Abstract
BMI and waist circumference (WC) are used to identify individuals with elevated obesity-related health risks. The current thresholds were derived largely in populations of European origin. This study determined optimal BMI and WC thresholds for the identification of cardiometabolic risk among white and African-American (AA) adults. The sample included 2,096 white women, 1,789 AA women, 1,948 white men, and 643 AA men aged 18–64 years. Elevated cardiometabolic risk was defined as ≥2 risk factors (blood pressure ≥130/85 mm Hg; glucose ≥100 mg/dl; triglycerides ≥150 mg/dl; high-density lipoprotein-cholesterol <40 mg/dl (men) or <50 mg/dl (women)). Receiver Operating Characteristic (ROC) curves were used to identify optimal BMI and WC thresholds in each sex-by-ethnicity group. The optimal BMI thresholds were 30 kg/m2 in white women, 32.9 kg/m2 in AA women, 29.1 kg/m2 white men, and 30.4 kg/ m2 in AA men, whereas optimal WC thresholds were 91.9 cm in white women, 96.8 cm in AA women, 99.4 in white men, and 99.1 cm in AA men. The sensitivities at the optimal thresholds ranged from 63.5 to 68.5% for BMI and 68.4 to 71.0% for WC and the specificities ranged from 64.2 to 68.8% for BMI and from 68.5 to 71.0% for WC, respectively. In general, the optimal BMI and WC thresholds approximated currently used thresholds in men and in white women. There are no apparent ethnic differences in men; however, in AA women the optimal BMI and WC values are ~3 kg/m2 and 5 cm higher than in white women.
IntroductIon
The use of anthropometric measures such as BMI and waist circumference (WC) to identify people who are at elevated risk of developing obesity-related disorders and conditions is currently a cornerstone of the clinical management of obesity (1-3). The utility of BMI and WC in identifying patients at increased obesity-related health risk is evidenced by their associations with more direct measures of adiposity (4), chronic disease risk factors (5-8), incidence of chronic disease (9-11), and rates of premature mortality (12-15).
The most commonly used BMI thresholds in adults for defining body weight status are 25 kg/m2 (overweight), and 30 kg/m2 (obesity) (1-3). Evidence that health risks accumulate at lower levels of BMI among Asian populations has resulted in lower clinical BMI thresholds for this group of 23 kg/m2 for moderate to high risk and 27.5 kg/m2 for high to very high risk (16). Formal recommendations for different BMI thresholds in populations other than Asians have not been made; however, levels of adiposity and obesity-related health risks may differ by ethnicity at the same level of BMI (17-20).
Among populations of European descent, the WC thresholds are 80 and 88 cm in women and 94 and 102 cm in men, to indicate high and very high levels of obesity-related health risk, respectively. These thresholds were developed based on the association between WC and BMI (at 25 and 30 kg/m2, respectively), rather than the association between WC and health risk per se (21). At the same level of WC, there is great heterogeneity across populations in sensitivity and specificity for identifying people who are considered overweight (BMI ≥25 kg/m2) (22). At present, there are no definitive WC thresholds proposed for other populations, although some have been suggested for Asian groups based on preliminary evidence (23).
The purpose of this study was to determine the optimal BMI and WC thresholds for the identification of cardiometabolic risk in a sample of white and African-American (AA) adults.
Methods and Procedures
Sample
The Pennington Center Longitudinal Study (PCLS) is an ongoing investigation of the effects of obesity and lifestyle factors on the development of chronic diseases such as type 2 diabetes, cardiovascular disease (CVD), and cancer. The sample is composed of volunteers who have participated in variety of clinical studies, including diet interventions, weight loss, and other metabolic/physiologic studies conducted at the Pennington Biomedical Research Center (PBRC) in Baton Rouge, LA since 1992. The current cross-sectional study included 6,476 participants (2,096 white women, 1,789 African-American women, 1,948 white men, and 643 African-American men) 18–64 years of age. Each participant provided their written informed consent and all PCLS procedures, including this analysis were approved by the PBRC institutional review board.
Anthropometry
Standardized anthropometric measures were obtained on all participants. Height was measured using standard methods with a stadiometer. Participants were required to remove shoes and asked to hold an inhaled breath, while a nurse lightly applied traction to the patient’s head in order to maintain alignment with the frankfort plane. A second nurse then lowered the slide until it reached the vertex of the skull and recorded the reading from the indicator. This process was repeated, and the average of the two heights was used in analysis (a third measurement was obtained if the first two measurements was >0.5 cm apart). Weight was measured in duplicate using a calibrated digital scale after all outer clothing, heavy pocket items, and shoes were removed. Weight was recorded to the nearest 0.1 kg. A third measurement was obtained if the difference between the first two measurements was >0.5 kg. BMI was calculated as the weight in kg divided by the height in m2 (kg/m2). WC was measured at the midpoint between the inferior border of the ribcage and the superior aspect of the iliac crest using an inelastic measuring tape. This process was repeated, and the average of the two values was used in analysis (a third measurement was obtained if the first two measurements was >0.5 cm apart).
Cardiometabolic risk factors
Blood pressure
All blood pressure measurements were taken manually using a stethoscope and standard sphygmomanometer or in some cases using a validated Omron automatic measuring device. Resting blood pressure measurements were obtained after a 5-min rest, with the participant in a semirecumbent position in a quiet room. Each measurement was taken twice. The nurse waited 1–2 min before repeating the measurement and the average of the two measurements was recorded. Participants were not allowed to engage in vigorous exercise, ingest food or caffeine, or smoke within 30 min of measurement. Upper-arm length and circumferences of the right arm were measured in order to establish the appropriate cuff size. The Korotkoff sounds were used to establish the first and 5th phases and all measurements were recorded in mm Hg. High blood pressure was defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, or reported treated hypertension.
Fasting blood lipid and glucose panel
Serum triglycerides, high-density lipoprotein-cholesterol, and plasma glucose were obtained from a 12-h fasting blood draw. Participants were asked to refrain from consuming alcohol or engaging in vigorous exercise at least 24 h before blood withdrawal. Samples were analyzed on a Beckman Coulter Chemistry Analyzer (Beckman Coulter, Brea, CA). High glucose was defined as fasting plasma glucose ≥100 mg/dl, or reported diabetes. High triglycerides were defined as ≥150 mg/dl. Low high-density lipoprotein-cholesterol was defined as <40 mg/dl for men or <50 mg/dl for women.
Covariates
Participant age was computed from birth and observation dates. Smoking status was self-reported during the screening process, and participants were classified as “nonsmokers,” “current smokers,” or “former smokers.” Menopausal status (premenopausal/postmenopausal) was determined in women from their age and responses to questions regarding their reproductive history. Women aged 55+ years of age or those who indicated that they can no longer have children because of achieving menopause were considered to be postmenopausal.
Statistical analysis
Logistic regression models were used to determine the odds of having abnormal risk factors and the presence of two or more cardiometabolic risk factors (systolic/diastolic blood pressure ≥130/85 mm Hg, fasting glucose ≥100 mg/dl, triglycerides ≥150 mg/dl, high-density lipoprotein-cholesterol <40 mg/dl (men), or <50 mg/dl (women)) in each sex-by-ethnicity group separately. Odds ratios with 95% confidence intervals were calculated to assess the odds of the risk factor abnormality with elevated BMI or WC relative to the odds without the elevation. All odds ratios are expressed per s.d. of BMI or WC. Age, smoking status, and menopausal status (in women) were included as covariates in the logistic regression models. Receiver Operating Characteristic (ROC) curves were used to select the optimal anthropometric thresholds that identified individuals with abnormal risk factor levels and risk factor clustering in each sex-by-ethnicity group. Because the area under the curve is considered as a measure of the utility of BMI or WC and represents the tradeoff between the correct identification of high-risk individuals (sensitivity) and the correct identification of low-risk individuals (specificity), the optimal threshold was considered to be the point of convergence between sensitivity and specificity. In order to investigate the effects of age on the optimal thresholds, the sample was divided into three age groups (18–34 years, 35–49 years, 50–64 years). SAS version 9.0 was used for data management and preliminary analyses, and PASW version 18.0 was used to perform the ROC analyses. The level of significance was set at P ≤ 0.05.
Results
The descriptive characteristics of the sample are presented in Table 1. The sample was composed of 60% women and 38% AA volunteers. The average age of the volunteers was 38.9 years (s.d. 12.9 years), and the prevalence of two or more cardiometabolic risk factors was 37.2%, ranging from 34% in white women to 43% in white men. Both BMI and WC were strongly associated with the presence of two or more cardiometabolic risk factors as well as individual cardiometabolic risk factors (Table 2). The odds ratios for having two or more cardiometabolic risk factors per s.d. of BMI ranged from 2.6 in white women to 2.0 in African-American women, and the odds ratios per s.d. of WC were 3.1 in white women and 2.4 in all other groups. These results indicate that for every s.d. higher BMI (~6 kg/m2) or WC (~16 cm), the odds of having two or more cardiometabolic risk factors was two to three times higher than someone with a correspondingly lower level of BMI or WC, respectively. In general, the strength of the associations between BMI and WC and cardiometabolic risk factors was similar across all sex-by-ethnicity groups.
Table 1. Descriptive characteristics of the sample.
| Women |
Men |
|||
|---|---|---|---|---|
| White | African American | White | African American | |
| Number of subjects | 2,096 | 1,789 | 1,948 | 643 |
| Age, years | 41.8 (12.8) | 39.0 (11.8) | 37.0 (13.3) | 34.9 (12.4) |
| BMI (kg/m2) | 29.6 (6.7) | 32.6 (6.7) | 29.2 (5.5) | 29.8 (5.8) |
| Waist circumference (cm) | 89.9 (16.3) | 95.7 (15.7) | 98.8 (15.4) | 96.6 (16.5) |
| Systolic blood pressure (mm Hg) | 116.4 (14.0) | 120.6 (15.7) | 120.2 (12.2) | 121.2 (12.4) |
| Diastolic blood pressure (mm Hg) | 74.5 (8.5) | 77.4 (9.7) | 77.3 (9.2) | 77.6 (9.4) |
| Triglycerides (mg/dl) | 122.2 (69.9) | 90.7 (50.2) | 137.5 (80.4) | 96.7 (55.0) |
| HDL-cholesterol (mg/dl) | 56.9 (13.9) | 56.5 (13.2) | 45.2 (11.0) | 49.5 (11.7) |
| Glucose (mg/dl) | 98.8 (21.0) | 104.0 (32.2) | 103.7 (25.8) | 102.1 (27.6) |
| High blood pressure (%) | 26.3 | 39.9 | 32.6 | 38.3 |
| High blood glucose (%) | 31.1 | 37.2 | 41.1 | 36.4 |
| High triglycerides (%) | 28.0 | 11.1 | 35.0 | 13.8 |
| Low HDL-cholesterol | 34.4 | 32.3 | 33.4 | 21.8 |
| 2+ Risk factors | 33.6 | 36.6 | 43.0 | 33.1 |
| Current smoking (%) | 3.4 | 4.0 | 2.6 | 6.8 |
| Postmenopausal (%) | 23.7 | 12.1 | --.-- | --.-- |
Mean values for continuous variables are presented as mean (s.d.) and categorical variables are presented as a percentage (%). High blood pressure ≥130/85 mm Hg or reported treated hypertension; high blood glucose ≥100 mg/dl or reported type 2 diabetes; high triglycerides ≥150 mg/dl; low HDL-cholesterol <40 mg/dl in men; <50 mg/dl in women.
HDL, high-density lipoprotein.
Table 2. Results of logistic regression analysis (odds ratios (95% confidence interval)) for BMI and waist circumference predicting individual risk factors and the presence of two or more cardiometabolic risk factors.
| Women |
Men |
|||
|---|---|---|---|---|
| White | African American | White | African American | |
| High blood pressure | ||||
| BMI | 1.8 (1.6–2.1) | 1.7 (1.5–1.9) | 1.7 (1.5–1.9) | 1.6 (1.3–1.9) |
| Waist circumference | 1.9 (1.7–2.1) | 1.8 (1.6–2.1) | 1.8 (1.6–2.0) | 1.6 (1.4–2.0) |
| High glucose | ||||
| BMI | 2.1 (1.9–2.4) | 1.8 (1.6–2.1) | 1.5 (1.3–1.7) | 1.6 (1.3–1.9) |
| Waist circumference | 2.4 (2.2–2.7) | 2.3 (2.0–2.6) | 1.6 (1.4–1.8) | 1.8 (1.4–2.2) |
| High triglycerides | ||||
| BMI | 1.8 (1.6–1.9) | 1.2 (1.0–1.4) | 1.9 (1.7–2.2) | 1.6 (1.3–2.1) |
| Waist circumference | 2.0 (1.8–2.2) | 1.5 (1.3–1.7) | 2.1 (1.9–2.4) | 1.8 (1.4–2.4) |
| Low HDL-cholesterol | ||||
| BMI | 1.9 (1.7–2.1) | 1.4 (1.3–1.6) | 1.6 (1.5–1.8) | 1.5 (1.3–1.9) |
| Waist circumference | 2.0 (1.8–2.3) | 1.5 (1.4–1.7) | 1.7 (1.5–1.9) | 1.7 (1.4–2.1) |
| 2+ Risk factors | ||||
| BMI | 2.6 (2.3–3.0) | 2.0 (1.7–2.2) | 2.2 (1.9–2.4) | 2.1 (1.7–2.6) |
| Waist circumference | 3.1 (2.7–3.5) | 2.4 (2.1–2.7) | 2.4 (2.1–2.7) | 2.4 (1.9–3.0) |
High blood pressure ≥130/85 mm Hg or reported treated hypertension; high blood glucose ≥100 mg/dl or reported type 2 diabetes; high triglycerides ≥150 mg/dl; low HDL-cholesterol <40 mg/dl in men; <50 mg/dl in women. Results are expressed per s.d. unit of BMI and waist circumference, respectively. Age, smoking status, and menopausal status (women only) were included as covariates in all logistic regression models.
HDL, high-density lipoprotein.
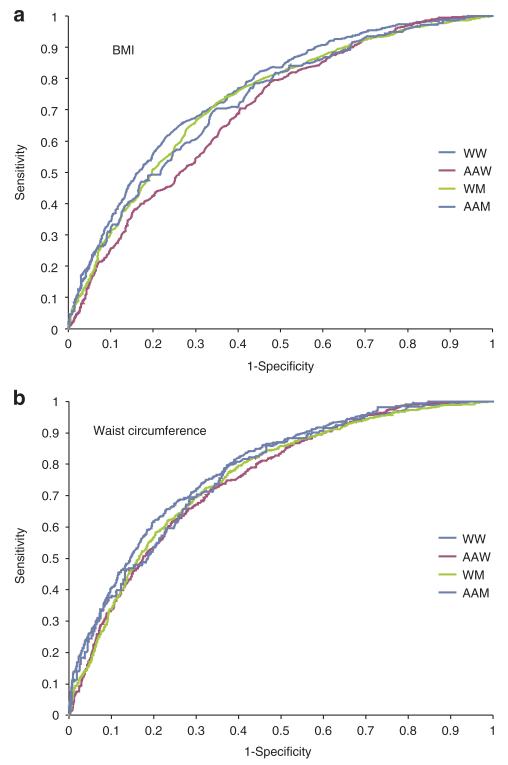
The sex- and ethnic-specific ROC curves are presented in Figure 1 for the clinical utility of BMI (panel A) and WC (panel B) in predicting two or more cardiometabolic risk factors. The areas under the curve were significantly >0.5 in all sex-by-ethnicity groups (Table 3), ranging from 0.697 to 0.755 for BMI and from 0.752 to 0.783 for WC. The optimal BMI thresholds were 30 kg/m2 in white women, 32.9 kg/m2 in AA women, 29.1 kg/m2 white men, and 30.4 kg/m2 in AA men, whereas optimal WC thresholds were 91.9 cm in white women, 96.8 cm in AA women, 99.4 cm in white men, and 99.1 cm in AA men. The sensitivities at the optimal thresholds ranged from 63.5 to 68.5% for BMI and 68.4 to 71.0% for WC and the specificities ranged from 64.2 to 68.8% for BMI and from 68.5 to 71.0% for WC, respectively.
Figure 1.
Receiver Operating Characteristics curves for (a) BMI and (b) waist circumference for predicting the presence of two or more cardiometabolic risk factors in 6,476 white and African-American adults in the Pennington Center Longitudinal Study.
Table 3. Results of Receiver Operating Characteristic (ROC) curve analyses for the utility of BMI and waist circumference in predicting two or more cardiometabolic risk factors.
| Area under the curvea |
95% Confidence interval |
Optimal threshold | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| BMI (kg/m2) | |||||
| White women | 0.755 | (0.734–0.777) | 30.0 | 68.5 | 68.8 |
| 18–34 years | 0.806 | (0.759–0.852) | 29.5 | 75.5 | 75.7 |
| 35–49 years | 0.722 | (0.685–0.758) | 30.5 | 65.4 | 65.9 |
| 50–64 years | 0.728 | (0.691–0.766) | 30.1 | 65.2 | 67.0 |
| African-American women | 0.697 | (0.672–0.721) | 32.9 | 63.5 | 64.2 |
| 18–34 years | 0.716 | (0.666–0.766) | 32.7 | 66.3 | 66.3 |
| 35–49 years | 0.673 | (0.634–0.711) | 32.9 | 62.6 | 62.0 |
| 50–64 years | 0.661 | (0.602–0.720) | 33.3 | 59.8 | 58.8 |
| White men | 0.732 | (0.710–0.754) | 29.1 | 68.7 | 67.7 |
| 18–34 years | 0.708 | (0.670–0.747) | 27.4 | 65.9 | 65.7 |
| 35–49 years | 0.655 | (0.612–0.699) | 30.6 | 60.6 | 61.1 |
| 50–64 years | 0.713 | (0.662–0.764) | 30.2 | 63.8 | 63.8 |
| African-American men | 0.726 | (0.686–0.767) | 30.4 | 67.1 | 66.7 |
| 18–34 years | 0.722 | (0.647–0.797) | 29.8 | 67.2 | 67.6 |
| 35–49 years | 0.655 | (0.578–0.732) | 30.6 | 59.8 | 60.6 |
| 50–64 years | 0.706 | (0.602–0.810) | 31.4 | 65.1 | 63.2 |
| Waist circumference (cm) | |||||
| White women | 0.783 | (0.763–0.803) | 91.9 | 71.0 | 71.0 |
| 18–34 years | 0.815 | (0.771–0.858) | 89.5 | 76.5 | 76.1 |
| 35–49 years | 0.750 | (0.715–0.785) | 93.2 | 68.4 | 68.3 |
| 50–64 years | 0.758 | (0.722–0.794) | 92.4 | 68.7 | 68.8 |
| African-American women | 0.752 | (0.730–0.775) | 96.8 | 69.9 | 69.5 |
| 18–34 years | 0.736 | (0.689–0.784) | 94.6 | 67.3 | 67.2 |
| 35–49 years | 0.722 | (0.685–0.758) | 96.4 | 66.5 | 66.4 |
| 50–64 years | 0.709 | (0.651–0.767) | 100.4 | 68.3 | 68.1 |
| White men | 0.759 | (0.738–0.780) | 99.4 | 68.4 | 68.5 |
| 18–34 years | 0.730 | (0.693–0.767) | 91.8 | 68.7 | 68.3 |
| 35–49 years | 0.676 | (0.633–0.719) | 104.0 | 65.6 | 65.4 |
| 50–64 years | 0.734 | (0.685–0.783) | 104.2 | 67.5 | 67.5 |
| African-American men | 0.769 | (0.732–0.806) | 99.1 | 70.0 | 69.8 |
| 18–34 years | 0.741 | (0.676–0.807) | 93.0 | 67.2 | 67.2 |
| 35–49 years | 0.682 | (0.607–0.757) | 101.2 | 62.0 | 62.6 |
| 50–64 years | 0.728 | (0.629–0.828) | 104.4 | 63.5 | 63.2 |
All areas under the curve were significantly >0.5.
Table 3 also presents the results of the age-specific analyses. In women, there were no appreciable effects of age on the optimal BMI thresholds, which ranged from 29.5 to 30.5 kg/m2 in white women and from 32.7 to 33.3 kg/m2 in AA women across the three age groups. The optimal WC thresholds tended to be lower in 18-34-year-old group vs. the older age groups in white women, and they increased from 94.6 to 100.4 cm across the three age groups in AA women. In men, the optimal BMI and WC thresholds were higher in the older age groups compared to the younger age groups. The optimal BMI thresholds were 27.4 and 30.4 kg/m2 in the younger vs. older age groups in white men and 29.8 kg/m2 and 31.4 kg/m2 in the younger vs. older AA men. The optimal WC thresholds were 91.8 and 104.2 cm in white men and 93.0 and 104.4 cm in black men, in the younger and older groups, respectively.
Discussion
This study presents optimal BMI and WC thresholds for the clinical identification of cardiometabolic risk factors in a sample of adult study volunteers. The results demonstrate the utility of BMI and WC to identify individuals with multiple cardiometabolic risk factors. The optimal thresholds identified for BMI closely approximate the currently recommended threshold for obesity (30 kg/m2) (1-3) in all sex-by-ethnicity groups with the exception of African-American women (~33 kg/m2). The optimal WC thresholds in men (~99 cm) were also close to the recommended threshold of 102 cm, and whereas the optimal threshold in white women (~92 cm) was about 4 cm higher than the recommended threshold of 88 cm, the optimal threshold was ~9 cm higher than the recommended threshold in African-American women (~97 cm).
A study using data from the Canadian Heart Health Surveys identified optimal BMI and WC thresholds for men and women for the identification of two or more CVD risk factors (24). Using a similar ROC approach as the present study, they identified optimal BMI thresholds of 26 and 24 kg/m2 in men and women, and WC thresholds as 91 and 78 cm in men and women, respectively. These thresholds are somewhat lower than those reported in the present study. It is difficult to determine the reasons for the differences in results between the two studies, but the Canadian Heart Health Surveys sample was older (18–74 years), had lower average BMI and WC, used different risk factor thresholds than the present study, and the surveys were conducted between 1986 and 1992. The Canadian Heart Health Surveys did not collect information on ethnicity; however, the majority of the participants were white (24).
There may be ethnic differences between white and AAs in the relationship between BMI and adiposity, CVD risk factors, and risk of mortality. The results from the present study demonstrated similar optimal BMI thresholds in white and African-American men, but African-American women had a higher threshold than white women. These observed relationships have some support from studies of BMI and mortality. Several studies in African-American men have shown a relationship between BMI and mortality similar to that of white men (15,25,26). However, in African-American women, the relationship between BMI and mortality is not as clear. Several studies that have included both AA and white women have shown a racial difference in the relationship between BMI and mortality (15,27-30). In these studies, a significant relationship was found for white women, but the association was much weaker or nonexistent in African-American women. The weighted evidence suggests that the relationship between BMI and mortality may be less strong in AA than white women (31). The degree to which racial differences in the relationship between BMI and mortality may be explained by differences in the association between BMI and adiposity or risk factors is not known.
Ethnic-specific WC thresholds that identify elevated CVD risk have previously been reported using data from National Health and Nutrition Examination Survey (NHANES) (1988–1994) (32). At BMI values between 25 and 40 kg/m2, the WC thresholds for white men were 5–6 cm higher than in African-American men; however, there were no differences between AA and white women. These results appear to be in contrast to those from the present study; however, the results are not directly comparable as the authors computed the WC thresholds associated with the CVD risk at established BMI thresholds within each sex-by-ethnicity group, rather than using a ROC approach to directly identify WC thresholds associated with cardiometabolic risk factor clustering.
Few studies to date have employed longitudinal prospective designs to study ethnic differences in the relationship between anthropometric markers of adiposity and the development of risk factors or morbidities (33). Data from the Atherosclerosis Risk in Communities (ARIC) Study indicated that the incidence of diabetes over a 9-year period was higher at all levels of BMI in AA compared with white adults (10). This is in contrast to results from the NHANES Epidemiologic Follow-up Study in which the 20-year incidence of diabetes was higher in AAs than white Americans at low BMI values and equivalent at higher BMI values (34). These results highlight the need to clarify racial differences in both the relative and absolute risk of disease, which is important to determine the need for ethnic-specific clinical guidelines.
A major strength of this study is the large biethnic sample of men and women who have direct measurements of anthropometry in addition to cardiometabolic risk factors. We were able to include information such as age, smoking, menopausal status; however, we did not have information on other variables such as socioeconomic status or the use of medications. The sample spans a wide range of ages (18–64 years), BMI (16–60 kg/m2), and WC (57–157 cm); however, the sample represents volunteers who have participated in a variety of clinical studies at the Pennington Biomedical Research Center, and it was not designed to be representative of other populations. Given that the determination of ROC curves is influenced by the prevalence of the exposure and its association with the outcome in the study sample, future validation of these results using representative population samples is required. Although mean values for the risk factors vary by ethnicity, and absolute CVD risk at the same risk factor level may vary by ethnicity, we chose to use a common threshold for the identification of elevated risk (i.e., 2+ risk factors exceeding current clinical criteria). Thus, we are unable to comment on the most appropriate anthropometric thresholds to identify absolute CVD risk levels in white and AA adults. Further, this study employed a cross-sectional design, so causal inferences should not be inferred from the results. Future studies using longitudinal designs are required to identify optimal anthropometric thresholds for predicting incident morbidity and mortality.
The results of this study support the existence of associations between BMI and WC and cardiometabolic risk factors, and demonstrate the utility of BMI and WC to identify indivi duals with multiple risk factors. There were no apparent ethnic differences in men; however, optimal BMI and WC values are ~3 kg/ m 2 and 5 cm higher in AA compared to white women. The need for ethnic-specific anthropometric thresholds to identify obesity-related health risks in women should be investigated further. These studies are important because if ethnic differences in BMI or WC as risk predictors are detected, the public health guidelines targeting risk reductions must be made specific for different ethnic groups.
ACKNOWLEDGMENTS
The Pennington Center Longitudinal Study is registered at ClinicalTrials.gov (Identifier NCT00959270). This research was supported by the Pennington Biomedical Research Center. P.T.K. is supported, in part, by the Louisiana Public Facilities Authority Endowed Chair in Nutrition and C.B. is funded, in part, by the john W. Barton Sr. Chair in Genetics and Nutrition. E.R. is funded, in part, by the Douglas L. Gordon Chair in Diabetes and Metabolism. Special thanks to Emily Mire and Connie Murla for data management, and to jennifer Rood and the staff of the Clinical Chemistry Laboratory, as well as the many clinical scientists and staff of the Pennington Biomedical Research Center who have contributed data to the development of the Pennington Center Longitudinal Study .
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.NIH . The Practical Guide to the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. US NIH; Bethesda, MD: 2000. [Google Scholar]
- 2.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997. World Health Organization; Geneva: 1998. [PubMed] [Google Scholar]
- 3.Lau DC, Douketis JD, Morrison KM, et al. Canadian clinical practice guidelines on the management and prevention of obesity in adults and children (summary) CMAJ. 2007;176:S1–S13. doi: 10.1503/cmaj.061409. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond) 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- 5.Can AS, Bersot TP, Gönen M. Anthropometric indices and their relationship with cardiometabolic risk factors in a sample of Turkish adults. Public Health Nutr. 2009;12:538–546. doi: 10.1017/S1368980008002474. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. The relation of BMI and skinfold thicknesses to risk factors among young and middle-aged adults: the Bogalusa Heart Study. Ann Hum Biol. 2010;37:726–737. doi: 10.3109/03014461003641849. [DOI] [PubMed] [Google Scholar]
- 7.Richelsen B, Pedersen SB. Associations between different anthropometric measurements of fatness and metabolic risk parameters in non-obese, healthy, middle-aged men. Int J Obes Relat Metab Disord. 1995;19:169–174. [PubMed] [Google Scholar]
- 8.Bosy-Westphal A, Geisler C, Onur S, et al. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes (Lond) 2006;30:475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 10.Stevens J, Couper D, Pankow J, et al. Sensitivity and specificity of anthropometrics for the prediction of diabetes in a biracial cohort. Obes Res. 2001;9:696–705. doi: 10.1038/oby.2001.94. [DOI] [PubMed] [Google Scholar]
- 11.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 12.Bigaard J, Tjønneland A, Thomsen BL, et al. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 13.Katzmarzyk PT, Craig CL, Bouchard C. Adiposity, adipose tissue distribution and mortality rates in the Canada Fitness Survey follow-up study. Int J Obes Relat Metab Disord. 2002;26:1054–1059. doi: 10.1038/sj.ijo.0802057. [DOI] [PubMed] [Google Scholar]
- 14.McGee DL, Diverse Populations Collaboration Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 18.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 19.Lear SA, Humphries KH, Kohli S, Birmingham CL. The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obesity (Silver Spring) 2007;15:2817–2824. doi: 10.1038/oby.2007.334. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Thornton JC, Burastero S, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996;4:377–384. doi: 10.1002/j.1550-8528.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 21.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Varying sensitivity of waist action levels to identify subjects with overweight or obesity in 19 populations of the WHO MONICA Project. J Clin Epidemiol. 1999;52:1213–1224. doi: 10.1016/s0895-4356(99)00114-6. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 24.Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001;25:652–661. doi: 10.1038/sj.ijo.0801582. [DOI] [PubMed] [Google Scholar]
- 25.Durazo-Arvizu R, Cooper RS, Luke A, et al. Relative weight and mortality in U.S. blacks and whites: findings from representative national population samples. Ann Epidemiol. 1997;7:383–395. doi: 10.1016/s1047-2797(97)00044-6. [DOI] [PubMed] [Google Scholar]
- 26.Carnethon MR, Lynch EB, Dyer AR, et al. Comparison of risk factors for cardiovascular mortality in black and white adults. Arch Intern Med. 2006;166:1196–1202. doi: 10.1001/archinte.166.11.1196. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JL, Heineman EF, Heiss G, Hames CG, Tyroler HA. Cardiovascular disease risk factors and mortality among black women and white women aged 40-64 years in Evans County, Georgia. Am J Epidemiol. 1986;123:209–220. doi: 10.1093/oxfordjournals.aje.a114230. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J, Keil JE, Rust PF, et al. Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med. 1992;152:1257–1262. [PubMed] [Google Scholar]
- 29.Stevens J, Plankey MW, Williamson DF, et al. The body mass index-mortality relationship in white and African American women. Obes Res. 1998;6:268–277. doi: 10.1002/j.1550-8528.1998.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 30.Abell JE, Egan BM, Wilson PW, et al. Differences in cardiovascular disease mortality associated with body mass between Black and White persons. Am J Public Health. 2008;98:63–66. doi: 10.2105/AJPH.2006.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens J. Obesity and mortality in Africans-Americans. Nutr Rev. 2000;58:346–353. doi: 10.1111/j.1753-4887.2000.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu S, Heymsfield SB, Toyoshima H, et al. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81:409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 33.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 34.Resnick HE, Valsania P, Halter JB, Lin X. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21:1828–1835. doi: 10.2337/diacare.21.11.1828. [DOI] [PubMed] [Google Scholar]