Abstract
Optimistic expectancies affect many psychosocial outcomes and may also predict immune system changes and health, but the nature and mechanisms of any such physiological effects have not been identified. The present study related law-school expectancies to cell-mediated immunity (CMI), examining the within- and between-person components of this relationship and affective mediators. First-year law students (N = 124) completed questionnaire measures of expectancies and affect and received delayed-type hypersensitivity skin tests at five time points. A positive relationship between optimistic expectancies and CMI occurred, in which that changes in optimism correlated with changes in CMI. Likewise, changes in optimism predicted changes in positive and, to a lesser degree, negative affect, but the relationship between optimism and immunity was partially accounted for only by positive affect. This dynamic relationship between expectancies and immunity has positive implications for psychological interventions to improve health, particularly those that increase positive affect.
Keywords: optimism, expectancy, affect, cell-mediated immunity
Expectations for the future are manifest at levels ranging from the broad and general (e.g., dispositional optimism), to the domain-specific (e.g., work or relationships), to the very particular (e.g., a specific goal or behavior). Domain-specific, situational expectancies may be particularly important insofar as they combine the power of specificity (cf. Ajzen & Fishbein, 1977) with the breadth to influence substantial outcomes, including persuasion, liking, happiness, goal achievement, relationship satisfaction, and clinical syndromes such as eating disorders and depression. The mechanisms by which situational expectancies affect psychosocial outcomes are fairly straightforward and transparent: For example, optimistic interpersonal expectancies affect interpersonal behavior and thereby improve relationship satisfaction.
Less transparently, expectancies may also influence physiological processes and outcomes. HIV patients who endorsed pessimistic health expectancies, such as, “I prepare myself for the worst,” had earlier symptom onset and shorter survival after AIDS diagnosis (Reed, Kemeny, Taylor, & Visscher, 1999; Reed, Kemeny, Taylor, Wang, & Visscher, 1994). Heart transplant patients who were more optimistic about their likelihood of 5-year survival before transplant had better health 6 months after transplant and longer median latency to first postoperative infection in analyses adjusted for preoperative health and health behavior (Leedham, Meyerowitz, Muirhead, & Frist, 1995). Optimistic expectancies in other domains (e.g., academic achievement or phobia treatment) also correlated with higher numbers of certain immune cells (Segerstrom, Taylor, Kemeny, & Fahey, 1998; Wiedenfeld et al., 1990). Therefore, optimistic expectancies not only may affect the corresponding psychosocial outcome (e.g., academic optimism predicts academic success; Solberg Nes, Evans, & Segerstrom, 2009) but also may predispose generally to more robust immune function and better health.
Important questions about whether and how expectancies affect the immune system remain. First, no study has shown that expectancy change is associated with immune change. Expectancy-immunity covariance between people has different implications from covariance within people. For example, temperament might both affect optimism and have neural and hormonal substrates that affect immunity and health (e.g., Cole, Kemeny, Fahey, Zack, & Naliboff, 2003). Such a case would undermine one important implication of an expectancy-immunity relationship: the possibility that interventions could change expectancies and thereby get “under the skin” to improve immunity and health. Evidence that expectancies and immunity covary within people could rule out many antecedent traits and at the same time demonstrate that expectancy change is accompanied by immune change.
Second, no study has identified the mechanism by which expectancies are associated with immunity. Distress, perceived stress, and health behaviors (e.g., sleep) did not account for the between-person relationship between expectancies and immunity in healthy young adults or HIV patients (Reed et al., 1994, 1999; Segerstrom et al., 1998). However, these between-person findings do not rule out the possibility that as a person's expectancies become more optimistic, he or she becomes less distressed, and his or her immunity may also change for the better. Affect is a good candidate to mediate expectancy effects on the immune system via relevant peripheral nervous system as well as neuroendocrine pathways (e.g., Davidson, 2003; Pressman & Cohen, 2005). Furthermore, previous studies have largely ignored the potential role of positive affect. Positive and negative affect can be largely independent (Feldman Barrett & Russell, 1999; Watson, 1988; Watson & Tellegen, 1985), and higher positive affect may predict better health and healthier physiological processes above and beyond the absence of negative affect (Pressman & Cohen, 2005).
Third, previous studies have examined in vitro immune measures, such as the number of immune cells of various types, which in most cases have limited implications for health. In contrast, in vivo measures can provide broader and more ecologically valid assessments of important immune responses such as cell-mediated immunity (CMI), which protects against microbes that survive within host cells (e.g., viruses). CMI can be measured in vivo using delayed-type hypersensitivity (DTH) skin testing. The DTH skin test elicits the coordinated response of several cell types, particularly macrophages and T lymphocytes, to an antigen (i.e., immune stimulus) injected into the skin. The ultimate response is recruitment and migration of immune cells into the skin and induration (i.e., swelling), the size of which indexes the robustness of the response (Rabin, 1999). Larger DTH responses predicted decreased morbidity and mortality in surgical patients, individuals with HIV, and older people (Christou et al., 1995; Dolan et al., 1995; Wayne, Rhyne, Garry, & Goodwin, 1990).
The present study examined the relationship between optimistic academic expectancies and in vivo immunity, focusing on the effects of changes in expectancies on immunity, as well as the candidate mechanisms of positive and negative affect. Academic optimism, positive and negative affect, and CMI were measured at each of five time points across the first year of law school. As such, the present study was able to test the within-person relationship between optimism change and immune change. Individual students' idiosyncratic experiences as they entered law school, encountered challenges (e.g., Socratic questioning), anticipated their first academic evaluations (final examinations), and received their first feedback (first-semester grades) were likely to affect optimism about their performance in law school. We expected that these changes in optimism would result in affective change that would influence immunity. Rather than a long-term, prospective, between-person relationship between optimism and immunity, we predicted a dynamic, within-person relationship, with changes in optimistic expectancies, affect, and immunity closely coupled and covarying with each other across assessment waves. It was predicted that optimistic academic expectancies during law school would be associated with more robust immunity. It was also predicted that optimistic expectancies would be associated with more positive affect and less negative affect and, in turn, positive affect would be associated with stronger immunity and negative affect would be associated with weaker immunity.
Method
Participants
Participants were 124 first-year law students recruited from entering classes from 2001 to 2005. The sample was 55% female and 45% male and predominantly White (90%), with minority representation including Asian American (1%), African American (7%), and more than one race (2%). Academically, the sample was representative of the law school as a whole. The median Law School Admission Test (LSAT) score in the sample was 159, which was the same as the entire entering classes in the same years, and the interquartile range differed by only 1 point. The median undergraduate grade point average (GPA) was 3.60, which was only slightly higher than that of the entire entering classes in the same years: 3.55. The interquartile range differed by only 0.04 point.
Procedure
Recruitment packets were mailed during the summer before the students started law school. If interested, participants returned a signed informed consent form, contact information, and a form that was used to screen for exclusion criteria related to mental health (e.g., self-reported history of impairment of function for 2 weeks or more), physical health (e.g., immunologically mediated disease), and substance use (e.g., more than two drinks of alcohol every day). At five time points, eligible participants completed questionnaires and received DTH skin tests. The five time points were August, at the beginning of the semester; October, midsemester; December, during final exams; January, at the beginning of the semester when most first-semester grades were available; and February, after all grades were available and interviews for summer internships were starting. During each 48-hr assessment period, the DTH skin test was administered between 7:00 and 9:00 a.m. on Day 1. Questionnaires were completed over Day 1 and Day 2 and returned when skin tests were read on the morning of Day 3, 48 hr after administration. Participants received $50 compensation at each wave.
Measures
Demographics, academic qualifications, and academic outcomes
Participants reported on their demographics (e.g., gender and race) as well as their LSAT scores and undergraduate GPAs in the first questionnaire. Class rank was reported in the fourth and fifth questionnaires.
Law-school optimism
Optimistic expectancies for law school were measured with a scale designed for use with law students (Segerstrom et al., 1998). Because one focus of the current investigation was the role of affect, two items that measured or implied affect (“I feel confident when I think about it” and “I feel very apprehensive when I think about it”) were removed, leaving eight items that tapped purely cognitive expectancies (e.g., “I will be less successful than most of my classmates” and “It's unlikely that I will fail”). Alpha reliability for these eight items at the five time points ranged from .75 to .89. Two terms for law-school optimism were created: a mean for each person (average law-school optimism) and a within-person deviation from that mean at each time point (change in law-school optimism). These terms represented, respectively, the between-person and within-person variance in optimism and were statistically and conceptually independent of each other (Enders & Tofighi, 2007).
Positive and negative affect
Affect was measured daily with the Positive and Negative Affect Schedule—Expanded Form (Watson & Clark, 1994). Participants completed the measure three times: in the morning of Day 1 for the previous day at the end of Day 1 for Day 1 and at the end of Day 2 for Day 2. The present analyses used the Positive Affect and Negative Affect subscales, which had good reliability in the validation sample with 1-day time instructions (.90 and .87, respectively; Watson, Clark, & Tellegen, 1988). A composite of the 3 days within waves in the present study had good reliability for both Positive Affect (.87) and Negative Affect (.86). As expected, Positive Affect and Negative Affect were essentially uncorrelated with each other (r = −.04). Between-person (mean across waves) and within-person (deviation at each wave) terms for positive and negative affect were created.
Dispositional optimism
Dispositional optimism was measured in the first questionnaire with the Life Orientation Test—Revised (Scheier, Carver, & Bridges, 1994). Alpha reliability for this sample was .84.
Health and behavior
At each wave, participants reported a number of their health behaviors. Details of this assessment are included in the Supplemental Material available on-line. At each skin test reading, participants were asked if they had symptoms of a cold, flu, or other infection; an allergy attack; or an asthma attack during the past 48 hr.
CMI
CMI was tested by intradermal injection of 0.1 ml of a commercially available DTH antigen preparation on the nondominant forearm. The antigen used in the first 2 years was derived from a heat-killed mumps virus (MSTA, Aventis Pasteur, Swiftwater, PA). After the discontinuation of its manufacture, an antigen derived from candida yeast was used for the last 3 years (Candin, Allermed, San Diego, CA). The type of antigen, however, was not a significant influence on the results (see Exclusions and confounds, later in the Method section). Skin test induration size was read at 48 hr after injection using the ballpoint-pen method, in which a pen is drawn toward the induration from each direction along the arm's parallel and orthogonal axes. Induration in the skin stops the pen, thereby marking its borders for measurement (Longfield et al., 1984).
Data analysis
The data were analyzed with multilevel models using SAS PROC MIXED (Singer, 2002). In a multilevel framework, CMI for person j at wave i can be partialed into a stable part associated with between-person variance (β0j), representing person j's average across waves, and a dynamic part associated with within-person variance (Rij), representing person j's deviation associated with wave i, CMIij = β0j + Rij. Between-person variance can be predicted by Level 2, between-person predictors; within-person variance can be predicted by Level 1, within-person predictors. At Level 1, therefore, deviation in academic optimism at wave i predicted deviation in immunity at the corresponding wave. This component of the model estimates the covariance between change in optimism and change in immunity within people:
The first Level 2 component of the model provided average academic optimism across waves as a predictor of average immunity across the five waves:
The second Level 2 component of the model allowed within-person effects of optimism (i.e., the optimism slope) to vary across people, that is, optimism was treated as a random variable:
Comparing models with random and nonrandom effects of optimism yielded Δ–2 log likelihood (LL) = 13.2, p < .001, indicating significant variance in the optimism slope. This component of the model prevented overly liberal estimates of the significance of the optimism slope.
Across all waves, then, by substitution for β0j and β1j:
The gamma weights, which are analogous to unstandardized beta weights, are reported, along with their corresponding t statistics. Because gamma weights are unstandardized, effect size (η) was estimated from the F statistic (using Type III sums of squares) associated with the effect; η can be interpreted in the same manner as r.
Effects of affect on CMI and of optimistic expectancies on affect were estimated similarly. Because they were not correlated, positive affect and negative affect were entered together as predictors. Affect was not treated as a random variable predicting CMI because the estimate of variance in the positive affect slope was only 0.118, with a standard error of 0.103, and including a random negative affect slope caused failure to correctly estimate variance components. When testing effects of optimistic expectancies on affect, optimism was not treated as a random variable because the estimate of variance in the optimism slope predicting positive affect was 0.048, with a standard error of 0.033; comparing models with random and nonrandom effects of optimism yielded Δ–2LL = 5.0, p >.05; and including optimism as a random variable predicting negative affect caused failure to correctly estimate variance components.
Exclusions and confounds
Eleven observations were excluded for use of idiosyncratic prescribed medications that could have affected skin test results. Twelve observations were excluded for use of unprescribed medications and illegal drugs. This resulted in 520 valid observations from 123 participants included in the models.
The following potential methodological, person, and behavioral covariates of skin test results were examined: antigen type (mumps vs. candida), antigen batch, sex, age, menstrual phase, prescription medications, alcohol use, caffeine use, smoking, exercise, cold symptoms, and acute allergy or asthma symptoms. Antigen batch (p < .0001), sex (p < .0003), and alcohol use (p < .005) all significantly predicted 48-hr DTH induration, where male sex and higher alcohol consumption predicted larger DTH induration. Because of the relatively large differences between antigen batches, DTH induration was standardized (M = 0, SD = 1) within batches for analyses. Analyses were repeated after controlling for the other factors without changing the substantive results, and so they are not discussed further.
Results
Law-school optimism and CMI
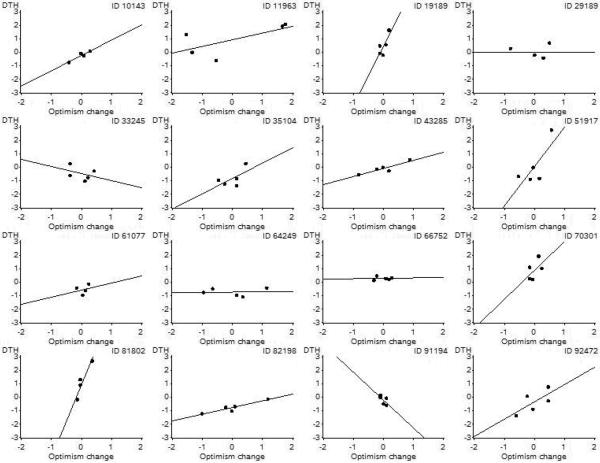
Mean optimism and the optimism deviation at each time point were entered as between-person and within-person predictors of CMI as measured by the DTH skin test, respectively. The between-person optimism term did not predict CMI. However, there was a significant within-person effect in which more optimistic expectancies about law school predicted better CMI (see Table 1). Therefore, although there were not mean differences in CMI between people that were attributable to mean levels of optimism, changes in CMI across time correlated with changes in optimism: When optimism increased, so did CMI; when optimism decreased, so did CMI. Figure 1 shows the relationship between within-person change in optimism and CMI for a sample of 16 participants selected randomly from those with at least four observations (n = 100). Most slopes were positive, but some were neutral or negative (e.g., ID 33245), and some apparently steep slopes were actually cases in which there was little change in optimism (e.g., IDs 19189 and 91194).
Table 1.
Results of the Multilevel Model With Law-School Optimism Predicting Cell-Mediated Immunity
| Measure | Parameter | Parameter value | SE |
|---|---|---|---|
| Intercept | β 00 | 0.03 | 0.07 |
| Within-person expectanciesa | β 10 | 0.36 | 0.09 |
| Between-person expectanciesb | β 01 | −0.02 | 0.12 |
| Intercept variability | τ 00 | 0.46 | 0.08 |
| Optimism slope variability | τ 11 | 0.30 | 0.15 |
| Intercept-slope covariance | τ 01 | .19 | .08 |
The effect size (η) for within-person expectancies was .19. This parameter value for this measure was significant, t(397) = 3.88, prep > .99.
The effect size (η) for between-person expectancies was .02.
Fig. 1.
Change in cell-mediated immunity (deviations from individual means, in standard deviation units) as a function of change in optimism (deviations from individual mean scores) for 16 participants selected randomly from those with at least four observations. CMI was measured with delayed-type hypersensitivity (DTH) skin testing. Regression lines are shown. See the text for an explanation of how the slopes were calculated.
Optimism, affect, and immunity
Following widely accepted conventions for testing mediation, optimism was subsequently tested as a predictor of affect, affect as predictors of CMI, and optimism as a predictor of CMI after controlling for affect (Baron & Kenny, 1986). Higher optimism correlated with more positive affect both between people, γ01 = 0.29, t(119) = 3.40, prep = .99, η = .30, and within people, γ10 = 0.35, t(418) = 8.28, prep > .99, η = .38. Optimism also correlated with less negative affect between people, γ01 = −0.50, t(119) = −7.22, prep > .99, η = .55, and within people, γ10 = −0.14, t(423) = −4.12, prep > .99, η = .20. As suggested by the effect sizes, however, optimism did not predict within- and between-person positive and negative affect equally well. Changes in optimism predicted positive affect better than negative affect, whereas stable individual differences in optimism predicted negative affect better than positive affect. Because the relationship between optimism and immunity occurred within people, this is the first piece of evidence that positive affect might be a mediator of the relationship between optimism and immunity.
Positive affect significantly predicted CMI, whereas negative affect did not, providing the second piece of evidence. Only within-person increases in positive affect significantly predicted increases in CMI, γ10 = 0.37, t(402) = 5.11, prep > .99, η = .25. Finally, the effect of optimistic expectancies on immunity was reduced when affect was included in the equation. The variances accounted for by optimism (η2 = .036) and positive affect (η2 = .061) were reduced by approximately half after controlling for the other (η2s = .018 and .030, respectively). However, each also continued to predict CMI after controlling for the other: optimism, γ30 = 0.26, t(393) = 2.71, prep = .96, η = .14; positive affect, γ10 = 0.27, t(393) = 3.46, prep = .99, η = .17, so this reduction in the optimism effect represented only partial mediation.
When and why does optimism change?
As the results discussed earlier would imply, although there was a reasonable amount of stability in law-school optimism, there was also change (intraclass correlation = .45). Rank-order changes in law-school optimism occurred as a consequence of differing influences of dispositional optimism, aptitude, and feedback (see Table 2). In the absence of information about one's performance, it is sensible to fall back on one's general expectancies and knowledge about one's aptitude. This was evident during the first semester of law school, when optimism about law school was largely influenced by dispositional optimism and, to a lesser extent, LSAT scores. Note that students' expectancies before class ranks were released (i.e., in August, October, and December) did not anticipate their later class ranks. However, once class ranks were released, expectancies correlated with actual performance. The source of expectancies, however, did not significantly influence their relationship to CMI, as there was not a significant interaction between law-school optimism and time, F(4, 396) = 2.00, prep = .82.
Table 2.
Correlations of Law-School Expectancies With Dispositional Optimism, Law School Aptitude Test (LSAT) Score, Class Rank, and Prior Optimism Over Five Waves
| Measure | Wave | ||||
|---|---|---|---|---|---|
| August | October | December | January | February | |
| Dispositional optimism | .61* | .44* | .27* | .14 | .08 |
| LSAT | .03 | .19 | .05 | .07 | .02 |
| Class rank | .05 | −.09 | .02 | −.41* | −.47* |
| Prior optimism (autocorrelation) | — | .65 | .59 | .50 | .75 |
Note: Dispositional optimism was measured with the Life Orientation Test—Revised (Scheier, Carver, & Bridges, 1994). Final class rank was known tentatively in January and definitely in February. Autocorrelations for prior optimism were calculated with respect to the previous time point.
prep > .90.
Discussion
These results provide the first evidence that changes in optimistic expectancies are accompanied by changes in immunity, as well as the first evidence for a mechanism by which this effect occurs. Changes in expectancies about law school predicted changes in cellular immune function, and this relationship could be partially accounted for by positive but not negative affect. The results support the validity of psychological interventions to improve immunity and health (e.g., Andersen et al., 2007) and suggest that efforts to correct irrationally pessimistic expectancies may be warranted, particularly if these efforts also increase positive affect. Previous investigations into the effects of expectancies on immunity and health have not found strong evidence for affect as a mediator of these effects. However, these investigations examined only differences between people and not changes within people, and they also failed to separate the effects of positive and negative affect, which may be largely independent of each other (Feldman Barrett & Russell, 1999; Watson & Tellegen, 1985). The present results support assertions and empirical evidence that the health effects of positive affect, such as greater longevity and better neuroendocrine and immune function, are more than the absence of negative affect (Danner, Snowdon, & Friesen, 2001; Lyubomirsky, King, & Diener, 2005; Pressman & Cohen, 2005; Stone, Cox, Valdimarsdottir, Jandorf, & Neale, 1987). In this case, negative affect could not account for any of the relationship between optimistic expectancies and immunity.
It is important to distinguish between the effects of situational expectancies and generalized expectancies on immunity. Peterson (2000) noted that “little” optimism (e.g., situational expectancies) and “big” optimism (p. 49; e.g., dispositional optimism; Scheier et al., 1994) may have different effects on health as well as mechanisms. In contrast to the generally positive effects of little optimism on health, there are some reports that big optimism predicts better CMI and resistance to viral disease and cancer (Allison, Guichard, Fung, & Gilain, 2003; Byrnes et al., 1998; Ironson et al., 2005), but many more examples of mixed or null results (Cohen et al., 1999; Milam, Richardson, Marks, Kemper, & McCutchan, 2004; Reed et al., 1994; Schofield et al., 2004; Schulz, Bookwala, Knapp, Scheier, & Williamson, 1996; Segerstrom, 2001, 2006; Sieber et al., 1992). With regard to immunity, big optimism often correlates with better cellular immunity under normal circumstances but with worse cellular immunity under difficult or highly stressful circumstances (see Segerstrom, 2005, for a review). Furthermore, effects of big optimism are not mediated by positive or negative affect but may be mediated by the energetic cost of increased coping efforts across the many domains implied by generalized expectancies (Segerstrom, 2006). In contrast, situational expectancies appear to correlate with affective states that, in turn, are associated with more robust immunity.
The present study is limited insofar as only one aspect of immunity was assessed. However, this parameter has important implications for the outcomes of health challenges (Christou et al., 1995; Dolan et al., 1995; Wayne et al., 1990). Another limitation of the study is the sample. Although the first year of law school provides a good opportunity for the study of variability in expectancies and the consequences thereof, law students are likely to be different from the population in a number of ways. The present results need to be extended to other samples, including clinical samples, measuring within- and between-person variability in expectancies and both positive and negative affect. The relationships in the present study among expectancies, affect, and immunity were entirely within-person, whereas other, prospective studies necessarily reported between-person effects (Leedham et al., 1995; Reed et al., 1994, 1999; Segerstrom et al., 1998). The lack of a between-person effect of expectancies in the present study could arise from between-person variability that could not be controlled, such as prior exposure to the antigen. Such exposure will increase DTH response but cannot be measured accurately by self-report, particularly in the case of candida. Therefore, it is possible that there were also between-person effects of expectancies that were obscured by random variance due to uncontrolled factors.
Optimistic expectancies have a number of psychosocial effects. These results add evidence that expectancies also affect immunity and, potentially, health. Importantly, this is the first evidence that expectancy change correlates with immunological change. The results also support the independence of positive and negative affect with regard to their relationships to both expectancies and immunity (Pressman & Cohen, 2005; Stone et al., 1987). Although optimistic expectancies associate with both increased positive affect and decreased negative affect, it may be as important for immunological health for people to be happy than to lack anxiety.
Acknowledgments
The authors thank research assistants Theresa Mickelwaite, Lise Solberg Nes, and Abbey Roach and supervising physicians Bann Kang and Beth Miller.
Funding
The study was supported by a grant from the National Institute of Mental Health (MH61531-R01).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interests with respect to their authorship and/or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Ajzen I, Fishbein M. Attitude-behavior relations: A theoretical analysis and review of empirical research. Psychological Bulletin. 1977;84:888–918. [Google Scholar]
- Allison PJ, Guichard C, Fung K, Gilain L. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. Journal of Clinical Oncology. 2003;21:543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Emery CF, Glaser R, Crespin T, Carson WE. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain, Behavior, and Immunity. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Byrnes DM, Antoni MH, Goodkin K, Efantis-Potter J, Asthana D, Simon T, et al. Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV+ Black women at risk for cervical cancer. Psychosomatic Medicine. 1998;60:714–722. doi: 10.1097/00006842-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, et al. The delayed hypersensitivity response and host resistance in surgical patients: 20 years later. Annals of Surgery. 1995;222:534–548. doi: 10.1097/00000658-199522240-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F, Kearney KA, Zegans LS, Kemeny ME, Neuhaus JM, Stites DP. Differential immune system changes with acute and persistent stress for optimists vs pessimists. Brain, Behavior, and Immunity. 1999;13:155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biological Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- Danner DD, Snowdon DA, Friesen WV. Positive emotions in early life and longevity: Findings from the nun study. Journal of Personality and Social Psychology. 2001;80:804–813. [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Dolan MJ, Clerici M, Blatt SP, Hendrix CW, Melcher GP, Boswell RN, et al. In vitro T cell function, delayed type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. Journal of Infectious Diseases. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L, Russell JA. The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science. 1999;8:10–14. [Google Scholar]
- Ironson G, Balbin E, Stuetzle R, Fletcher MA, O'Cleirigh C, Laurenceau JP, et al. Dispositional optimism and the mechanisms by which it predicts slower disease progression in HIV: Proactive behavior, avoidant coping, and depression. International Journal of Behavioral Medicine. 2005;12:86–97. doi: 10.1207/s15327558ijbm1202_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedham B, Meyerowitz BE, Muirhead J, Frist WH. Positive expectations predict health after heart transplantation. Health Psychology. 1995;14:74–79. doi: 10.1037//0278-6133.14.1.74. [DOI] [PubMed] [Google Scholar]
- Longfield JN, Margileth AM, Golden SM, Lazoritz S, Bohan JS, Cruess DF. Interobserver and method variability in tuberculin skin testing. Pediatric Infectious Disease. 1984;3:323–326. doi: 10.1097/00006454-198407000-00010. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: Does happiness lead to success? Psychological Bulletin. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Milam JE, Richardson JL, Marks G, Kemper CA, McCutchan AJ. The roles of dispositional optimism and pessimism in HIV disease progression. Psychology and Health. 2004;19:167–181. [Google Scholar]
- Peterson C. The future of optimism. American Psychologist. 2000;55:44–55. doi: 10.1037//0003-066x.55.1.44. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Rabin BS. Stress, immune function, and health: The connection. Wiley-Liss; New York: 1999. [Google Scholar]
- Reed GM, Kemeny ME, Taylor SE, Visscher BR. Negative HIV-specific expectancies and AIDS-related bereavement as predictors of symptom onset in asymptomatic HIV-positive gay men. Health Psychology. 1999;18:354–363. doi: 10.1037//0278-6133.18.4.354. [DOI] [PubMed] [Google Scholar]
- Reed GM, Kemeny ME, Taylor SE, Wang HYJ, Visscher BR. Realistic acceptance as a predictor of decreased survival time in gay men with AIDS. Health Psychology. 1994;13:299–307. doi: 10.1037//0278-6133.13.4.299. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schofield P, Ball D, Smith JG, Borland R, O'Brien P, Davis S, et al. Optimism and survival in lung cancer patients. Cancer. 2004;100:1276–1282. doi: 10.1002/cncr.20076. [DOI] [PubMed] [Google Scholar]
- Schulz R, Bookwala J, Knapp JE, Scheier M, Williamson GM. Pessimism, age, and cancer mortality. Psychology and Aging. 1996;11:304–309. doi: 10.1037//0882-7974.11.2.304. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism, goal conflict, and stressor-related immune change. Journal of Behavioral Medicine. 2001;24:441–467. doi: 10.1023/a:1012271410485. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and immunity: Do positive thoughts always lead to positive effects? Brain, Behavior, and Immunity. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC. How does optimism suppress immunity? Evaluation of three affective pathways. Health Psychology. 2006;25:653–657. doi: 10.1037/0278-6133.25.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping, and immune change in response to stress. Journal of Personality and Social Psychology. 1998;74:1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- Sieber WJ, Rodin J, Larson L, Ortega S, Cummings N, Levy S, et al. Modulation of human natural killer cell activity by exposure to uncontrollable stress. Brain, Behavior, and Immunity. 1992;6:141–156. doi: 10.1016/0889-1591(92)90014-f. [DOI] [PubMed] [Google Scholar]
- Singer JD. Fitting individual growth models using SAS PROC MIXED. In: Moskowitz DS, Hershberger SL, editors. Modeling intraindividual variability with repeated measures data: Methods and applications. Erlbaum; Mahwah, NJ: 2002. pp. 135–170. [Google Scholar]
- Solberg Nes L, Evans DR, Segerstrom SC. Optimism and college retention: Mediation by motivation, performance, and adjustment. Journal of Applied Social Psychology. 2009;9:1887–1912. [Google Scholar]
- Stone AA, Cox DS, Valdimarsdottir H, Jandorf L, Neale JM. Evidence that secretory IgA antibody is associated with daily mood. Journal of Personality and Social Psychology. 1987;52:988–993. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- Watson D. Intraindividual and interindividual analyses of positive and negative affect: Their relation to health complaints, perceived stress, and daily activities. Journal of Personality and Social Psychology. 1988;54:1020–1030. doi: 10.1037//0022-3514.54.6.1020. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X manual for the Positive and Negative Affect Schedule—expanded form. University of Iowa; Iowa City: 1994. Unpublished manuscript. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. Journal of Gerontology: Medical Sciences. 1990;45:M45–M48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- Wiedenfeld SA, O'Leary A, Bandura A, Brown S, Levine S, Raska K. Impact of perceived self-efficacy in coping with stressors on components of the immune system. Journal of Personality and Social Psychology. 1990;59:1082–1094. doi: 10.1037//0022-3514.59.5.1082. [DOI] [PubMed] [Google Scholar]