Abstract
Hempseed is rich in polyunsaturated fatty acids (PUFAs), which have potential as therapeutic compounds for the treatment of neurodegenerative and cardiovascular dis-ease. However, the effect of hempseed meal (HSM) intake on the animal models of these diseases has yet to be elucidated. In this study, we assessed the effects of the intake of HSM and PUFAs on oxidative stress, cytotoxicity and neurological phenotypes, and cholesterol uptake, using Drosophila models. HSM intake was shown to reduce H2O2 toxicity markedly, indicating that HSM exerts a profound antioxidant effect. Meanwhile, intake of HSM, as well as linoleic or linolenic acids (major PUFA components of HSM) was shown to ameliorate Aβ42-induced eye degeneration, thus suggesting that these compounds exert a protective effect against Aβ42 cytotoxicity. On the contrary, locomotion and longevity in the Parkinson’s disease model andeye degeneration in the Huntington’s disease model were unaffected by HSM feeding. Additionally, intake of HSM or linoleic acid was shown to reduce cholesterol uptake significantly. Moreover, linoleic acid intake has been shown to delay pupariation, and cholesterol feeding rescued the linoleic acid-induced larval growth delay, thereby indicating that linoleic acid acts antagonistically with cholesterol during larval growth. In conclusion, our results indicate that HSM and linoleic acid exert inhibitory effects on both Aβ42 cytotoxicity and cholesterol uptake, and are potential candidates for the treatment of Alzheimer’s disease and cardiovasculardisease.
Keywords: Alzheimer’s disease, cholesterol, Drosophila, hempseed meal, polyunsaturated fatty acids
INTRODUCTION
A number of previous studies have shown that polyunsatu-rated fatty acids (PUFAs) and phytosterols are critically important for human health (Fedor and Kelley, 2009; Holub and Holub, 2004; Patch et al., 2006; Youdim et al., 2000). In particular, PUFAs have been implicated in a variety of neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) (Das and Vaddadi, 2004;Delattre et al., 2010; Florent-Béchard et al., 2009). In the various experimental models and the brains of human neurodegenerative disease patients, membrane loss of PUFAs was assessed, which has been recognized as a consequence of increased lipid peroxidation induced by elevated reactive oxygen species in these diseases (Das and Vaddadi, 2004; You-dim et al., 2000). Accordingly, the moderate consumption of fish, a rich source of n-3 PUFAs, or treatment with PUFAs have all been reported to exert a beneficial effect on cognitive functions via a variety of molecular mechanisms and kinetic functions in human and animal models involving dyskinesia (Das and Vaddadi, 2004; Florent-Béchard et al., 2009). Moreover, do-cosahexaenoic acid (DHA), a major PUFA found in the brain, has been determined to reduce levels of Aβ in AD cellular models (Florent-Béchard et al., 2009) and to stimulate doparminergic turnover in a rat PD model (Delattre et al., 2010). Additionally, both PUFAs and phytosterols have been previously implicated in the prevention of cardiovascular diseases, owing to their ability to lower total cholesterol and low density lipo-protein-cholesterol levels in serum (Harris, 2008; Malini and Vanithakumari, 1990; Miettinen and Gylling, 2004; Patch et al., 2006). Although a great deal of evidence has been collected to suggest that PUFAs have beneficial effects on brain functions and the regulation of cholesterol, some other studies have raised questions regarding their effects (Florent et al., 2006; Florent-Béchard et al., 2009; Lee et al., 2009); currently, the molecular mechanisms underlying the activities of PUFAs remain to be clearly elucidated.
Hempseed is a rich source of plant oil, which contains more than 80% PUFAs and 3922-6719 mg/kg of phytosterols (e.g., sitosterol and campesterol) (Matthäus and Brühl, 2008). The fatty acids in hempseed oil include a variety of essential fatty acids, including linoleic acid (18:2n6) and alpha-linolenic acid (18:3n3), as well as gamma-linolenic acid (18:3n6) (Callaway, 2004). An excellent source of PUFAs and phytosterols, hempseed has also been recognized as a potentially useful drug for a variety of lipid-associated diseases (Callaway, 2004). Indeed, hempseed has been employed as a medicine in Asia for at least 3000 years (Callaway, 2004; de Padua et al., 1999;Kim and Mahlberg, 2003). Moreover, the findings of a series of recent studies have suggested the possibility that hempseed might be used as a therapeutic compound for the treatment of several diseases: cardioprotective effects of hempseed intake during postischemic reperfusion injury in rats and rabbits (Al-Khalifa et al., 2007; Prociuk et al., 2006), the inhibition of cholesterol-induced stimulation of platelet aggregation by a hempseed-enriched diet (Prociuk et al., 2008), and improvement of clinical symptoms in atopic dermatitis patients (Callaway et al., 2005).
Previously, we have reported that dietary hempseed meal (HSM) intake accelerates body growth by increasing cell num-bers and egg laying, and shortens the larval stage of Drosophila (Lee et al., 2010). However, the effects of HSM on neurodegenerative diseases and high cholesterolemia have yet to be clearly elucidated. In this study, based on the beneficial effects of PUFAs and phytosterols on the neurodegenerative disease and cardiovascular disease, we assessed the effects of the intake of HSM and linoleic acid on oxidative stress, cytotoxic phenotypes, and cholesterol uptake, using Drosophila models. Interestingly, HSM and linoleic acid reduced the cytotoxicity of Aβ and cholesterol uptake, thus suggesting that HSM may exert beneficial effects on AD and cardiovascular disease.
MATERIALS AND METHODS
Fly strains
The wild-type strain, glass multimer reporter (GMR)-GAL4 and sevenless (sev)-GAL4 were acquired from the Bloomington Drosophila Stock Center (Bloomington, USA). UAS-hemip-terousCA (hepCA, the constitutively active form of Drosophila JNKK) was a gift from Dr. K. Matsumoto (Nagoya University, Japan). The GMR-Aβ42 line has been previously described (Finelli et al., 2004). The Dpark1 fly line has also been previously described (Cha et al., 2005). UAS-poly127Q line was a gift from Dr. Kazemi-Esfarjani (University of Buffalo, USA).
HSM media and Drosophila feeding with cholesterol, PUFAs, campesterol
HSM media was manufactured according to the method previ-ously described by Lee et al. (2010). In an effort to confirm the effects of the lipid components of HSM on the eye degenera-tion of Aβ42 overexpression, the files were reared in cornmeal-soybean standard (CTL) media with 5.76 mg/ml of linoleic acid (Sigma-Aldrich), 2.26 mg/ml of linolenic acid (Sigma-Aldrich), 7.32 μ g/ml of campesterol (Sigma-Aldrich), or 400 μg/ml of γ-linolenic acid (Sigma-Aldrich). In order to evaluate the effects of HSM and linoleic acid on cholesterol uptake, wild-type larvae were reared in HSM media with 35.1 μg/ml of cholesterol or CTL media with 5.76 mg/ml of linoleic acid and 35.1 μg/ml of cholesterol (Sigma-Aldrich). In order to measure the effects of linoleic acid and cholesterol on larval growth, the wild-type larvae were reared in 5.76 mg/ml of linoleic acid, or 5.76 mg/ml of linoleic acid combined with 0.351 μg/ml of cholesterol-contain-ing CTL media. These are the same amounts contained in HSM (Callaway, 2004; Matthäus and Brühl, 2008). For the control experiments, same amount of ethanol as in the cholesterol media was added to the appropriate media, respectively.
Oxidative stress test
The effects of oxidative stress on the survival of the flies were assessed by feeding with Drosophila food containing hydrogen peroxide. Ten male 3-day-old flies were starved for 6 h, and then transferred to vials containing the appropriate media with 3% hydrogen peroxide. The surviving flies were counted semi-diurnally. The experiments were repeated more than 19 times for each medium.
Ectopic gene expression with the UAS-GAL4 system
The UAS-GAL4 system was used to assess the phenotypes induced by the overexpression of several genes, including Aβ42, p127Q, and hepCA. The GAL4 gene was placed near a tissue-specific enhancer, allowing for the ectopic expression of the target gene in the desired tissue. GMR-GAL4 and sev-GAL4 were employed to induce target gene expression in the eye. The external eyes were visualized under an AxioCam MRc5 (Carl Zeiss, Germany).
Climbing assay
The climbing assay was conducted as described (Cha et al., 2005; Feany and Bender, 2000) with some modifications. Ten male flies were transferred to the climbing ability test vials and incubated for 1 h at room temperature to allow for environmen-tal acclimation. After tapping the flies down to the bottom, we counted the number of flies that climbed to the top of the vial within 8 sec. Ten trials were conducted for each group.
Measuring longevity
The measurement of longevity at Dpark1 was conducted as follows. Ten flies were reared per vial with the CTL or HSM media. The flies were then transferred to vials containing fresh media every 3 days, and the numbers of live flies were counted. More than 200 flies per medium were used to measure the lifespan.
Sterol assay
The quantification of sterol content in the larvae was conducted in accordance with the previously published protocols (Fluegel et al., 2006). The Amplex Red cholesterol assay kit (Molecular Probes, USA) was employed to determine sterol content in the wandering third-instar larvae. Ten larvae were collected and washed prior to being weighed and homogenized in 150 mM NaCl, 2 mM EGTA, 50 mM Tris pH 7.5, to prepare a 100 mg/ml larval homogenate. The homogenate was spun at 5000 rpm for 5 min to pellet the cuticle debris, and the supernatant was employed for Amplex Red reactions for 30 min at 37℃. Fluore-scence was measured with a UVM 340 fluorescence spectrophotometer (Asys Hitech Gmbh, Austria) with a 560/585 nm filter set.
Measuring pupariation time
To measure pupariation time, the wild-type embryos (0-3 h) were gathered in embryo collection media (Sullivan et al., 2000). These embryos were subsequently transferred to vials containing the indicated media. In order to avoid crowding, 50 embryos were placed into each vial. The numbers of pupa were subsequently counted every 12 h.
RESULTS
HSM reduced the toxicity of H2O2
Although lipid peroxidation is a marker for neurodegenerative disease, several recent studies have demonstrated that PUFAs exert antioxidant effects in some disease models (Bouzidi et al., 2010; Casós et al., 2010; Kim et al., 2010; Zúñiga et al., 2010). Because HSM is a rich source of PUFAs, we assessed the possible functions of HSM on the reduction of oxidative toxicity. In order to confirm the antioxidant effects of HSM, flies were reared in HSM with H2O2, and their survival rates were compared to those of the flies reared in cornmeal-soybean standard media (CTL) with H2O2. Interestingly, the survival rates of flies reared in HSM-H2O2 media were increased as compared to the flies reared in CTL-H2O2 media (Fig. 1A), thereby suggesting that HSM has antioxidant properties.
Fig. 1. Inhibitory effect of HSM and linoleic acid against hydrogen peroxide toxicity. (A, B) Comparison of survival rates among wild-type flies reared in appropriate media with 3% hydrogen peroxide. (A) HSM media evidenced higher antioxidant ability than cornmeal soybean standard (CTL) media (n ≥ 190). (B) The CTL media contains linoleic acid, which evidenced an effect similar to that of HSM media against hydrogen peroxide toxicity (n ≥ 120). Error bars represent ± SE. CTL, cornmeal-soybean standard media; HSM, hempseed meal media; LI, CTL with 5.76 mg/ml linoleic acid.
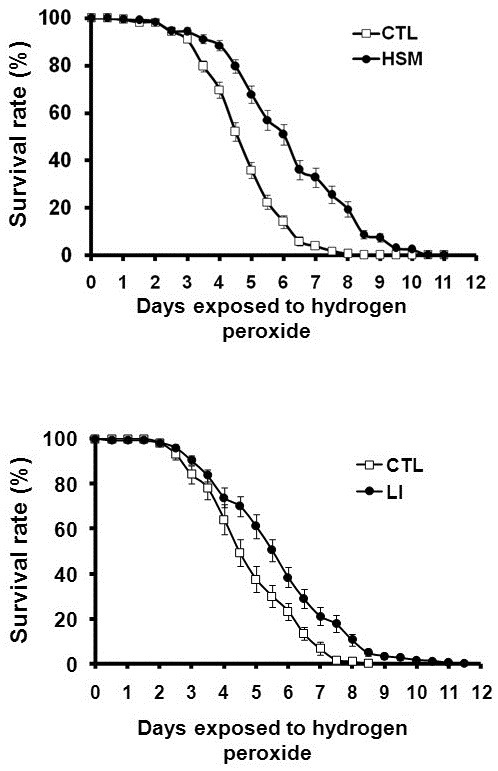
In an effort to understand which components of hempseed mediate its antioxidant activity, we evaluated the antioxidant effect of linoleic acid, a major nonpolar component of hemp-seed. As shown in Fig. 1B, feeding with CTL-H2O2 media containing 5.76 mg/ml of linoleic acid, the same amount as was contained in HSM, increased the survival rates of the flies. This finding demonstrates that the linoleic acid in HSM is responsible, at least in part, for the antioxidative effects of HSM. How-ever, the degree of increase in the survival rate of linoleic acid-fed flies was lower than that observed in the flies fed on HSM, thereby implying that the antioxidant activity of HSM is not caused solely by linoleic acid, but rather, as the result of the complex effects of various HSM components.
HSM intake ameliorated the eye degeneration induced by Aβ42
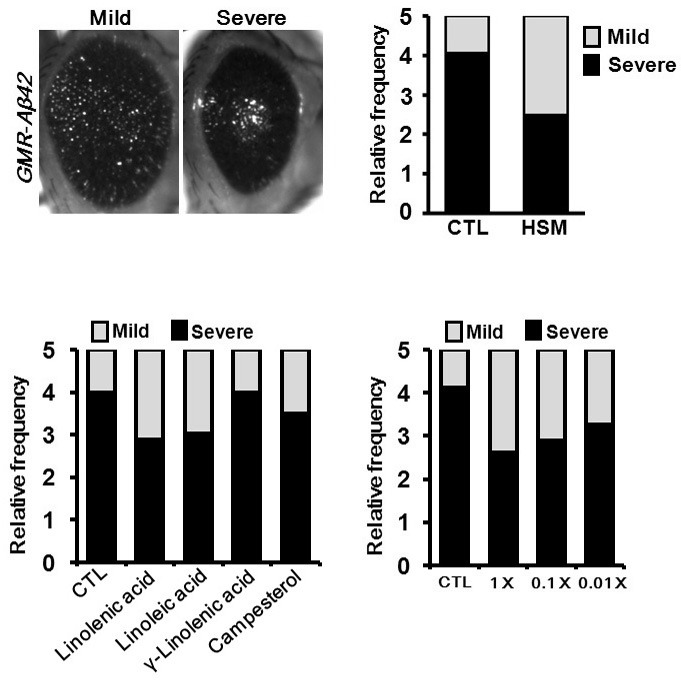
As hempseed evidences strong antioxidant activity and PUFAs have been associated with a variety of neurodegenerative diseases including AD (Florent-Béchard et al., 2009), the possible effects of dietary HSM intake on the disease-like phenotype of the Drosophila AD model was investigated. As shown in Fig. 2A, when Aβ42 was expressed in the fly eye, profound eye degeneration was noted. Additionally, the eyes can be divided into 2 classes - mild or severe - according to their size. Where-as 80% of the AD flies reared in CTL media exhibited a severe phenotype, only 50% of the flies fed on HSM exhibited the severe phenotype (Fig. 2B), thereby indicating that HSM intake suppressed Aβ42 cytotoxicity.
Fig. 2. Suppression of eye degeneration phenotype of AD model flies by dietary intake of HSM. In particular, some com-ponents of HSM (linoleic acid, linolenic acid, and campesterol) affect the eye degeneration phenotype. (A, B) The eye degeneration phenotype caused by Aβ42 overexpression was strongly suppressed by HSM intake. (A) Microscopic images of adult eyes showing the sub-types (mild and severe) of Aβ42-overexpressing flies divided by their phe-notypic severity. (B) Bar graphs represent the ratio of eye phenotypes (n ≥ 16). Phenotypic severity was expressed as the rate of mild (white) and severe (black). (C, D) The effects of the various compo-nents of HSM (C) and dose-dependent effect of linoleic acid (D) on the eye degeneration of Aβ42 overexpressing flies (n ≥ 20). The files were reared in CTL media with linoleic acid, linolenic acid, campesterol, or γ-linolenic acid. All fly strains were maintained at 29℃. CTL, cornmeal-soybean standard media; HSM, hempseed meal media; Linolenic acid, CTL with 2.26 mg/ml of linolenic acid; Linoleic acid, CTL with 5.76 mg/ml of linoleic acid; γ-Linolenic acid, CTL with 400 μg/ml of γ-linolenic acid; Campesterol, CTL with 7.32 μg/ml of campesterol; 1×, CTL with 5.76 mg/ml of linoleic acid; 0.1×, CTL with 0.576 mg/ml of linoleic acid; 0.01×, CTL with 57.6 μg/ml of linoleic acid. The genotype of the sample is GMR-Aβ42; GMR-Aβ42.
Linoleic acid and linolenic acid ameliorates the eye degeneration of the AD model
Next, we evaluated the effects of four representative components of HSM - linoleic acid, linolenic acid, γ-linolenic acid, and campesterol - on the eye degeneration of the AD model flies. As shown in Fig. 2C, feeding CTL with 5.76 mg/ml of linoleic acid or 2.26 mg/ml of linolenic acid, amounts identical to the amounts in HSM, improved the eye degeneration phenotype induced by Aβ42. The effect of linoleic acid is dose-dependent (Fig. 2D). However, CTL with campesterol or γ-linolenic acid feeding did not induce any prominent changes. These results indicated that the linoleic acid and the linolenic acid in HSM are responsible for the protective effects of HSM on the eye degenerative phenotype.
HSM intake did not affect the disease-like phenotypes of Drosophila PD and HD models
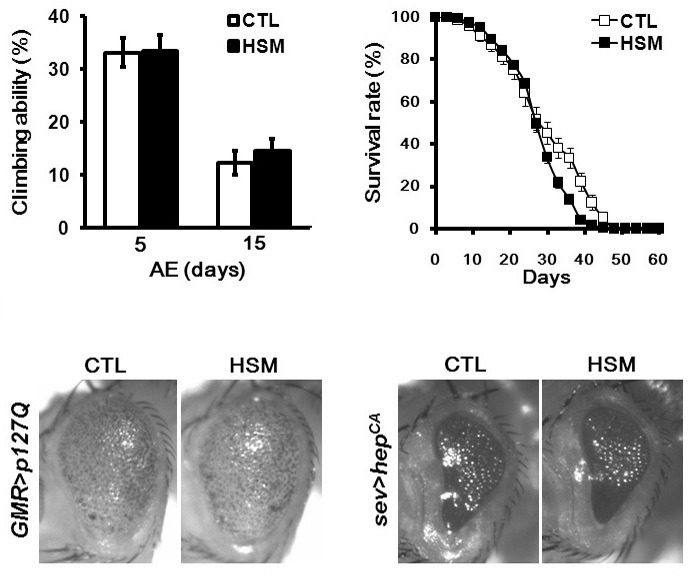
As oxidative stress has been extensively implicated in a variety of neurodegenerative diseases, such as PD and HD, and HSM showed strong antioxidant activity as shown in Fig. 1, we attempted to determine whether or not HSM intake ameliorates the disease-like phenotypes of the fly models of PD and HD. When reared in CTL media, a Drosophila parkin mutant, a PD model, evidenced a locomotive defect, which was measured by climbing ability, as well as reduced lifespan as previously described (Cha et al., 2005), and these phenotypes were unaffected by feeding HSM (Figs. 3A and 3B). Moreover, HSM feeding did not mitigate polyglutamine (polyQ)-induced eye degeneration, which is a major phenotype of the Drosophila HD model (Fig. 3C). We then evaluated the effects of HSM feeding on the eye degeneration induced JNK activation, because JNK has been implicated in oxidative stress-induced cell death. Counter to our expectations, HSM feeding also exerted no protective effects on the eye degeneration induced by hyper-activated JNK signaling (Fig. 3D).
Fig. 3. The effect of HSM intake on the phenotype of PD and HD model flies. (A, B) The effect of HSM intake on the phenotypes of PD model flies. The climbing ability (n ≥ 140) (A) and the longevity (n ≥ 200) (B) of parkin mutant flies were unaffected by HSM intake. Error bars represent ± SE. (C, D) The effect of HSM intake on the phenotype of HD and JNK hyperactivation model flies. Microscopic images of the adult eyes of HD (C) and JNK hyperacti-vation (D) model flies demonstrate that HSM intake do not influence their cytotoxic phenotypes. CTL, cornmeal-soybean standard media; HSM, hempseed meal media; AE, after eclosion. The genotypes of the samples are Dpark1/Dpark1, GMR-GAL4/+; UAS-p127Q/+, sev-GAL4/+; UAS- hepCA/+.
HSM and linoleic acid intake reduce cholesterol uptake level in Drosophila
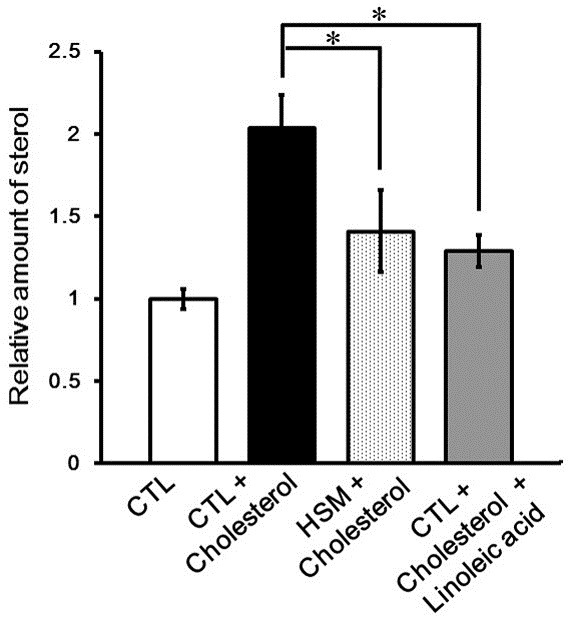
In order to verify the role of linoleic acid and HSM in the cholesterol uptake, we evaluated the effects of linoleic acid or HSM feeding on the accumulation of cholesterol in the flies fed on a high-cholesterol diet. As shown in Fig. 4, feeding with cholesterol-containing CTL food profoundly increased body cholesterol levels. However, the cholesterol levels of flies fed on linoleicacid or HSM together with cholesterol was significantly reduced relative to the controls (Fig. 4). These results demonstrate that linoleic acid and HSM inhibit cholesterol uptake.
Fig. 4. Reduction of cholesterol uptake by HSM feeding. The graph represents the relative quantity of sterol in larvae reared in CTL media with/without cholesterol, HSM media with cholesterol, or CTL media with cholesterol and linoleic acid. (*p < 0.01, n ≥ 3, Student’s t-test). Error bars represent ± SE. CTL, cornmeal-soybean standard media; CTL + Cholesterol, CTL media with 35.1 μg/ml cholesterol; HSM + Cholesterol, hempseed meal media with 35.1 μg/ml of cholesterol; CTL + Cholesterol + Linoleic acid, CTL media with 35.1 μg/ml cholesterol and 5.76 mg/ml linoleic acid.
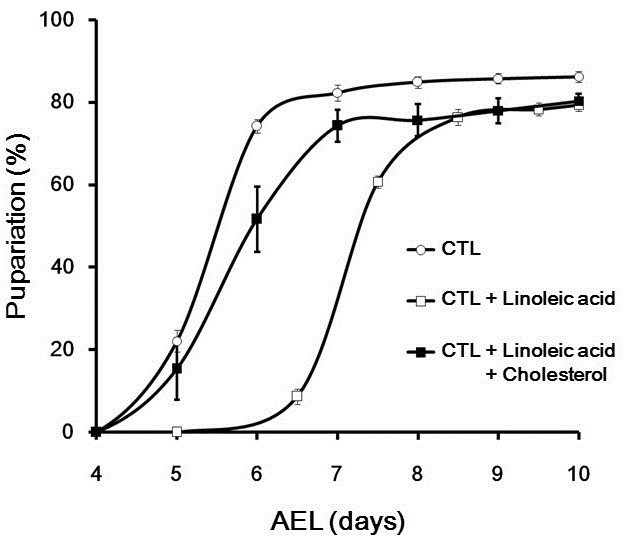
Considering that the cholesterol level is crucially important for metamorphosis, as cholesterol is a precursor of ecdysone, a molting hormone, we next attempted to ascertain whether or not linoleic acid affects larval growth. Previously, we reported that Drosophila larval growth was delayed by intake of a PUFA mixture, and enhanced by cholesterol (Lee et al., 2010). Similar to the PUFA mixture, linoleic acid intake prolonged the larval stage (Fig. 5). Interestingly, the delayed larval growth induced by linoleic acid feeding was almost completely rescued by cholesterol intake (Fig. 5), suggesting that the linoleic acid acts antagonistically with cholesterol during Drosophila development.
Fig. 5. The antagonistic effect of linoleic acid against cholesterol on larval growth. The graph represents pupariation time of larvae reared in the CTL media with/without linoleic acid or CTL media with cholesterol and linoleic acid (n ≥ 300, Student’s t-test). Error bars represent ± SE. CTL, cornmeal-soybean standard media; CTL + Linoleic acid, CTL media with 5.76 mg/ml of linoleic acid; CTL + Cholesterol + Linoleic acid, CTL media with 0.351 μg/ml of cholesterol and 5.76 mg/ml of linoleic acid; AEL, after egg laying.
DISCUSSION
Although hempseed is a rich source of PUFAs and phytosterols, both of which have been reported to exert a disease-controlling effect in neurodegenerative and cardiovascular diseases, the effects of dietary hempseed intake on these diseases have yet to be clearly understood. In this study, we attempted to characterize the effects of hempseed meal (HSM) and linoleic acid, a major compo-nent of hempseed oil, on disease-like phenotypes of Drosophila neurodegenerative disease models and choles-terol uptake. We determined that HSM and linoleic acid appeared to significantly reduce the cytotoxicity of Aβ42 and cholesterol uptake. These results show that HSM has potential for therapeutic use to control AD and high cholesterol-related diseases, and may exert beneficial effects on human health.
In this study, we demonstrated that HSM or PUFA intake restored the eye degenerative phenotype caused by Aβ42 in a Drosophila AD model. Growing data suggest that essential fatty acids play a crucial role in neurodegenerative diseases including AD (Florent-Béchard et al., 2009; Youdim et al., 2000). Lipids have been implicated in neuronal susceptibility to amyloid stress via several independent mechanisms (Florent-Béchard et al., 2009). In particular, docosahexaenoic acid (DHA), a fatty acid essential for cerebral function in mammals, has been extensively implicated in amyloid production and toxicity, the supplementation potential of which has been previously demonstrated in AD models (Florent-Béchard et al., 2009). However, analyses of the fatty acids composition of Drosophila has revealed a complete lack of C20 and C22 PUFAs such as DHA, and no genes encoding for Δ-6/Δ-5 desaturases, the key enzymes for the synthesis of C20/C22 PUFA, were detected in Drosophila (Shen et al., 2010). Therefore, we surmised that the effects of HSM on neuroprotection against amyloid would be mediated by other PUFAs, with the exception of DHA. Indeed, we determined that linoleic acid and linolenic acid, both major components of hempseed oil, exerted a similar effect against Aβ42 toxicity. Additionally, we determined that γ-linolenic acid exerted no ameliorating effects against Aβ42 toxicity. Considering that γ-linolenic acid is essentially absent in the head of Drosophila, including the brain and compound eyes (Yoshioka et al., 1985), this difference in the protective effect against Aβ42 cytotoxicity may also result from the different lipid contents in the fly tissues, rather than from its biochemical characteristics. Collectively, our data suggest both that the neuroprotective effect of PUFAs against Aβ42 toxicity is dependent on its level in the affected tissues, and that hempseed has therapeutic potential as a rich source of various PUFAs.
With regard to in vivo oxidative stress, the role of PUFAs remains controversial. Some studies have suggested that supplementation with PUFAs can result in antioxidant effects in a neurodegenerative disease model or cell culture system (Hashimoto et al., 2002; Sarsilmaz et al., 2003; Wang et al., 2003). On the contrary, other researchers have detected elevated lipid peroxidation under high oxidative stress conditions, including aging and neurodegenerative diseases (Youdim et al., 2000); with this modification, PUFA treatment exerted no protective effects against oxidative stress (Florent et al., 2006; Florent-Béchard et al., 2009). The data presented in this study demonstrated that both HSM and PUFA possess profound antioxidant capacity. These results imply that HSM and PUFAs may have a beneficial effect on a variety of oxidative stress-related diseases, including PD and HD. However, despite the finding that hempseed exhibited strong antioxidant properties ex vivo, it failed to ameliorate the disease-like phenotypes of PD and HD. This may occur as the result of low-level uptake or transport of antioxidant factor(s), such as PUFAs, in the fly intestine. However, we previously demonstrated that HSM intake significantly alters sterol and triglyceride levels, thereby suggesting that its components were absorbed successfully (Lee et al., 2010). Thus, it can be surmised that the failure of HSM to reduce the disease-like phenotypes is attributable to the loss of its antioxidant activity during digestion and absorption in our models. Further studies conducted to find a way to maintain its antioxidant activity during absorption and transport to internal tissues such as neurons will be required for the use of hempseed as an antioxidant medicine for the treatment of neurodegenerative diseases.
Although hempseed harbors only small quantities of cholesterol and some effects of HSM are expected to be mediated by cholesterol, the data presented in this study demonstrated that hempseed intake along with high cholesterol reduces cholesterol uptake. This effect may be attributable to the anticholesterol effect of some hempseed components, including PUFAs. Indeed, linoleic acid and phytosterol intake delayed and reduced the molting (Lee et al., 2010 and Fig. 5 in this study), which is enhanced by cholesterol intake (Lee et al., 2010). Furthermore, we showedtudy that feeding with cholesterol coupled with linoleic acid completely rescued the delay in the molting rate induced by linoleic acid, thereby suggesting that the suppressive effect of linoleic acid on the molting is attributable to a reduction in the cholesterol level. Our results are consistent with the results of human studies, which have demonstrated that the substitution of PUFA (the vast majority being linoleic acid) for carbohydrates or saturated fatty acids is associated with a reduction in LDL-cholesterol levels (Harris, 2008). Therefore, the results of this study demonstrate that the role of linoleic acid in the regulation of cholesterol level is well conserved in Drosophila, although the nutritional intake characteristics of this species differ greatly from those of humans. Addi-tionally, as insects cannot synthesize cholesterol de novo and cholesterol is crucial for insect growth and molting, Drosophila can be considered a useful model system for finding foods with inhibitory effects on cholesterol uptake.
How, then, does linoleic acid inhibit cholesterol function? Thus far, the mechanism underlying the effects of linoleic acid on cholesterol levels is uncertain. However, a limited amount of evidence has been collected pointing to the relationship between PUFA and cholesterol uptake. It has also been suggested that the lipid-lowering effects of PUFAs are dependent on their role in the regulation of gene expression (Sampath and Ntambi, 2004). For example, PUFAs have been previously suggested to interact with, and thus activate, transcription factors (Sampath and Ntambi, 2004). Moreover, the results of a recent study have demonstrated that PUFAs downregulate the expression of the Niemann-Pick C1-like 1 gene NPC1L1, a key intestinal cholesterol absorption protein (Alvaro et al., 2010). Therefore, linoleic acid probably controls cholesterol levels via the regulation of gene expression of the Drosophila orthologue of mammalian NPC1L1. Indeed, Drosophila npc1b, an ortho-logue of mammalian NPC1L1, is also critically important for cholesterol uptake (Horner et al., 2009). In addition to Drosophila npc1b, Drosophila DHR96 nuclear receptor has been identified as a cholesterol homeostasis regulator (Horner et al., 2009). It will be interesting to determine whether linoleic acid regulates DHR96 and npc1b expression activity.
In conclusion, in this study, we demonstrated that HSM, as a prominent source of PUFAs, exerts protective effects against Aβ42-induced cytotoxicity and hypercholesterolemia. These results show that HSM may prove of great utility as a health food, with potential for the prevention of AD and cardiovascular disease.
Acknowledgments
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries, Republic of Korea, and by a grant of the Traditional Korean Medicine R&D Project, Ministryfor Health & Welfare & Family Affairs, Republicof Korea (B090068).
References
- 1.Al-Khalifa A., Maddaford T.G., Chahine M.N. Effect of dietary hempseed intake on cardiac ischemia-reperfusion injury. American journal of physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. (2007);292:1198–1203. doi: 10.1152/ajpregu.00661.2006. [DOI] [PubMed] [Google Scholar]
- 2.Alvaro A., Rosales R., Masana L., Vallvé J.C. Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J. Nutr. Biochem. (2010);21:518–525. doi: 10.1016/j.jnutbio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Bouzidi N., Mekki K., Boukaddoum A., Dida N., Kaddous A., Bouchenak M. Effects of omega-3 polyunsaturated fatty-acid supplementation on redox status in chronic renal failure patients with dyslipidemia. J. Ren. Nutr. (2010);5:321–328. doi: 10.1053/j.jrn.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Callaway J.C. Hempseed as a nutritional resource: an overview. Euphytica. (2004);140:65–72. [Google Scholar]
- 5.Callaway J., Schwab U., Harvima I., Halonen P., Mykkänen O., Hyvönen P., Järvinen T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatolog. Treat. (2005);16:87–94. doi: 10.1080/09546630510035832. [DOI] [PubMed] [Google Scholar]
- 6.Casós K., Zaragozá M.C., Zarkovic N., Zarkovic K., Andrisic L., Portero-Otín M., Cacabelos D., Mitjavila M.T. A fish oil-rich diet reduces vascular oxidative stress inapoE (-/-) mice. Free Radic. Res. (2010);44:821–829. doi: 10.3109/10715762.2010.485992. [DOI] [PubMed] [Google Scholar]
- 7.Cha G.H., Kim S., Park J., Lee E., Kim M., Lee S.B., Kim J.M., Chung J., Cho K.S. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc. Natl. Acad. Sci. USA. (2005);102:10345–10350. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das U.N., Vaddadi K.S. Essential fatty acids in Huntington’s disease. Nutrition. (2004);20:942–947. doi: 10.1016/j.nut.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Delattre A.M., Kiss A., Szawka R.E., Anselmo-Franci J.A., Bagatini P.B., Xavier L.L., Rigon P., Achaval M., Iagher F., de David C., et al. Evaluation of chronic omega-3 fatty acids supplementation on behavioral and neurochemical alterations in 6-hydroxydopamine-lesion model of Parkinson’s disease. Neurosci. Res. (2010);66:256–264. doi: 10.1016/j.neures.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 10.de Padua L.S., Bunyaprafatsara N., Lemmens R.H.M.J. Plant resources of South-East Asia: medicinal and poisonous plants. Backhuys Publishers; Leiden: (1999). [Google Scholar]
- 11.Feany M.B., Bender W.W. A Drosophila model of Parkinson’s disease. Nature. (2000);404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 12.Fedor D., Kelley D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. (2009);12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 13.Finelli A., Kelkar A., Song H.J., Yang H., Konsolaki M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. (2004);26:365–375. doi: 10.1016/j.mcn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Florent S., Malaplate-Armand C., Youssef I., Kriem B., Koziel V., Escanyé M.C., Fifre A., Sponne I., Leininger-Muller B., Olivier J.L., et al. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J. Neuroche. (2006);96:385–895. doi: 10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 15.Florent-Béchard S., Desbène C., Garcia P., Allouche A., You- ssef I., Escanyé M.C., Koziel V., Hanse M., Malaplate-Armand C., Stenger C., et al. The essential role of lipids in Alzheimer’s disease. Biochimie. (2009);91:804–809. doi: 10.1016/j.biochi.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Fluegel M.L., Parker T.J., Pallanck L.J. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics. (2006);172:185–196. doi: 10.1534/genetics.105.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris W.S. Linoleic acid and coronary heart disease. Prostaglandins Leukot. Essent. Fatty Acids. (2008);79:169–171. doi: 10.1016/j.plefa.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto M., Hossain S., Shimada T., Sugioka K., Yamasaki H., Fujii Y., Ishibashi Y., Oka J., Shido O. Doco-sahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats. J. Neurochem. (2002);81:1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 19.Holub D.J., Holub B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell. Biochem. (2004);263:217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 20.Horner M.A., Pardee K., Liu S., King-Jones K., Lajoie G., Edwards A., Krause H.M., Thummel C.S. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. (2009);23:2711–2716. doi: 10.1101/gad.1833609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E.S., Mahlberg P.G. Secretory vesicle formation in the secretory cavity of glandular trichomes of Cannabis sativa L. (cannabaceae). Mol. Cells. (2003);15:387–395. [PubMed] [Google Scholar]
- 22.Kim S.J., Zhang Z., Saha A., Sarkar C., Zhao Z., Xu Y., Mukherjee A.B. Omega-3 and omega-6 fatty acids suppress ER- and oxidative stress in cultured neurons and neuronal progenitor cells from mice lacking PPT1. Neurosci. Lett. (2010);479:292–296. doi: 10.1016/j.neulet.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K.M., Jang J.H., Park J.S., Kim D.S., Han H.S. Effect of mild hypothermia on blood brain barrier disruption induced by oleic acid in rats. Genes Genomics. (2009);31:89–98. [Google Scholar]
- 24.Lee M.J., Park M.S., Hwang S., Hong Y.K., Choi G., Suh Y.S., Han S.Y., Kim D., Jeun J., Oh C.T., et al. Dietary hempseed meal intake increases body growth and shortens the larval stage via the upregulation of cell growth and sterol levels in Drosophila melanogaster. Mol. Cells. (2010);30:29–36. doi: 10.1007/s10059-010-0085-0. [DOI] [PubMed] [Google Scholar]
- 25.Malini T., Vanithakumari G. Rat toxicity studies with β-sitosterol. J. Ethnopharmacol. (1990);28:221–234. doi: 10.1016/0378-8741(90)90032-o. [DOI] [PubMed] [Google Scholar]
- 26.Matthäus B., Brühl L. Virgin hempseed oil: an interesting niche product. Eur. J. Lipid Sci. Technol. (2008);110:655–661. [Google Scholar]
- 27.Miettinen T.A., Gylling H. Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann. Med. (2004);36:126–134. doi: 10.1080/07853890310021625. [DOI] [PubMed] [Google Scholar]
- 28.Patch C.S., Tapsell L.C., Williams P.G., Gordon M. Plant sterols as dietary adjuvants in the reduction of cardiovascular risk: theory and evidence. Vasc. Health Risk Manag. (2006);2:157–162. doi: 10.2147/vhrm.2006.2.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prociuk M., Edel A., Gavel N., Deniset J., Ganguly R., Austria J., Ander B., Lukas A., Pierce G. The effects of dietary hempseed on cardiac ischemia/reperfusion injury in hypercholesterolemic rabbits. Exp. Clin. Cardiol. (2006);11:198–205. [PMC free article] [PubMed] [Google Scholar]
- 30.Prociuk M.A., Edel A.L., Richard M.N., Gavel N.T., Ander B.P., Dupasquier C.M., Pierce G.N. Choles-terol-induced stimulation of platelet aggregation is pre-vented by a hempseed-enriched diet. Can. J. Physiol. Pharmacol. (2008);86:153–159. doi: 10.1139/Y08-011. [DOI] [PubMed] [Google Scholar]
- 31.Sampath H., Ntambi J.M. Polyunsaturated fatty acid regulation of gene expression. Nutr. Rev. (2004);62:333–339. doi: 10.1111/j.1753-4887.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarsilmaz M., Songur A., Ozyurt H., Kuş I., Ozen O.A., Ozyurt B., Söğüt S., Akyol O. Potential role of dietary omega-3 essential fatty acids on some oxidant/antioxidant parameters in rats’ corpus striatum. Prostaglandins Leukot. Essent. Fatty Acids. (2003);69:253–259. doi: 10.1016/s0952-3278(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 33.Shen L.R., Lai C.Q., Feng X., Parnell L.D., Wan J.B., Wang J.D., Li D., Ordovas J.M., Kang J.X. Drosophila lacks C20 and C22 PUFAs. J. Lipid Res. (2010);51:2985–2992. doi: 10.1194/jlr.M008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan W., Ashburner M., Hawley R.S. Droso-phila protocols. Cold Spring Harbor Laboratory Press; New York: (2000). [Google Scholar]
- 35.Wang X., Zhao X., Mao Z.Y., Wang X.M., Liu Z.L. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. (2003);14:2457–2461. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 36.Yoshioka T., Inoue H., Kasama T., Seyama Y., Nakashima S., Nozawa Y., Hotta Y. Evidence that arachidonic acid is deficient in phosphatidylinositol of Drosophila heads. J. Biochem. (1985);98:657–662. doi: 10.1093/oxfordjournals.jbchem.a135322. [DOI] [PubMed] [Google Scholar]
- 37.Youdim K.A., Martin A., Joseph J.A. Essential fatty acids and the brain: possible health implications. Int. J. Dev. Neurosci. (2000);18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 38.Zúñiga J., Venegas F., Villarreal M., Núñez D., Chandía M., Valenzuela R., Tapia G., Varela P., Videla L.A., Fernán-dez V. Protection against in vivo liver ischemia-reper-fusion injury by n-3 long-chain polyunsaturated fatty acids in the rat. Free Radic. Res. (2010);44:854–863. doi: 10.3109/10715762.2010.485995. [DOI] [PubMed] [Google Scholar]


