Abstract
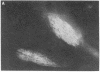
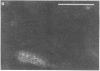
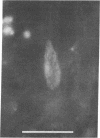
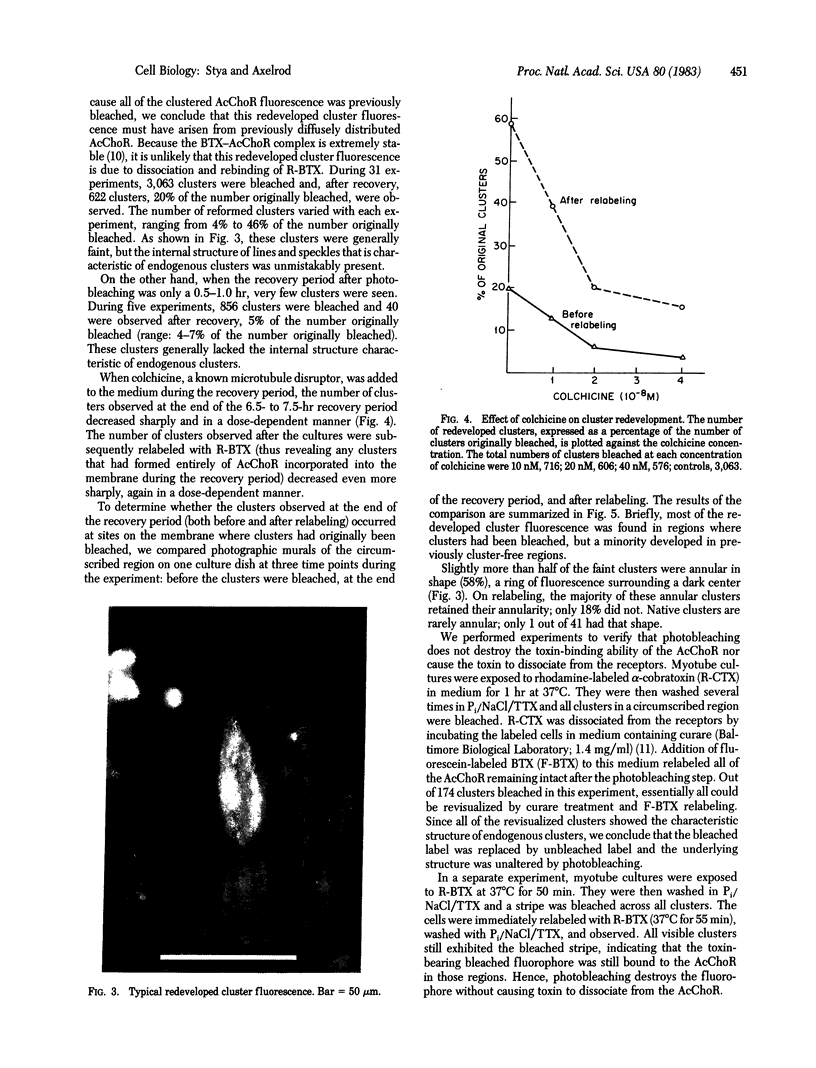
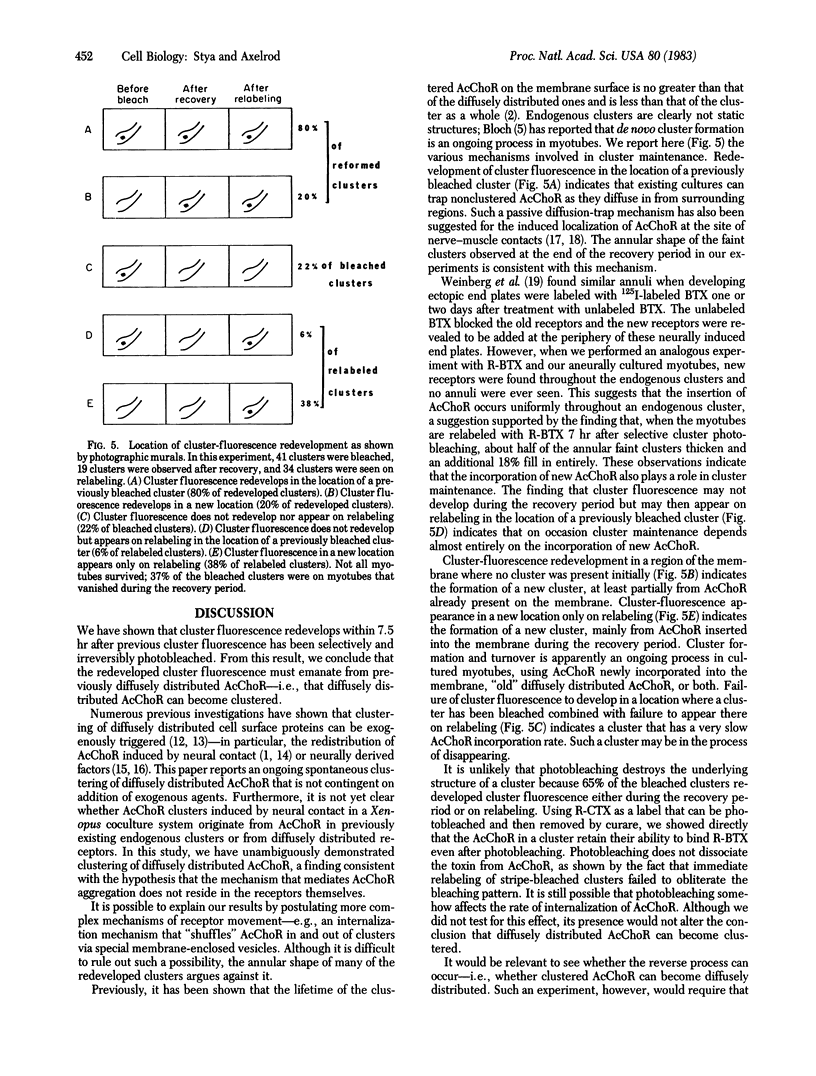
On aneurally cultured rat primary myotubes, 10% of the acetylcholine receptors (AcChoR) are found to be aggregated and immobilized in endogenous clusters while the remainder are diffusely distributed and partially mobile. This paper reports that AcChoR in clusters can be gathered from AcChoR in diffuse areas during the course of normal myotube development. AcChoR were fluorescently labeled with rhodamine-conjugated alpha-bungarotoxin, and all existing clusters in a circumscribed region of the culture dish were irreversibly photobleached by a slightly defocused laser beam, the movement of which was controlled by a lens mounted on a joystick translator. This procedure leaves intact only the fluorescent label on the diffusely distributed AcChoR. Observation of the myotubes after several hours of incubation revealed cluster fluorescence redevelopment. This cluster fluorescence must have consisted of AcChoR that previously were diffusely distributed. The majority (but not all) of cluster fluorescence redevelopment occurred in the location of a previously bleached cluster. About half of the redeveloped clusters have an annular shape. The major conclusions of this study are (i) diffusely distributed AcChoR can become clustered; (ii) endogenous clusters appear to form, at least in part, by "trapping" receptors as they diffuse in from surrounding regions; (iii) cluster formation is an ongoing process in cultured rat myotubes; and (iv) colchicine (a microtubule-disrupting agent) inhibits cluster formation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Crosslinkage and visualization of acetylcholine receptors on myotubes with biotinylated alpha-bungarotoxin and fluorescent avidin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4823–4827. doi: 10.1073/pnas.77.8.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P. M., Podleski T. R. Control of acetylcholine receptor mobility and distribution in cultured muscle membranes. A fluorescence study. Biochim Biophys Acta. 1978 Jul 20;511(1):23–38. doi: 10.1016/0005-2736(78)90062-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R. J. Dispersal and reformation of acetylcholine receptor clusters of cultured rat myotubes treated with inhibitors of energy metabolism. J Cell Biol. 1979 Sep;82(3):626–643. doi: 10.1083/jcb.82.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R. J., Geiger B. The localization of acetylcholine receptor clusters in areas of cell-substrate contact in cultures of rat myotubes. Cell. 1980 Aug;21(1):25–35. doi: 10.1016/0092-8674(80)90111-7. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Frisch H. L. A model for the localization of acetylcholine receptors at the muscle endplate. J Neurobiol. 1976 Jul;7(4):377–381. doi: 10.1002/neu.480070409. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleski T. R., Axelrod D., Ravdin P., Greenberg I., Johnson M. M., Salpeter M. M. Nerve extract induces increase and redistribution of acetylcholine receptors on cloned muscle cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2035–2039. doi: 10.1073/pnas.75.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. Rapid lateral diffusion of functional A Ch receptors in embryonic muscle cell membrane. Nature. 1982 Jan 28;295(5847):332–334. doi: 10.1038/295332a0. [DOI] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Tipnis U. R., Malhotra S. K. Junctional and extrajunctional acetylcholine receptors. Can J Physiol Pharmacol. 1980 May;58(5):445–458. doi: 10.1139/y80-076. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Reiness C. G., Hall Z. W. Topographical segregation of old and new acetylcholine receptors at developing ectopic endplates in adult rat muscle. J Cell Biol. 1981 Jan;88(1):215–218. doi: 10.1083/jcb.88.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]