites are only present in ASIC1 but not in other ASIC isoforms. ASIC1a interacts with α-actinin-1 and α-actinin-4 [64]. α-actinin-4 does not affect cell surface expression of ASIC1a but reduces ASIC1a current density and increases the pH sensitivity [64]. It was not clear whether the reduction in current density is due to increased steady-state desensitization. In CHO cells, co-expression of α-actinin-4 with ASIC1a accelerates the recovery of the channel from desensitization. On the contrary, rod-actinin, a dominant negative construct produces opposite effects such as increased ASIC1a current and decreased pH sensitivity [64]. The association between α-actinin and ASIC1a could have implications in neuronal injury during cerebral ischemia.
AKAP79/150
A-Kinase Anchoring Proteins (AKAPs) are a family of intracellular scaffolding proteins that tether cAMP-dependent protein kinase A (PKA) to cellular membranes and organelles and orchestrate signaling and other second-messenger mediated processes [51, 82]. Chai and colleagues reported that the N or C terminus of these channels is also involved in the regulation of function or surface expression of ASICs [12]. They were able to show that AKAP79/150 interacts with ASIC1a and ASIC2a as well as being co-localized to dendrites. When the AKAP79/150 is disrupted with Ht-31 inhibitor peptide it significantly limits ASIC current [12]. Thus, targeting the interaction of AKAP79/150 and ASIC1a could be a potential therapeutic strategy for stroke.
Genetic Modification
Genetic manipulations have been useful in studying the function of ASICs. Targeted disruption of ASIC subunits in vivo [9, 26, 39, 43, 84] has allowed for the observation of changes in acid-evoked currents in various CNS/PNS neurons and the impact on the pathological outcomes yielding insight to the functions of the specific subunit. In a mouse model of multiple sclerosis, Friese and colleagues observed a decrease in clinical deficit in ASIC1a−/− mice associated with reduced axonal degeneration in comparison with WT mice [26]. In a transient focal ischemia model, in which the middle cerebral artery was occluded, targeted disruption of ASIC1a−/− provided significant neuroprotection with incremental decreases in infarct volume seen in ASIC1a+/− and ASIC1a−/− animals [84]. Others have utilized KO animals in conjunction with pharmacologic tools to evaluate the composition and physiology of ASICs in specific brain regions, such as the hippocampus [66]. Additionally, RNA interference (RNAi) has been used to silence ASIC gene expression through short hairpin RNA (shRNA) or small interfering RNA (siRNA) [21, 66]. Likewise, transfection and overexpression of specific ASIC subunit using expression systems have also proven to be helpful [9]. The recently resolved crystal structure of ASIC1a has given insight to the structural topology of the protein and more importantly has led to identification of key residues which contribute to alteration of channel gating and kinetics. Thus, mutations of specific residues and chimeras have enabled detailed investigation of ASIC function and regulation. In addition to targeting ASIC directly, acidosis-induced neurotoxicity has been investigated by siRNA targeted to other regulatory proteins, e.g. adaptor protein 2, a membrane protein that interacts with clathrin and promotes endocytosis of ASIC1a [87].
ASIC Trafficking
Constitutive Endocytosis
Clathrin-mediated endocytosis is a process by which specific cargoes (such as ion channels) are internalized from the plasma membrane into clathrin-coated vesicles [70]. Membrane protein trafficking pathways generally originate from the endoplasmic reticulum (ER) and, via the Golgi, are either antero-trafficked, towards the cell membrane, or retrograde trafficked back to the ER. The process itself can be branched, rapidly or slowly carried out and even bidirectional. Endocytosis is an important factor in cell surface expression for several receptors and ion channels. Within individual neurons, the subcellular distribution of ASIC1a and ASIC2a is preferentially located to the postsynaptic membrane at terminal and also has somatodendritic localization [25]. Trafficking of ASIC1a to the plasma membrane increases the density of dendritic spines, whereas other ASIC subtypes did not see similar increases [86]. Antero-trafficking of ASIC1a is critical for acidosis mediated injury especially when increased dendritic levels prevail, leading to spine loss [38]. Thus, membrane trafficking process is fundamental to cellular homeostasis. Alteration of this process may be implicated in ASIC mediated pathology.
AP2α2/β1
ASIC1a is usually localized to the ER in neurons and Chinese Hamster Ovarian cells (CHO) cells and is regulated by ER retention mechanisms that preserve a reservoir for surface delivery of ASIC1a [11]. ASIC1a undergoes constitutive endocytosis in CHO cells and cultured cortical neurons. GST pull-down based mass spectroscopy identified adaptor protein 2 as interacting protein that might be regulating ASIC1a function [86]. Adaptor protein 2 is a heterotetrametric clathrin adaptor that is associated with coated pits at the plasma membrane [69]. AP2 binds to the cytoplasmic domains of receptors and attaches membrane proteins to clathrin, promoting assembly of coated pits. The identification of AP2α2 and AP2β1 proteins that binds to ASIC1a c-terminus indicates that ASIC1a undergoes endocytosis via clathrin-dependent pathway and supports findings that endocytosis of ENaC is clathrin-dependent [81].
Dynamin/Dynasore
Dynamin works in vesicle scission reactions and aids in the detachment of clathrin-coated pits [59]. ASIC1a endocytosis is driven by clathrin-mediated and dynamin dependent processes in which the binding of AP2 to the c-terminus of ASIC1a imitates removal of the protein from the membrane [86]. Dynasore, a dynamin inhibitor, blocks ASIC1a endocytosis and increases surface retention of ASIC1a shows that ASIC1a is internalized via dynamin-dependent pathway [86]. The reduction of clathrin-dependent endocytosis of ASIC1a during neurodegeneration could lead to severe acidosis-induced neuronal injury [86]. This was demonstrated by the finding that dynasore pretreatment resulted in an significant increase of acidosis-induced cell damages [86]. Thus, enhancing dynamin-mediated endocytosis of ASIC1a channels could provide protection to neurons during acidosis while retention of ASIC1a protein during acidosis is expected to enhance acidosis-induced neuronal injury.
Trafficking Inhibition
Inhibition of ER transport proteins may be a potential mechanism by which ASIC surface expression is limited. Brefeldin A, an inhibitor of protein trafficking, prevents the surface accumulation of ASIC1a thus suggesting that ASIC1a is trafficked from the ER to the cell surface [11]. As anticipated, increased ASIC1a surface trafficking potentiates acidosis-induced neuronal death [86]. It has been shown that forward trafficking of ASIC1a to the cell membrane is enhanced by BNDF in central nervous system neurons [24]. Blocking related downstream pathways like, TrKB and PI3 kinase, abolishes the enhancing effect of BDNF recruitment of ASIC1a to the cell surface [24]. Therefore, blocking BDNF or its downstream pathways may reduce ASIC1a-mediated injury. Conversely, enhancing endocytosis of ASIC1a would also limit acidosis mediated cellular injury. In contrast, inhibition of ASIC1a internalization by Tyr A23, a small molecular inhibitor of clathrin-dependent endocytosis, perpetuates neuronal injury [86]. Perhaps it is possible to target more ER related trafficking mechanisms to prevent acidosis mediated cell injury.
Insulin
ASICs have already been known to be substantially potentiated during in vitro cell culture models of ischemia, like that of oxygen and glucose deprivation [84]. Hyperglycemia, a condition of elevated blood glucose, exacerbates ischemic stroke [60]. It is not clear whether this occurs through ASIC activation. In diabetes, low levels of serum insulin may be a key factor in the increased surface expression of ASIC1a [11]. Therefore, in dually prevalent situations where ischemia and low serum insulin levels are manifested, there is the potential for increased neuronal injury beyond that of typical ischemic stroke possibly due to increased surface expression and activity of ASIC1a. [11].
Summary and Conclusion
Ischemic stroke is a devastating neurologic injury and a leading cause of death and long term disabilities among other neurologic diseases. Ion channels are now well established in ischemic stroke pathology. Pharmacologic agents such as amiloride, NSAIDs, and Zinc are invaluable tools to study ASICs which can modulate ASIC properties (Figure 1). Still, translational studies are not without difficulties; there are limited pharmacologic agents, like PcTX1, that offer specific ASIC subunit inhibition that may be exploited for therapeutic benefit. New avenues have yet to be thoroughly investigated and are at the forefront of ASIC and neuroprotection. One such emerging translational strategy to limit neuronal injury may be the modulation of ASIC activity, for example, taking advantage of molecular mechanisms regulating desensitization. Further exploration to find endogenous antagonist remains to be done. Alterations in the regulatory machinery leading to a decrease in expression or activation during an ischemic event would improve stroke prognosis. A series of molecules, such as AKAP, are closely linked to ASIC regulation and trafficking (Figure 2). Comprehension of ASIC physiology and pathology will be necessary to translate findings, novel and existing drugs to progress therapeutic advances.
Acknowledgments
The work in ZGX's laboratory was partially supported by NIH R01NS066027, NIMHD S21MD000101, U54 NS083932, AHA 0840132N, and ALZ IIRG-10-173350.
Footnotes
Disclosure: Zaven O'Bryant declares no conflict of interest.
Kiara Vann declares no conflict of interest.
Zhi-Gang Xiong declares no conflict of interest.
Reference List
- 1.Akopian ANFAU, Chen CCFAU, Ding YF, Cesare PF, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–22. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 2.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 3.Auer RN. Progress review: hypoglycemic brain damage. Stroke. 1986;17:699–708. doi: 10.1161/01.str.17.4.699. [DOI] [PubMed] [Google Scholar]
- 4.Auer RN, Siesjo BK. Hypoglycaemia: brain neurochemistry and neuropathology. Baillieres Clin Endocrinol Metab. 1993;7:611–625. doi: 10.1016/s0950-351x(05)80210-1. [DOI] [PubMed] [Google Scholar]
- 5.Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 6.Babinski KF, Le KTFAU, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–7. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 7.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassler EL FAU - Ngo-Anh, Ngo-Anh TJ FAU - Geisler, Geisler HSFAU, Ruppersberg JPFAU, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–7. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 9.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capasso M, Jeng JM, Malavolta M, Mocchegiani E, Sensi SL. Zinc dyshomeostasis: a key modulator of neuronal injury. J Alzheimers Dis. 2005;8:93–108. doi: 10.3233/jad-2005-8202. [DOI] [PubMed] [Google Scholar]
- 11.Chai S, Li M, Branigan D, Xiong ZG, Simon RP. Activation of acid-sensing ion channel 1a (ASIC1a) by surface trafficking. J Biol Chem. 2010;285:13002–13011. doi: 10.1074/jbc.M109.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai S, Li M, Lan J, Xiong ZG, Saugstad JA, Simon RP. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem. 2007;282:22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CCFAU, England S FAU - Akopian, Akopian ANFAU, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–5. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Kalbacher H, Gründer S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Grunder S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J Physiol. 2007;579:657–670. doi: 10.1113/jphysiol.2006.120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng YD, Al-Khory L, Zivin JA. Neuroprotection for Ischemic Stroke: Two Decades of Success and Failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu XP, Papasian CJ, Wang JQ, Xiong ZG. Modulation of acid-sensing ion channels: molecular mechanisms and therapeutic potential. Int J Physiol Pathophysiol Pharmacol. 2011;3:288–309. [PMC free article] [PubMed] [Google Scholar]
- 18.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson RJ, Benz J, Stohler P, Tetaz T, Joseph C, Huber S, Schmid G, Hügin D, Pflimlin P, Trube G, Rudolph MG, Hennig M, Ruf A. Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat Commun. 2012;3:1–8. doi: 10.1038/ncomms1917. [DOI] [PubMed] [Google Scholar]
- 20.de Weille JR FAU - Bassilana, Bassilana FF, Lazdunski MF, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 1998;433:257–60. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 21.Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem. 2005;280:38666–38672. doi: 10.1074/jbc.M505981200. [DOI] [PubMed] [Google Scholar]
- 23.Dorofeeva NA, Barygin OI, Staruschenko A, Bolshakov KV, Magazanik LG. Mechanisms of non-steroid anti-inflammatory drugs action on ASICs expressed in hippocampal interneurons. J Neurochem. 2008;106:429–441. doi: 10.1111/j.1471-4159.2008.05412.x. [DOI] [PubMed] [Google Scholar]
- 24.Duan B, Liu DS, Huang Y, Zeng WZ, Wang X, Yu H, Zhu MX, Chen ZY, Xu TL. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci. 2012;32:6351–6363. doi: 10.1523/JNEUROSCI.4479-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277:5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 26.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2012;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA Receptor and Acid-Sensing Ion Channel Contributes to Ischemic Neuronal Death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Anoveros JF, Derfler BF, Neville-Golden JF, Hyman BTFAU, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–64. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267–273. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 30.Garty H, Edelman IS. Amiloride-sensitive trypsinization of apical sodium channels. Analysis of hormonal regulation of sodium transport in toad bladder. The Journal of General Physiology. 1983;81:785–803. doi: 10.1085/jgp.81.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilmore RM, Stead LG. The Role of Hyperglycemia in Acute Ischemic Stroke. Neurocritical Care. 2006;5:153–158. doi: 10.1385/ncc:5:2:153. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunder S FAU - Geissler, Geissler HSFAU, Bassler ELFAU, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–11. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 34.Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–35. doi: 10.2741/1522. Print;%2005 Jan 1.:216-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecquet C, Tan F, Marcic BM, Erdos EG. Human bradykinin B(2) receptor is activated by kallikrein and other serine proteases. Mol Pharmacol. 2000;58:828–836. doi: 10.1124/mol.58.4.828. [DOI] [PubMed] [Google Scholar]
- 36.Ishibashi KF, Marumo F. Molecular cloning of a DEG/ENaC sodium channel cDNA from human testis. [DOI] [PubMed]
- 37.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 38.Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci. 2012;32:4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS One. 2012;7:e35225. doi: 10.1371/journal.pone.0035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 41.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 42.Kuno T, Mukai H, Ito A, Chang CD, Kishima K, Saito N, Tanaka C. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem. 1992;58:1643–1651. doi: 10.1111/j.1471-4159.1992.tb10036.x. [DOI] [PubMed] [Google Scholar]
- 43.Kusama N, Gautam M, Harding AM, Snyder PM, Benson CJ. Acid-sensing ion channels (ASICs) are differentially modulated by anions dependent upon their subunit composition. Am J Physiol Cell Physiol. 2012:1–35. doi: 10.1152/ajpcell.00216.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusama N, Harding AM, Benson CJ. Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a) J Biol Chem. 2010;285:17425–17431. doi: 10.1074/jbc.M109.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson AA, Kitto KF. Manipulations of zinc in the spinal cord, by intrathecal injection of zinc chloride, disodium-calcium-EDTA, or dipicolinic acid, alter nociceptive activity in mice. J Pharmacol Exp Ther. 1997;282:1319–1325. [PubMed] [Google Scholar]
- 46.Lewis SA, Alles WP. Urinary kallikrein: a physiological regulator of epithelial Na+ absorption. Proceedings of the National Academy of Sciences. 1986;83:5345–5348. doi: 10.1073/pnas.83.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q, Dai XQ, Shen PY, Wu Y, Long W, Chen CX, Hussain Z, Wang S, Chen XZ. Direct binding of alpha-actinin enhances TRPP3 channel activity. J Neurochem. 2007;103:2391–2400. doi: 10.1111/j.1471-4159.2007.04940.x. [DOI] [PubMed] [Google Scholar]
- 48.Li T, Yang Y, Canessa CM. Impact of recovery from desensitization on acid-sensing ion channel-1a (ASIC1a) current and response to high frequency stimulation. J Biol Chem. 2012;287:40680–40689. doi: 10.1074/jbc.M112.418400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lingueglia E FAU - de Weille, de Weille JR FAU - Bassilana, Bassilana FF, Heurteaux CF, Sakai HF, Waldmann RF, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–83. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Zhang R, Liu K, Zhou H, Yang X, Liu X, Tang M, Su J, Dong Q. Tissue kallikrein protects cortical neurons against in vitro ischemia-acidosis/reperfusion-induced injury through the ERK1/2 pathway. Exp Neurol. 2009;219:453–465. doi: 10.1016/j.expneurol.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Logue JS, Scott JD. Organizing signal transduction through A-kinase anchoring proteins (AKAPs) FEBS J. 2010;277:4370–4375. doi: 10.1111/j.1742-4658.2010.07866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maruoka ND, Steele DF, Au BP, Dan P, Zhang X, Moore ED, Fedida D. alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells. FEBS Lett. 2000;473:188–194. doi: 10.1016/s0014-5793(00)01521-0. [DOI] [PubMed] [Google Scholar]
- 53.Munshi A, Babu S, Kaul S, Shafi G, Rajeshwar K, Alladi S, Jyothy A. Depletion of serum zinc in ischemic stroke patients. Methods Find Exp Clin Pharmacol. 2010;32:433–436. doi: 10.1358/mf.2010.32.6.1487084. [DOI] [PubMed] [Google Scholar]
- 54.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 55.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 56.Pignataro G, Cuomo O, Esposito E, Sirabella R, Di RG, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 58.Poirot OF, Vukicevic MF, Boesch AF, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem. 2004;279:38448–57. doi: 10.1074/jbc.M407381200. [DOI] [PubMed] [Google Scholar]
- 59.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 60.Prakash A, Matta BF. Hyperglycaemia and neurological injury. Curr Opin Anaesthesiol. 2008;21:565–569. doi: 10.1097/ACO.0b013e32830f44e4. [DOI] [PubMed] [Google Scholar]
- 61.Price MPFAU, Snyder PMFAU, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. [DOI] [PubMed]
- 62.Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, Russo-Marie F, Lewit-Bentley A. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat Struct Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- 63.Sadeghi A, Doyle AD, Johnson BD. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins alpha-actinin and dystrophin. Am J Physiol Cell Physiol. 2002;282:C1502–C1511. doi: 10.1152/ajpcell.00435.2001. [DOI] [PubMed] [Google Scholar]
- 64.Schnizler MK, Schnizler K, Zha XM, Hall DD, Wemmie JA, Hell JW, Welsh MJ. The cytoskeletal protein alpha-actinin regulates acid-sensing ion channel 1a through a C-terminal interaction. J Biol Chem. 2009;284:2697–2705. doi: 10.1074/jbc.M805110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. J Neurosci. 2011;31:16076–16085. doi: 10.1523/JNEUROSCI.3454-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric Acid-Sensing Ion Channels (ASICs) Composed of ASIC2b and ASIC1a Display Novel Channel Properties and Contribute to Acidosis-Induced Neuronal Death. The Journal of Neuroscience. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherwood TW, Askwith CC. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J Biol Chem. 2008;283:1818–1830. doi: 10.1074/jbc.M705118200. [DOI] [PubMed] [Google Scholar]
- 68.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smythe E. Regulating the clathrin-coated vesicle cycle by AP2 subunit phosphorylation. Trends Cell Biol. 2002;12:352–354. doi: 10.1016/s0962-8924(02)02333-4. [DOI] [PubMed] [Google Scholar]
- 70.Sorkin A, von ZM. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su J, Tang Y, Liu L, Zhou H, Dong Q. Regulation of acid-sensing ion channel 1a function by tissue kallikrein may be through channel cleavage. Neurosci Lett. 2011;490:46–51. doi: 10.1016/j.neulet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 72.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 73.Thomson RM, Anderson DC. Aspirin and clopidogrel for prevention of ischemic stroke. Curr Neurol Neurosci Rep. 2013;13:327–0327. doi: 10.1007/s11910-012-0327-y. [DOI] [PubMed] [Google Scholar]
- 74.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 75.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs) Curr Drug Targets Inflamm Allergy. 2004;3:71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 76.Voilley N, de WJ, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vukicevic M, Weder G, Boillat Al, Boesch A, Kellenberger S. Trypsin Cleaves Acid-sensing Ion Channel 1a in a Domain That Is Critical for Channel Gating. Journal of Biological Chemistry. 2006;281:714–722. doi: 10.1074/jbc.M510472200. [DOI] [PubMed] [Google Scholar]
- 78.Waldmann RF, Champigny GF, Voilley NF, Lauritzen IF, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. [DOI] [PubMed]
- 79.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Ye SD, Zhou KQ, Wu LM, Huang Y. High Doses of Salicylate and Aspirin Are Inhibitory on Acid-Sensing Ion Channels and Protective Against Acidosis-Induced Neuronal Injury in the Rat Cortical Neuron. Journal of Neuroscience Research. 2011;90:267–277. doi: 10.1002/jnr.22742. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281:14129–14135. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 82.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiong ZG. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels (2004) Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 85.Xiong ZG, Chu XP, Simon RP. Ca2+ -permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209:59–68. doi: 10.1007/s00232-005-0840-x. [DOI] [PubMed] [Google Scholar]
- 86.Zeng WZ, Liu DS, Duan B, Song XL, Wang X, Wei D, Jiang W, Zhu MX, Li Y, Xu TL. Molecular mechanism of constitutive endocytosis of Acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death. J Neurosci. 2013;33:7066–7078. doi: 10.1523/JNEUROSCI.5206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng WZ, Liu DS, Duan B, Song XL, Wang X, Wei D, Jiang W, Zhu MX, Li Y, Xu TL. Molecular mechanism of constitutive endocytosis of Acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death. J Neurosci. 2013;33:7066–7078. doi: 10.1523/JNEUROSCI.5206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]