Abstract
The ATPases associated with various cellular activities (AAA) proteins are widespread in living organisms. Some of the AAA-type ATPases possess metalloprotease activities. Other members constitute the 26S proteasome complexes. In recent years, a few AAA members have been implicated in vesicle-mediated secretion, membrane fusion, cellular organelle biogenesis, and hypersensitive responses (HR) in plants. However, the physiological roles and biochemical activities of plant AAA proteins have not yet been defined at the molecular level, and regulatory mechanisms underlying their functions are largely unknown. In this study, we showed that overexpression of an Arabidopsis gene encoding a mitochondrial AAA protein, ATPase-in-Seed-Development (ASD), induces morphological and anatomical defects in seed maturation. The ASD gene is expressed at a high level during the seed maturation process and in mature seeds but is repressed rapidly in germinating seeds. Transgenic plants overexpressing the ASD gene are morphologically normal. However, seed formation is severely disrupted in the transgenic plants. The ASD gene is induced by abiotic stresses, such as low temperatures and high salinity, in an abscisic acid (ABA)- dependent manner. The ASD protein possesses ATPase activity and is localized into the mitochondria. Our observations suggest that ASD may play a role in seed maturation by influencing mitochondrial function under abiotic stress.
Keywords: AAA-type ATPase, abiotic stress, abscisic acid, Arabidopsis, seed development
INTRODUCTION
The life cycle of higher plants is not continuous but is interrupted by developmental arrests (Goldberg et al., 1989; Kermode, 1990). A mature embryo undergoes a quiescent, desiccated stage. The discontinuous development mediated by seed maturation confers an evolutionary advantage that enables plants to cope with unfavorable environmental conditions and facilitates dispersal of the plant species (Goldberg et al., 1989; Holdsworth et al., 2008; West et al., 1994).
Seed development is divided into 3 distinct phases (Gutierrez et al., 2007; Parcy et al., 1994; West and Harada, 1993). The first developmental phase is characterized by cell division and tissue differentiation. Embryogenesis is initiated by double fertilization. While the zygote divides asymmetrically to form the embryo proper and the suspensor, the endosperm constantly proliferates (Natesh and Rau, 1984; West and Harada, 1993). In the second developmental phase, which is also called the maturation phase, the growth of the embryo and cell cycle activities are arrested, and storage molecules, such as proteins, carbohydrates, and lipids, accumulate in the embryo, particularly in the cotyledons (Goldberg et al., 1989; Huang et al., 1992; Raz et al., 2001). The seeds finally become dehydrated in the third developmental phase, designated the late maturation phase. Mature seeds in the quiescent state are tolerant to desiccation (Crouch, 1987; Harada et al., 1988; Kermode, 1990; McCarty and Carson, 1991).
ABA is a central phytohormone mediating seed maturation and development. The endogenous content of ABA is low during early embryogenesis, but the ABA level is gradually elevated during the first half of seed maturation (Gutierrez et al., 2007). After seed maturation, the ABA content decreases. During the maturation phase, ABA inhibits precocious germination and vivipary, thereby rendering the seed dormancy (Finch- Savage and Leubner-Metzger, 2006; Nambara and Marion-Poll, 2003). In addition, ABA promotes the accumulation of seed storage compounds, such as carbohydrates, proteins, and oils, which would be required for seed germination and seedling growth prior to becoming an autotroph (Verdier and Thompson, 2008; Wobus and Weber, 1999). It also confers desiccation tolerance on mature seeds. The induction of late embryogenesis abundant (LEA) proteins by ABA is involved in the desiccation tolerance response (Finkelstein and Somerville, 1990; Keith et al., 1994; Meinke et al., 1994).
LEAFY COTYLEDON 1 (LEC1), LEC2, FUSCA 3 (FUS3), and ABA INSENSITIVE 3 (ABI3) are known to be key transcriptional regulators of seed maturation (Keith et al., 1994; Luerssen et al., 1998; Meinke et al., 1994; Parcy et al., 1997; Stone et al., 2001; West et al., 1994). LEC1, LEC2, and FUS3 are involved in the determination of embryonic cell fates during embryogenesis (Gutierrez et al., 2007; Verdier and Thompson, 2008; Wobus and Weber, 1999). These transcription factors, together with ABI3, also regulate initiation and maintenance of the maturation phase (Kagaya et al., 2005; Verdier and Thompson, 2008; Wobus and Weber, 1999). Consequently, seeds having mutations in these transcription factors are less resistant to desiccation, and accumulation of storage compounds is re-duced in the mutant seeds (Finkelstein and Somerville, 1990; Parcy et al., 1994; West et al., 1994). However, these transcrip-tion factor genes are differentially regulated during the devel-opmental stages, and phenotypes of the mutants are not identi-cal, supporting that these transcription factors play both redun-dant and independent roles (Parcy et al., 1997; Wobus and Weber, 1999).
The ATPases associated with various cellular activities (AAA) family members play roles in diverse cellular activities (Hanson and Whiteheart, 2005). They contain a highly conserved AAA domain consisting of Walker A and B motifs and a second-region-of-homology (SRH) domain (Karata et al., 1999). The Walker A motif, which is known as the P-loop, binds to phos-phates in NTP. The Walker B motif is associated with Mg2+. These 2 motifs are cooperatively involved in NTP hydrolysis (Hanson and Whiteheart, 2005). The SRH domain contributes to the maintenance of ATPase activity. Mutations to the con-served amino acid residues significantly decrease ATPase activity (Karata et al., 1999). The SRH domain is characteristic of the AAA protein family. It does not exist in the Walker-type ATPases, which have only 2 consensus motifs, Walker A and B (Karata et al., 1999).
The AAA-type ATPases are widely conserved in archaebacteria, prokaryotes, and eukaryotes, suggesting that they play a critical role in cellular activities. They are involved in various cellular activities and can thus be divided into several subfamilies according to their biochemical activities and physio-logical functions, including proteolytic activity, proteasome func-tions, vesicle-mediated secretion, membrane fusion, peroxi-some biogenesis, and mitochondrial functions (Karata et al., 1999; Lupas and Martin, 2002; Patel and Latterich, 1998). More than 60 AAA-type ATPase genes have been identified in the Arabidopsis genome (Sugimoto et al., 2004). They regulate diverse aspects of cellular function, such as protein degradation (Lindahl et al., 1996), 26S proteasome activity (Fu et al., 1999), vesicle trafficking (Rancour et al., 2002), peroxisome biogene-sis (Olsen, 1998), and hypersensitive responses in plants (Sugimoto et al., 2004).
In this study, we investigated an Arabidopsis AAA-type ATPase gene, ATPase-in-Seed-Development (ASD), which is highly expressed in seeds. The expression pattern of the ASD gene correlated with the seed maturation process. Transgenic plants overexpressing the ASD gene (35S:ASD) showed ab-normal seed development. The ASD gene was induced by abiotic stresses in an ABA-dependent manner. Subcellular localization assays and biochemical activity assays revealed that the ASD protein is a mitochondrial ATPase. We propose that the ASD protein is involved in stress regulation of seed maturation.
MATERIALS AND METHODS
Plant materials, growth conditions, and Arabidopsis transformation
All Arabidopsis thaliana lines used were in the Columbia back-ground (Col-0). Plants were grown in a controlled culture room set at 22℃ with a relative humidity of 55% under long days (16-h light/8-h dark) with white light illumination (120 μmol photons/ m2s) provided by fluorescent FLR40D/A tubes (Osram, Korea). The ASD-deficient asd-1 mutant (SALK-026454) was obtained from a T-DNA insertional mutant pool deposited into the Ara-bidopsis Biological Resource Center (ABRC, Ohio State Uni-versity). Absence of gene expression in the mutants was verified by reverse-transcriptase polymerase chain reaction (RT-PCR) before use.
To produce transgenic plants overexpressing the ASD gene, a full-size ASD cDNA was subcloned into the binary pH2GW7 vector under the control of the Cauliflower Mosaic Virus (CaMV) 35S promoter. Agrobacterium-mediated Arabidopsis transformation was carried out according to a modified floral dip method (Clough and Bent, 1998).
Subcellular localization
The ASD cDNA sequence was subcloned into the XbaI/BamHI-digested 326-RFP expression vector (Lee et al., 2001). The expression construct was transformed into Arabidopsis proto-plasts by a polyethylene glycol-calcium transfection method (Yoo et al., 2007). The mt-yk CD3-989 binary plasmid (Nelson et al., 2007) was used as a mitochondria-localized marker. Subcellular distribution of the ASD protein was visualized by differential interference contrast microscopy (DIC) and fluores-cence microscopy using the Zeiss LSM510 confocal micro-scope (Carl Zeiss, MicroImaging GmbH, Germany).
Transcript level analysis
Quantitative real-time RT-PCR (qRT-PCR) was employed for measuring transcript levels. RNA sample preparation, reverse transcription, and quantitative PCR were carried out on the basis of the rules that have been proposed recently to ensure reproducible and accurate measurements (Udvardi et al., 2008). Extraction of total RNA from appropriate plant materials and RT-PCR conditions have been described previously (Kim et al., 2006). The RNA samples were pretreated extensively with an RNAse-free DNAse to eliminate any contaminating genomic DNA before use.
qRT-PCR was carried out in 96-well blocks with an Applied Biosystems 7500 Real-Time PCR System using the SYBR Green I master mix in a volume of 25 μl. The PCR primers were designed using the software Primer Express: the primer sequences are listed in Supplementary Table 1. The 2-step thermal cycling profile used was 15 s at 94℃ and 1 min at 68℃. An eIF4A gene (At3g13920) was included in the assays as an internal control for normalizing the variations in cDNA quantities used (Gutierrez et al., 2008). The qRT-PCR reactions were carried out in biological triplicates using RNA samples extracted from 3 independent plant materials grown under iden-tical growth conditions. The comparative ΔΔCT method was used to evaluate the relative quantities of each amplified prod-uct in the samples. The threshold cycle (CT) was automatically determined for each reaction by the System set with default parameters. The specificity of the PCR was determined by melt curve analysis of the amplified products using the standard method installed in the System.
Extraction of total RNA from seeds
Total RNA was extracted from dry seeds according to the pro-cedure described previously (Suzuki et al., 2004). Seeds of 10-15 mg were routinely used, and total RNA was extracted using 700 μl of extraction buffer (100 mM Tris-Cl, pH 9.5, 10 mM EDTA, 0.6 M NaCl, 0.4 M trisodium citrate, 2% (w/v) lithium dodecyl sulfate, and 5% (w/v) β-mercaptoethanol) after grinding in liquid nitrogen. The RNA samples were finally cleaned up using the Qiagen Plant Total RNA Isolation Kit (Qiagen, USA).
Histochemical staining
The primers used for subcloning of the ASD gene promoter were PASD:GUS-F (5’-AAAAAGCAGGCTTTCTTTTATTGGGC CTTATTATAGCC) and PASD:GUS-R (5’-AGAAAGCTGGGTTTTTTTGGATGCTCTGTTCGG). The PCR product was subcloned into the pHGWFS7 vector (Invitrogen, USA).
For histochemical analysis of β-glucuronidase (GUS) activity, plant materials were incubated in 90% acetone for 20 min on ice, washed twice with rinsing solution [50 mM sodium phos-phate, pH 7.2, 0.5 mM K3Fe(CN)6, and 0.5 mM K4Fe(CN)6], and subsequently incubated at 37℃ for 18-24 h in rinsing solu-tion containing 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucu-ronide (X-Gluc) (Duchefa, The Netherlands). The plant materi-als were then incubated in a series of ethanol solutions ranging from 15% to 80% in order to remove chlorophylls from plant tissues. They were then mounted on microscope slides and visualized using a Nikon SMZ 800 microscope (Nikon, Japan).
Treatments with growth hormones and abiotic stresses
Two-week-old plants grown on 1/2 X Murashige and Skoog (MS)-agar plates (hereafter referred to as MS-agar plates) were transferred to fresh MS-agar plates supplemented with appro-priate growth hormones. ABA and methyl jasmonic acid (MeJA) were used at a final concentration of 20 μM. Salicylic acid (SA) was used at a final concentration of 100 μM.
For the assays on the effects of drought on gene expression, 2-week-old plants grown on MS-agar plates were put on a dry 3 MM paper at room temperature for the indicated time periods. To examine the effects of high salinity on gene expression, 2-week-old plants grown on MS-agar plates were soaked in MS liquid cultures containing 200 mM NaCl and incubated under constant light for the indicated time periods. For cold treatments, 2-week-old plants grown on MS-agar plates were transferred to a cold chamber set at 4℃ and incubated for the indicated time periods before harvesting plant materials. Whole plants were used for RNA extraction, unless otherwise specified.
Preparation of recombinant proteins
The wild-type ASD gene and a mutated ASD gene, in which K256 was mutated to either arginine or alanine, were fused in-frame to the 5’ end of the maltose binding protein (MBP)-coding sequence in the pMBP-GW vector (Invitrogen, USA). The ex-pression constructs were transformed into Escherichia coli strain BL21 cells. Cell cultures, induction, and protein purifica-tion were carried out according to the manufacture’s procedure (Novagen, Germany).
ATPase activity assay
The ATPase activity assay was carried out as previously de-scribed with minor modifications (Perlin and Spanswick, 1981). A reaction mixture containing 50 mM Tris-Cl, pH 7.5, 3 mM ATP, 3 mM MgCl2, and 1 μg of recombinant or MBP protein was incubated at 30℃ for appropriate time periods. The reaction was terminated by adding a developing reagent (0.42% ammo-nium molybdate in 1 N H2SO4:10% ascorbic acid at 5:1 [v/v] ratio). After incubation at 25℃ for 30 min, the absorbance was measured at 820 nm. To determine the amount of released phosphate in the reaction mixture, potassium dihydrogen phos-phate was used as the standard.
RESULTS
ASD is an AAA-type protein
The AAA proteins constitute a large superfamily (Snider et al., 2008). Among the AAA genes identified in Arabidopsis, the ASD gene is of particular interest. Gene expression analysis using the GENEVESTIGATOR database (https://www.gene-vestigator.com/gv/index.jsp) showed that the ASD gene is highly expressed in mature seeds and is influenced by environmental stress conditions.
Web-based bioinformatics tools were used to predict the pro-tein structure of ASD. The analysis revealed that the ASD pro-tein has a transmembrane motif in the N-terminal region and an AAA domain in the C-terminal region (Fig. 1A).
Fig. 1. Protein structure of ASD and phylogenetic analysis of ASD homologues in plants. (A) Protein structure of ASD. The ASD pro-tein contains a TM motif in the N-terminal region (black box) and an AAA domain in the C-terminal region (white box). The protein struc-ture was analyzed using the software available in the ExPASY da-tabase (http://us.expasy.org/tools/). (B) Phylogenetic analysis of ASD and its homologues from several plant species. The accession numbers of the protein sequences analyzed are XP-002275941 (grape vine, Vitis vinifera), CAH10209 (wheat, Triticum aestivum), CAJ13559 (wheat, Triticum turgidum), AAV49988 (barley, Hordeum vulgare), ABA97668 (rice, Oryza sativa), and ABO99982 (potato, Solanum tuberosum). (C) Sequence alignment of the AAA domain from ASD and its homologues. Black boxes indicate identical resi-dues, and gray boxes indicate biochemically conserved residues. The Walker A and B and SRH motifs are indicated above the se-quences. The amino acid sequence alignment was carried out using the ClustalW server (http://www.ebi.ac.uk/clustalw/).
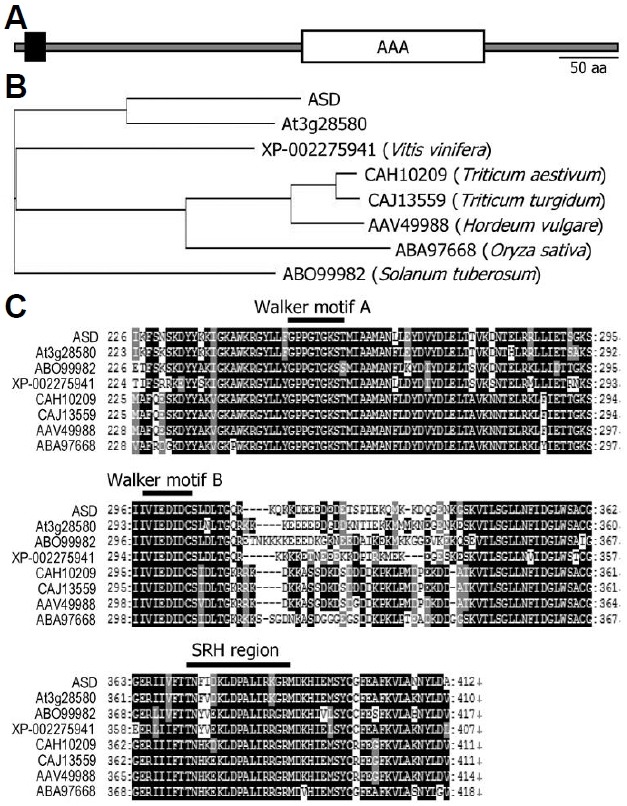
Protein homologues of ASD were identified in diverse plant species using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/blast/). They included XP-0022 75941 from grape vine (Vitis vinifera), CAH10209 and CAJ 13559 from wheat (Triticum aestivum and Triticum turgidum, respectively), AAV49988 from barley (Hordeum vulgare), ABA97668 from rice (Oryza sativa), and ABO99982 from potato (Solanum tuberosum), suggesting that the ASD protein is con-served throughout the plant kingdom (Fig. 1B). Multiple se-quence alignments showed that ASD and its homologues share high levels of amino acid sequence identities, particularly within the AAA domains (Figs. 1B and 1C).
Typical AAA proteins contain 3 conserved protein motifs with-in the AAA domain: Walker A and B motifs and the SRH motif (Karata et al., 1999; Patel and Latterich, 1998). These protein motifs were conserved in ASD and its homologues, indicating that the ASD protein is an AAA-type protein (Fig. 1C).
The ASD gene is highly expressed in seeds
To obtain clues as to the role played by the ASD protein, we analyzed the temporal expression pattern of the ASD gene using qRT-PCR. The ASD gene was expressed predominantly in seeds (Fig. 2A). ASD gene expression was particularly high in dry seeds but rapidly decreased as seeds germinate, sug-gesting that the ASD gene plays a role in maintaining seed dormancy or regulating the seed maturation process (Fig. 2B).
Fig. 2. Temporal and tissue-specific expression patterns of the ASD gene. In (A), (B), and (D), transcript levels were examined by using qRT-PCR. Biological triplicates were averaged. Bars represent standard error of the mean. (A) Temporal expression pattern. Total RNA was extracted from seeds or whole seedlings harvested at the indicated time points. d, days after germination. (B) Expression pattern in germinating seeds. Total RNA was extracted from the seeds harvested at the indicated time points. h, hours after cold imbibition. (C) Distribution of GUS activity in seeds. The pASD-GUS fusion construct, in which the GUS-coding sequence was transcrip-tionally fused to the ASD gene promoter sequence covering an approximately 1-kb region upstream of the transcription start site, was transformed into Col-0 plants. GUS activities were detected exclusively in the embryo. (D) Expression pattern during silique development. Total RNA was extracted from the siliques harvested at the indicated time points. Silique development covered from anthesis (0 DAP) to dry seed stage (21 DAP). d, days after pollina-tion (DAP).
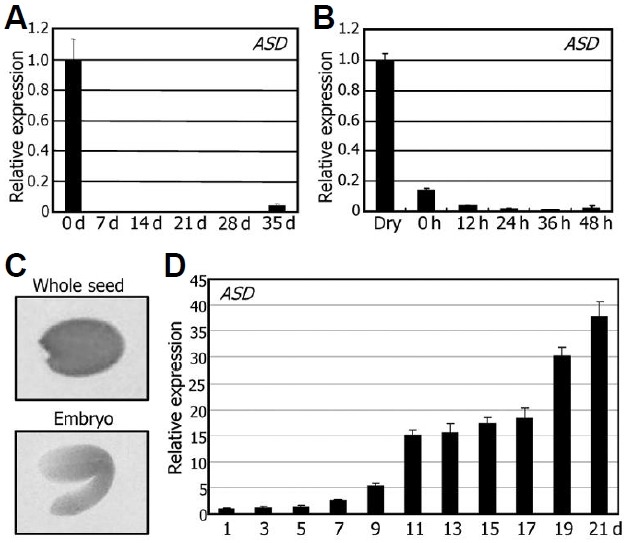
We also examined the localized expression pattern of the ASD gene in seeds using a promoter-GUS gene fusion, in which the GUS-coding sequence was transcriptionally fused to the ASD gene promoter sequence covering an approximately 1-kb region upstream of the transcription start site. The fusion construct was transformed into Col-0 plants. The GUS activity was detected in seeds (Fig. 2C) but not in seedlings and fully grown plants (data not shown). Furthermore, ASD gene ex-pression was observed only in the embryo but not in the seed coat (Fig. 2C).
The ASD gene was highly expressed in seeds, but its expression rapidly decreased in germinating seeds, suggesting that it is regulated by the seed maturation process. We there-fore analyzed the expression kinetics of the ASD gene during silique development. ASD gene expression increased gradually during the seed developmental process and reached a peak at the late developmental stage [21 days after pollination (DAP)] (Fig. 2D), supporting that the ASD gene plays a role in seed development, particularly in seed maturation step.
The ASD protein is involved in seed maturation
To investigate the physiological role of the ASD gene in seed development and maturation, we produced transgenic plants (35S:ASD) that overexpress the ASD gene under the control of the CaMV 35S promoter. A T-DNA insertional knockout mutant asd-1 was also obtained from the public database (Fig. 3A). Overexpression of the ASD gene in the 35S:ASD transgenic plants and lack of gene expression in the asd-1 mutant were verified by RT-PCR before further analysis (Fig. 3B).
Fig. 3. Seed phenotypes of the 35S:ASD transgenic and asd-1 mutant plants. (A) Mapping of the T-DNA insertion site in the asd-1 mutant. (B) Transcript levels of the ASD gene. Transcript levels were examined by RT-PCR using RNA samples extracted from 2-week-old whole plants grown on MS-agar plates. (C) Seed pheno-types. Mature seeds were photographed. (D) Quantification of per-centages of abnormal seeds. Approximately 200 seeds were used from each counting to calculate percentages of abnormal seeds. Bars represent standard error of the mean (t-test, *P < 0.01). (E) Silique development. Images show representative siliques from plants grown in soil under long days for 50 days after germination. Scale bars = 1 mm. (F) Measurements of silique lengths. Silique lengths were calculated using 50 siliques for each plant group. Bars represent standard error of the mean (t-test, *P < 0.01).
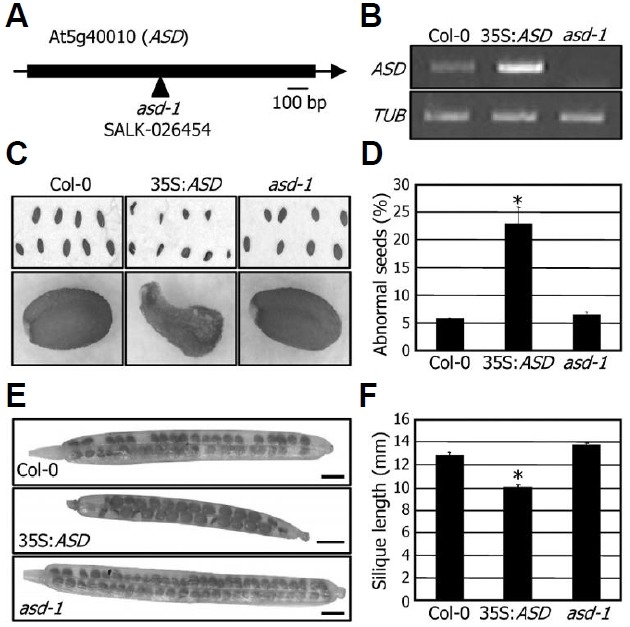
Although the phenotypes of the 35S:ASD transgenic plants were indistinguishable from wild-type plant, the transgenic plants produced a higher amount of abnormal seeds with dis-turbed morphology (Fig. 3C). Whereas approximately 5% of the seeds were morphologically abnormal in Col-0 plants, more than 20% of the seeds exhibited a distorted morphology in the 35S:ASD transgenic plants (Fig. 3D), further supporting the role of ASD in seed maturation. Seed maturation was not discernibly affected in the asd-1 mutant, which may be due to functional redundancy among the AAA proteins.
Silique development was also influenced in the 35S:ASD transgenic plants. Length of the 35S:ASD transgenic siliques was shorter than that of Col-0 and asd-1 mutant siliques (Fig. 3E). Whereas the silique length was reduced by approximately 23% in the 35S:ASD transgenic plants, it was slightly, but re-producibly, elongated in the asd-1 mutant (Fig. 3F). Seed matu-ration is essential for seed viability and germination (Lee et al., 2010; Parcy et al., 1994). Consistent with the disturbed seed maturation in the 35S:ASD transgenic plants, germination of the transgenic seeds was significantly delayed (Fig. 4), indicat-ing that the 35S:ASD transgenic seeds had defects in seed maturation processes. Overall, these observations indicated that the ASD gene is involved in the late stages of seed devel-opment, such as seed maturation and silique development.
Fig. 4. Delayed germination of the 35S:ASD transgenic seeds. Seeds were air-dried for 2 weeks after harvesting and imbibed at 4℃ on MS-agar plates for 3 days. The cold-imbibed seeds were allowed to germinate at 22℃ under long days. Appearance of visi-ble radicles was used as a morphological marker for germination. Three measurements, each consisting of 80-100 seeds, were aver-aged. Bars represent standard error of the mean. h, hours after cold imbibition.

Seed developmental marker genes are influenced in the transgenic and mutant seeds
To look into the molecular mechanisms underlying the function of the ASD gene in seed maturation, we isolated total RNAs from mature seeds and examined transcript levels of key tran-scription factor genes involved in seed development and matu-ration by using qRT-PCR. Expression of LEC1, LEC2, FUS3, and ABI3 genes were reduced 2- to 5-fold in the 35S:ASD transgenic seeds but elevated 2- to 3-fold in the asd-1 mutant seeds, consistent with the notion that the ASD gene plays a role in seed development and maturation (Fig. 5).
Fig. 5. Transcript levels of seed developmental marker genes in the 35S:ASD transgenic and asd-1 mutant seeds. Total RNA was ex-tracted from dry seeds. Transcript levels were examined by using qRT-PCR. Biological triplicates were averaged. Bars represent standard error of the mean. The y-axis is presented on a logarithmic scale for better comparison of fold changes.
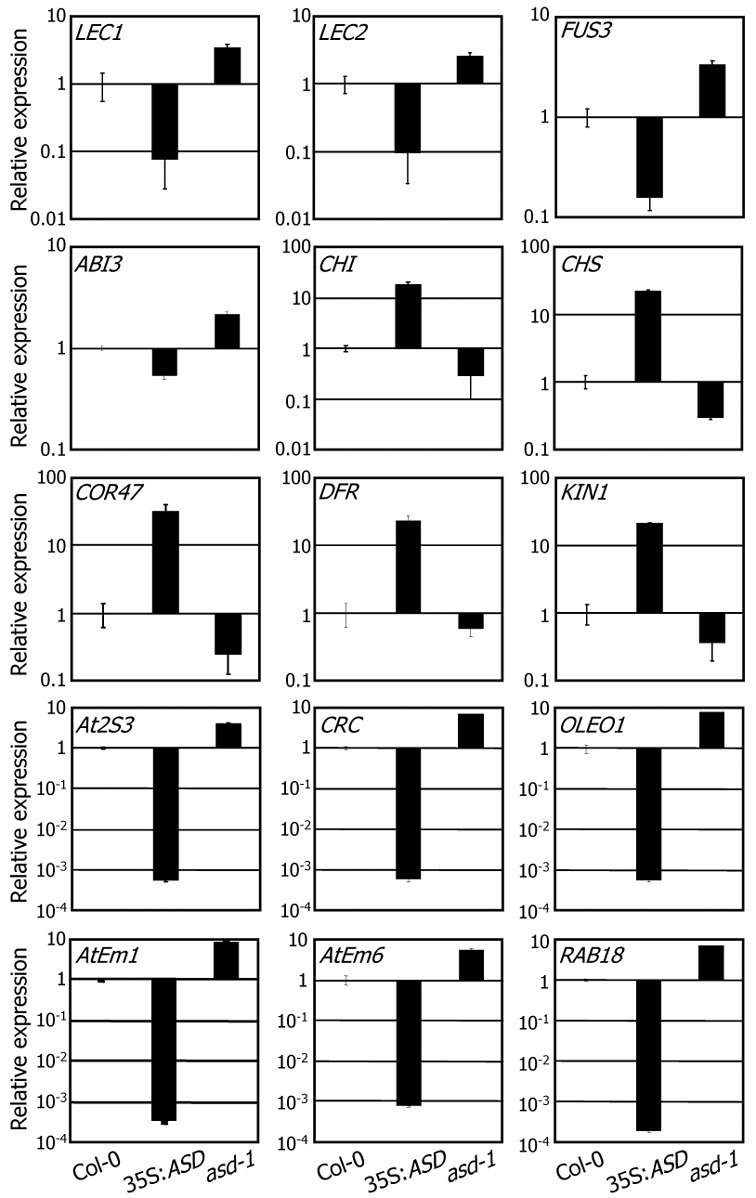
Seed developmental stages are characterized by distinct sets of marker genes (Parcy et al., 1994). The marker genes are grouped into 3 classes according to their temporal expression patterns: class I, maturation (MAT), and LEA. Cold-regulated 47 (COR47), kold-induced 1 (KIN1), chalcone synthase (CHS), chalcone flavanone isomerase (CHI), and dihydroflavonol 4-reductase (DFR) genes constitute the ‘class I’ gene family (Feinbaum and Ausubel, 1988; Gilmour et al., 1992; Kurkela and Franck, 1990; Parcy et al., 1994; Shirley et al., 1992). The-se genes were expressed predominantly during the earliest seed developmental stages, covering 0-10 DAP (Parcy et al., 1994). Whereas expression of the ‘class I’ genes was signifi-cantly elevated in the 35S:ASD transgenic seeds, it was downregulated in the asd-1 mutant seeds (Fig. 5).
The marker genes of the maturation (MAT) phase include At2S3, which encodes the Arabidopsis napin 3 protein, cruciferin C (CRC), and oleosin 1 (OLEO1) genes (Parcy et al., 1994). Their expression is initiated at 9 DAP and peaks at 15-18 DAP, functioning in the accumulation of storage proteins and lipids (Guerche et al., 1990; Pang et al., 1988; Parcy et al., 1994; van Rooijen et al., 1992). Expression patterns of the MAT members were distinct from those of the ‘class I’ marker genes in the 35S:ASD transgenic and asd-1 mutant seeds. Whereas their expression was downregulated in the 35S:ASD transgenic seeds, it was upregulated in the asd-1 mutant seeds (Fig. 5).
The LEA genes are expressed from 13 to 18 DAP, and their expression reaches a peak in the final days of silique develop-ment. The LEA gene group includes Arabidopsis thaliana late embryogenesis abundant 1 (AtEm1), AtEm6, and responsive to ABA 18 (RAB18) genes (Finkelstein, 1993; Gaubier et al., 1993; Lång and Palva, 1992; Parcy et al., 1994). Expression patterns of the LEA gene group members were similar to those of the MAT genes in the 35S:ASD transgenic and asd-1 mutant seeds (Fig. 5). Together with the seed phenotypes of the 35S:ASD transgenic plants, these results indicate that the ASD gene plays a role in the seed maturation stage and that seed development is arrested prior to completion of seed maturation in the 35S:ASD transgenic plants.
The ASD protein is localized in mitochondria
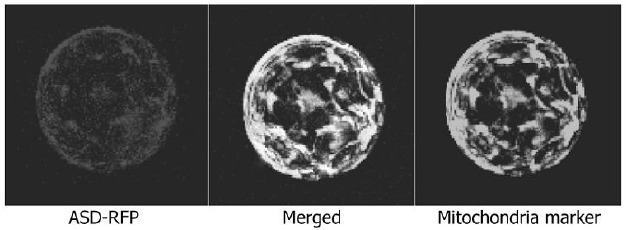
The AAA proteins are involved in diverse cellular activities (Lupas and Martin, 2002; Patel and Latterich, 1998). According-ly, their subcellular localizations vary. To determine the subcellu-lar localization of the ASD protein, a red fluorescence protein (RFP)-coding sequence was fused in frame to the 3’ end of the ASD gene, and the fusion construct was expressed transiently in Arabidopsis protoplasts. RFP signals were broadly distribut-ed within the cell, in a manner similar to the distribution of mito-chondrial proteins (Nelson et al., 2007).
To examine whether the ASD protein is localized into the mi-tochondria, organelle-specific marker constructs were coexpressed with the ASD-RFP gene fusion. RFP signals were clearly overlaid with the subcellular distribution of the mitochon-drial marker CD3-989, which is fused to yellow fluorescence protein (YFP) (Fig. 6), indicating that the ASD protein is a mito-chondrial AAA protein.
Fig. 6. Mitochondrial localization of the ASD protein in Arabidopsis protoplasts. An ASD-RFP gene fusion, in which the RFP-coding sequence was fused in-frame to the 3’ end of the ASD gene, was transiently expressed in Arabidopsis protoplasts and visualized by fluorescence microscopy. The mt-yk CD3-989 construct was coexpressed as a mitochondrial marker (Nelson et al., 2007).
The ASD protein has ATPase activity
ASD is a mitochondrial AAA protein having conserved protein motifs required for ATPase activity. To examine whether the ASD protein has ATPase activity, a recombinant ASD protein was purified as an MBP fusion from E. coli cells. We also gen-erated mutated ASD proteins (mASDs), in which the absolutely conserved lysine residue within the Walker A domain, K256, was mutated to either alanine or arginine, resulting in K256A or K256R. Immunoblot analysis using an anti-MBP antibody re-vealed that the purified recombinant proteins had an estimated molecular mass of 102 kDa (ASD 60 kDa + MBP 42 kDa = 102 kDa) (Fig. 7A).
Fig. 7. ATPase activity assays of recombinant ASD and mASD proteins. (A) Purified ASD proteins. Recombinant MBP-ASD and -mASD fusion proteins were prepared in Escherichia coli cells and partially purified. The recombinant proteins were detected by Coomassie staining (left panel) and Western blot analysis using an anti-MBP antibody (right panel). kDa, kilodaltons. (B) ATPase activi-ty assays. ATP hydrolysis activity of recombinant proteins was esti-mated in the presence (+) or absence (-) of 3 mM MgCl2. MBP protein was also included in the assays. Three measurements were averaged (t-test, *P < 0.01). Bars represent standard error of the mean.
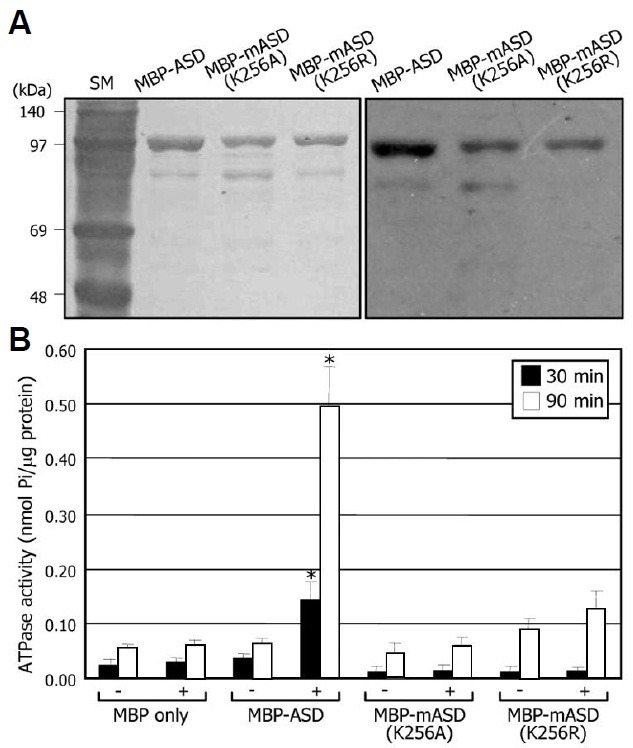
The purified recombinant ASD proteins were subject to ATPase activity assays. The MBP-ASD recombinant protein showed remarkable ATPase activity (Fig. 7B). In contrast, The mutated K256A or K256R proteins exhibited significantly sup-pressed ATPase activities, demonstrating that the ASD protein possesses ATPase activity and that K256 within the Walker A motif is critical for ATP hydrolysis. In addition, ATP hydrolytic activity was greatly enhanced in the presence of Mg2+, showing that the ASD protein is an Mg2+-dependent ATPase (Fig. 7B). MBP protein itself did not exhibit any detectable activity in ATP hydrolysis.
The ASD gene is induced by abiotic stresses in an ABA-dependent manner
To determine the nature of environmental signals regulating the ASD gene, we examined the effects of various environmental stress conditions and growth hormones on ASD gene expres-sion. ASD transcription was upregulated significantly by drought, cold, and salt stresses (Fig. 8A). The ASD gene was also in-duced significantly by ABA but unaffected by SA and MeJA (Fig. 8B), suggesting that the ASD gene is involved in abiotic stress responses.
Fig. 8. Effects of abiotic stresses and growth hormones on ASD gene expression. Two-week-old plants grown on MS-agar plates were used for treatments with abiotic stresses and growth hor-mones. Transcript levels were determined by qRT-PCR. Biological triplicates were averaged. Bars indicate standard error of the mean. (A) Effects of abiotic stresses. Plants were exposed to drought, 4℃, or 150 mM NaCl for the indicated time periods before harvesting plant materials for total RNA extraction. (B) Effects of growth hor-mones. Plants were treated with 20 μM ABA, 100 μM SA, or 20 μM MeJA for the indicated time periods before harvesting plant materi-als. (C) Effects of high salinity on ASD gene expression in the aba3-1 mutant.
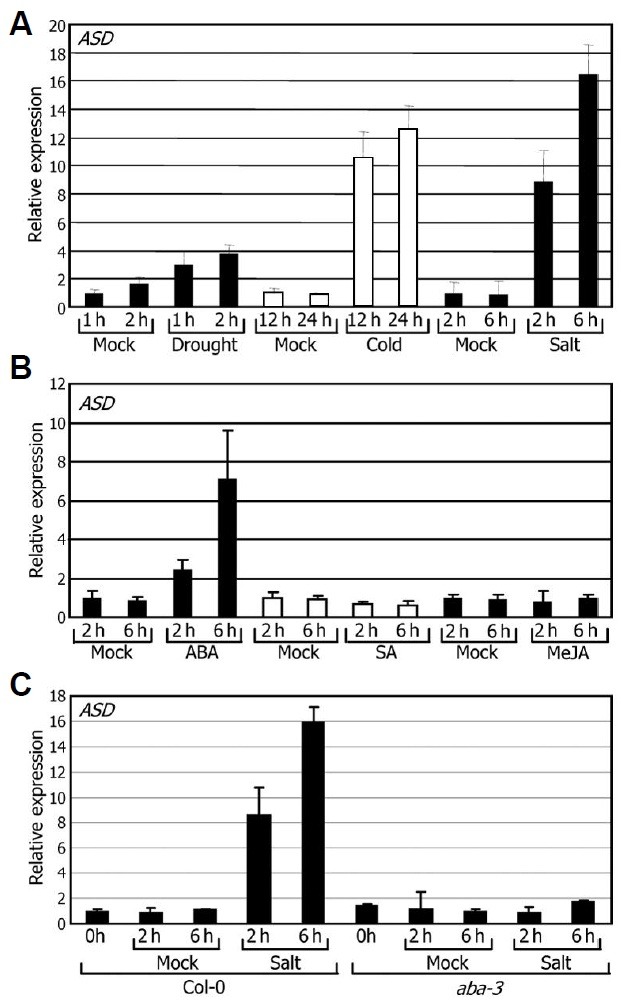
We next examined whether induction of the ASD gene by abiotic stresses depends on ABA. Salt induction of the ASD gene completely disappeared in the ABA-deficient aba3-1 mu-tant (Fig. 8C), indicating that the ASD gene is regulated by high salinity in an ABA-dependent manner.
DISCUSSION
Some members of the AAA-type ATPase family possess sev-eral biochemical activities, such as metalloprotease activities, in addition to intrinsic ATPase activity and are functional constitu-ents of 26S proteasome complexes. Consistent with the di-verse biochemical activities exerted by the AAA-type ATPases, they have been implicated in a variety of cellular and physiolog-ical processes, including biogenesis of peroxisomes and mito-chondria and HR responses (Olsen, 1998; Sugimoto et al., 2004).
Here, we identified an Arabidopsis AAA-type ATPase gene, ASD, which is involved in the seed maturation process, thereby further extending the repertoire of the roles played by AAA-type ATPases in Arabidopsis. ASD gene expression is regulated by both developmental and environmental cues. Overexpression of the ASD gene leads to disrupted seed development and maturation.
The ASD gene is expressed predominantly in developing seeds. It is notable that the ASD gene is also induced under abiotic stress conditions, which certainly affect seed maturation processes. Seed development is known to be influenced by environmental factors. In cowpea (Vigna unguiculata), drought stress reduces seed yields because of defects in seed matura-tion (Summerfield et al., 1976). Stress regulation of seed devel-opment has also been observed in other plant species (Cooper et al., 2003; Martin et al., 2010). Reproduction or seed produc-tion is an energy-consuming process. Acquisition of stress resistance also requires a high input of metabolic energy, implying that proper deposition of energy and metabolites is necessary to complete reproduction under stress conditions. The mito-chondrial ASD ATPase may play a role in mediating develop-mental and environmental signals to maintain proper seed mat-uration process, contributing to plant fitness to energy and nu-trient distributions.
The AAA-type ATPases regulate diverse cellular functions with versatile biochemical activities. One subfamily of the AAA-type ATPases possesses metalloprotease activity, as exempli-fied by FtsH (Karata et al., 1999; Langer, 2000). Membrane-bound organelles serve as membrane-integrated quality-control systems, which are involved in protein folding and selective degradation of non-native polypeptides (Langer, 2000). Mito-chondria are representative subcellular organelles that harbor ATP-dependent proteases and chaperone proteins (Langer et al., 2001). The AAA-type proteases form large protein com-plexes consisting of identical or closely related subunits in as-sociation with the membranes. Such protease complexes are thought to be involved in the degradation of mitochondrial membrane proteins (Leonhard et al., 2000). Most of the mito-chondrial AAA-type ATPases contain transmembrane (TM) motifs. Whereas the i-AAA-type members have 1 TM motif, the m-AAA-type members have 2 TM motifs (Urantowka et al., 2005). The N-terminal TM motif is required for oligomerization of the m-AAA-type members (Urantowka et al., 2005).
The ASD ATPase contains the conserved AAA domain. It al-so has 1 TM motif in the N-terminal region. The presence of these structural components and the localization of ASD in the mitochondria suggest that a certain protease activity resides in the ASD protein, although this activity was not determined in this study. According to this view, it is possible that the ASD protein may play a role in membrane-mediated protein degra-dation in mitochondria and may contribute to the proper regula-tion of seed development and maturation.
Mitochondria play a central role in ATP production via the tricarboxylic acid (TCA) cycle and the integration of carbon and nitrogen metabolism (Pellny et al., 2008; van Aken et al., 2009). Controlled distribution and availability of metabolic energy and nutrients is important for cellular and organismal adaptation under environmental stress conditions. In this regard, mito-chondria are the target of environmental stresses. Studies have shown that mitochondria respond to diverse environmental stresses (Grelet et al., 2004; van Aken et al., 2009) and that mitochondria may regulate cellular stress responses (Clifton et al., 2006; van Aken et al., 2009). The prevention of excessive reactive oxygen species (ROS) accumulation and the regula-tion of energy metabolism are well-known examples. In addition, some mitochondrial proteins, such as alternative oxidases, nicotinamide adenine dinucleotide [NAD(P)H] dehydrogenases, and heat shock proteins, are stress-responsive (van Aken et al., 2009). Although the protein targets of the ASD ATPase are still elusive, ASD-mediated signaling may be associated with mito-chondrial stress responses during seed maturation.
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Acknowledgments
This work was supported by the Brain Korea 21 and Biogreen 21 (20080401034001) Programs and by grants from the Plant Signaling Network Research Center, the National Research Foundation of Korea (2009-0087317 and 2007-03415), and from the Agricultural R&D Promotion Center (309017-5), Korea Ministry for Food, Agriculture, Forestry and Fisheries.
References
- 1.Clifton R., Millar A.H., Whelan J. Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochim. Biophys. Acta. (2006);1757:730–741. doi: 10.1016/j.bbabio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis tha-liana. Plant J. (1998);16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper B., Clarke J.D., Budworth P., Kreps J., Hutchison D., Park S., Guimil S., Dunn M., Luginbühl P., Ellero C., et al. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA. (2003);100:4945–4950. doi: 10.1073/pnas.0737574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouch M.L., Browder L.W. Regulation of gene expression during seed development in flowering plants. In Developmental Biology: A Comprehensive Synthesis, Vol. 5. Plenum Press; New York: (1987). pp. 367–404. [DOI] [PubMed] [Google Scholar]
- 5.Feinbaum R.L., Ausubel F.M. Transcriptional regula-tion of the Arabidopsis thaliana chalcone synthase gene. Mol. Cell. Biol. (1988);8:1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch-Savage W.E., Leubner-Metzger G. Seed dor-mancy and the control of germination. New Phytol. (2006);171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein R.R. Abscisic acid-insensitive mutations provide evidence for stage-specific signal pathways regulating expres-sion of an Arabidopsis late embryogenesis-abundant (lea) gene. Mol. Gen. Genet. (1993);238:401–408. doi: 10.1007/BF00291999. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein R.R., Somerville C.R. Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. (1990);94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu H., Doelling J.H., Rubin D.M., Vierstra R.D. Struc-tural and functional analysis of the six regulatory particle triple-A ATPase subunits from the Arabidopsis 26S proteasome. Plant J. (1999);18:529–539. doi: 10.1046/j.1365-313x.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaubier P., Raynal M., Hull G., Huestis G.M., Grellet F., Arenas C., Pagès M., Delseny M. Two different Em-like genes are expressed in Arabidopsis thaliana seeds during matu-ration. Mol. Gen. Genet. (1993);238:409–418. doi: 10.1007/BF00292000. [DOI] [PubMed] [Google Scholar]
- 11.Gilmour S.J., Artus N.N., Thomashow M.F. cDNA se-quence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol. Biol. (1992);18:13–21. doi: 10.1007/BF00018452. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg R.B., Barker S.J., Perez-Grau L. Regulation of gene expression during plant embryogenesis. Cell. (1989);56:149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- 13.Grelet J., Benamar A., Teyssier E., Avelange-Macherel M.H., Grun- wald D., Macherel D. Identification in pea seed mi-tochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. (2004);137:157–167. doi: 10.1104/pp.104.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerche P., Tire C., De Sa F.G., De Clercq A., Van Montagu M., Krebbers E. Differential expression of the Ara-bidopsis 2S albumin genes and the effect of increasing gene family size. Plant Cell. (1990);2:469–478. doi: 10.1105/tpc.2.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez L., Van Wuytswinkel O., Castelain M., Bellini C. Combined networks regulating seed maturation. Trends Plant Sci. (2007);12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez L., Mauriat M., Guénin S., Pelloux J., Lefebvre J.F., Louvet R., Rusterucci C., Moritz T., Guerineau F., Bellini C. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. (2008);6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanson P.I., Whiteheart S.W. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. (2005);6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 18.Harada J.J., Dietrich R.A., Comal L., Baden C.S., Verma D.P.S., Goldberg R.B. Regulation of gene expression during seed germination and post germinative development. In Plant Gene Research: Tem-poral and Spatial Regulation of Plant Genes, Vol. 5. Springer-Verlag; Wien: (1988). pp. 27–39. [Google Scholar]
- 19.Holdsworth M.J., Bentsink L., Soppe W.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. (2008);179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang N., Reinl S.J., Rodriguez R.L. RAmy2A; a novel alpha-amylase-encoding gene in rice. Gene. (1992);111:223–228. doi: 10.1016/0378-1119(92)90690-q. [DOI] [PubMed] [Google Scholar]
- 21.Kagaya Y., Toyoshima R., Okuda R., Usui H., Yamamoto A., Hattori T. LEAFY COTYLEDON1 controls seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant Cell Physiol. (2005);46:399–406. doi: 10.1093/pcp/pci048. [DOI] [PubMed] [Google Scholar]
- 22.Karata K., Inagawa T., Wilkinson A.J., Tatsuta T., Ogura T. Dissecting the role of a conserved motif (the second re-gion of homology) in the AAA family of ATPases. Site-directed mutagenesis of the ATP-dependent protease FtsH. J. Biol. Chem. (1999);274:26225–26232. doi: 10.1074/jbc.274.37.26225. [DOI] [PubMed] [Google Scholar]
- 23.Keith K., Kraml M., Dengler N.G., McCourt P. fusca3: a heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell. (1994);6:589–600. doi: 10.1105/tpc.6.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kermode A.R. Regulatory mechanisms involved in the transition from seed development to germination. Crit. Rev. Plant Sci. (1990);81:280–288. [Google Scholar]
- 25.Kim Y.S., Kim S.G., Park J.E, Park H.Y., Lim M.H., Chua N.H, Park C.M. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell. (2006);18:3132–3144. doi: 10.1105/tpc.106.043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurkela S, Franck M. Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol. Biol. (1990);15:137–144. doi: 10.1007/BF00017731. [DOI] [PubMed] [Google Scholar]
- 27.Lång V., Palva E.T. The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclima-tion process of Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. (1992);20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- 28.Langer T. AAA proteases: cellular machines for degrading membrane proteins. Trends Biochem. Sci. (2000);25:247–251. doi: 10.1016/s0968-0004(99)01541-8. [DOI] [PubMed] [Google Scholar]
- 29.Langer T., Käser M., Klanner C., Leonhard K. AAA proteases of mitochondria: quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochem. Soc. Trans. (2001);29:431–436. doi: 10.1042/bst0290431. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.J., Kim D.H., Kim Y.-W., Hwang I. Identification of a signal that distinguishes between the chloroplast outer en-velope membrane and the endomembrane system in vivo. Plant Cell. (2001);13:2175–2190. doi: 10.1105/tpc.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.J., Cho D.I., Kang J.Y., Kim M.D., Kim S.Y. AtNEK6 interacts with ARIA and is involved in ABA response during seed germination. Mol. Cells. (2010);29:559–566. doi: 10.1007/s10059-010-0070-7. [DOI] [PubMed] [Google Scholar]
- 32.Leonhard K., Guiard B., Pellecchia G., Tzagoloff A., Neupert W., Langer T. Membrane protein degradation by AAA proteases in mitochondria: extraction of substrates from either membrane surface. Mol. Cell. (2000);5:629–638. doi: 10.1016/s1097-2765(00)80242-7. [DOI] [PubMed] [Google Scholar]
- 33.Lindahl M., Tabak S., Cseke L., Pichersky E., Andersson B., Adam Z. Identification, characterization, and molecular cloning of a homologue of the bacterial FtsH protease in chloro-plasts of higher plants. J. Biol. Chem. (1996);271:29329–29334. doi: 10.1074/jbc.271.46.29329. [DOI] [PubMed] [Google Scholar]
- 34.Luerssen H., Kirik V., Herrmann P., Miséra S. FUSCA3 encodes a protein with a conserved VP1/AB13-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J. (1998);15:755–764. doi: 10.1046/j.1365-313x.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- 35.Lupas A.N., Martin J. AAA proteins. Curr. Opin. Struct. Biol. (2002);12:746–753. doi: 10.1016/s0959-440x(02)00388-3. [DOI] [PubMed] [Google Scholar]
- 36.Martin R.C., Liu P.P., Goloviznina N.A., Nonogaki H. microRNA, seeds, and Darwin?: diverse function of miRNA in seed biology and plant responses to stress. J. Exp. Bot. (2010);61:2229–2234. doi: 10.1093/jxb/erq063. [DOI] [PubMed] [Google Scholar]
- 37.McCarty D.R., Carson C.B. The molecular genetics of seed maturation in maize. Physiol. Plant. (1991);81:267–272. [Google Scholar]
- 38.Meinke D.W., Franzmann L.H., Nickle T.C., Yeung E.C. Leafy cotyledon mutants of Arabidopsis. Plant Cell. (1994);6:1049–1064. doi: 10.1105/tpc.6.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nambara E., Marion-Poll A. ABA action and interac-tions in seeds. Trends Plant Sci. (2003);8:213–217. doi: 10.1016/S1360-1385(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 40.Natesh S., Rau M.A. The embryo. In Embryology of Angiosperms, B.M., Johri, ed. Springer-Verlag; Berlin: (1984). pp. 377–443. [Google Scholar]
- 41.Nelson B.K., Cai X., Nebenführ A. A multicolored set of in vivo organelle markers for co-localization studies in Ara-bidopsis and other plants. Plant J. (2007);51:1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x. [DOI] [PubMed] [Google Scholar]
- 42.Olsen L.J. The surprising complexity of peroxisome bio-genesis. Plant Mol. Biol. (1998);38:163–189. [PubMed] [Google Scholar]
- 43.Pang P.P., Pruitt R.E., Meyerowitz E.M. Molecular cloning, genomic organization, expression and evolution of 12S seed storage protein genes of Arabidopsis thaliana. Plant Mol. Biol. (1988);11:805–820. doi: 10.1007/BF00019521. [DOI] [PubMed] [Google Scholar]
- 44.Parcy F., Valon C., Raynal M., Gaubier-Comella P., Delseny M., Giraudat J. Regulation of gene expression pro-grams during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. (1994);6:1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parcy F., Valon C., Kohara A., Miséra S., Giraudat J. The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell. (1997);9:1265–1277. doi: 10.1105/tpc.9.8.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel S., Latterich M. The AAA team: related ATPases with diverse functions. Trends Cell Biol. (1998);8:65–71. [PubMed] [Google Scholar]
- 47.Pellny T.K., Van Aken O., Dutilleul C., Wolff T., Groten K., Bor M., De Paepe R., Reyss A., Van Breusegem F., Noctor G., et al. Mitochondrial respiratory pathways modulate nitrate sensing and nitrogen-dependent regulation of plant architecture in Nicotiana sylvestris. Plant J. (2008);54:976–992. doi: 10.1111/j.1365-313X.2008.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perlin D.S., Spanswick R.M. Characterization of ATPase activity associated with corn leaf plasma membranes. Plant Physiol. (1981);68:521–526. doi: 10.1104/pp.68.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rancour D.M., Dickey C.E., Park S., Bednarek S.Y. Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol. (2002);130:1241–1253. doi: 10.1104/pp.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raz V., Bergervoet J.H., Koornneef M. Sequential steps for developmental arrest in Arabidopsis seeds. Develop-ment. (2001);128:243–252. doi: 10.1242/dev.128.2.243. [DOI] [PubMed] [Google Scholar]
- 51.Shirley B.W., Hanley S., Goodman H.M. Effects of ionizing radiation on a plant genome: analysis of two Arabidop-sis transparent testa mutations. Plant Cell. (1992);4:333–347. doi: 10.1105/tpc.4.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snider J., Thibault G., Houry W.A. The AAA+ super-family of functionally diverse proteins. Genome Biol. (2008);9:216. doi: 10.1186/gb-2008-9-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone S.L., Kwong L.W., Yee K.M., Pelletier J., Lepiniec L., Fischer R.L., Goldberg R.B., Harada J.J. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. USA. (2001);98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugimoto M., Yamaguchi Y., Nakamura K., Tatsumi Y., Sano H. A hypersensitive response-induced ATPase associat-ed with various cellular activities (AAA) protein from tobacco plants. Plant Mol. Biol. (2004);56:973–985. doi: 10.1007/s11103-004-6459-y. [DOI] [PubMed] [Google Scholar]
- 55.Summerfield R.J., Huxley P.A., Dart P.J., Hughes A.P. Some effects of environmental stress on seed yield of cowpea (Vigna unguiculata (L.) walp.) cv. Prima. Plant Soil. (1976);44:527–546. [Google Scholar]
- 56.Suzuki Y., Kawazu T., Koyama H. RNA isolation from siliques, dry seeds, and other tissues of Arabidopsis thaliana. Biotechniques. (2004);37:542–544. doi: 10.2144/04374BM03. [DOI] [PubMed] [Google Scholar]
- 57.Udvardi M.K., Czechowski T., Scheible W.R. Eleven golden rules of quantitative RT-PCR. Plant Cell. (2008);20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urantowka A., Knorpp C., Olczak T., Kolodziejczak M., Janska H. Plant mitochondria contain at least two i-AAA-like complexes. Plant Mol. Biol. (2005);59:239–252. doi: 10.1007/s11103-005-8766-3. [DOI] [PubMed] [Google Scholar]
- 59.van Aken O., Zhang B., Carrie C., Uggalla V., Paynter E., Giraud E., Whelan J. Defining the mitochondrial stress re-sponse in Arabidopsis thaliana. Mol. Plant. (2009);2:1310–1324. doi: 10.1093/mp/ssp053. [DOI] [PubMed] [Google Scholar]
- 60.van Rooijen G.J., Terning L.I., Moloney M.M. Nucleo-tide sequence of an Arabidopsis thaliana oleosin gene. Plant Mol. Biol. (1992);18:1177–1179. doi: 10.1007/BF00047721. [DOI] [PubMed] [Google Scholar]
- 61.Verdier J., Thompson R.D. Transcriptional regulation of storage protein synthesis during dicotyledon seed filling. Plant Cell Physiol. (2008);49:1263–1271. doi: 10.1093/pcp/pcn116. [DOI] [PubMed] [Google Scholar]
- 62.West M., Harada J.J. Embryogenesis in higher plants: An overview. Plant Cell. (1993);5:1361–1369. doi: 10.1105/tpc.5.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West M., Yee K.M., Danao J., Zimmerman J.L., Fischer R.L., Goldberg R.B., Harada J.J. LEAFY COTYLE-DON1 is an essential regulator of late embryogenesis and coty-ledon identity in Arabidopsis. Plant Cell. (1994);6:1731–1745. doi: 10.1105/tpc.6.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wobus U., Weber H. Seed maturation: genetic pro-grammes and control signals. Curr. Opin. Plant Biol. (1999);2:33–38. doi: 10.1016/s1369-5266(99)80007-7. [DOI] [PubMed] [Google Scholar]
- 65.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. (2007);2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]


