Abstract
IMPORTANCE
Pharmacologic activation of mucociliary clearance (MCC) represents an emerging therapeutic strategy for patients with chronic rhinosinusitis, even in the absence of congenital mutations of the CFTR gene. Drug discovery efforts have identified small molecules that activate the cystic fibrosis transmembrane conductance regulator (CFTR), including potentiators under development for treatment of cystic fibrosis.
OBJECTIVE
To evaluate the properties of CFTR modulators and their effects on ciliary beat frequency (CBF) in human sinonasal epithelium (HSNE).
DESIGN
Primary HSNE cultures (wild type and F508del/F508del) were used to compare stimulation of CFTR-mediated Cl− conductance and CBF by the CFTR modulators genistein, VRT-532, and UCCF-152.
MAIN OUTCOMES AND MEASURES
Increase in CFTR-dependent anion transport and CBF.
RESULTS
HSNE cultures were analyzed using pharmacologic manipulation of ion transport (change in short-circuit current [ΔISC]) and high-speed digital imaging (CBF). Activation of CFTR-dependent anion transport was significantly different among agonists (P < .001), with genistein exerting the greatest effect (mean [SD] ΔISC, genistein, 23.1 [1.8] µA/cm2 > VRT-532, 8.1 [1.0] µA/cm2 > UCCF-152, 3.4 [1.4] µA/cm2 > control, 0.7 [0.2] µA/cm2; Tukey-Kramer P < .05) in the absence of forskolin. Genistein and UCCF-152 augmented CBF (under submerged conditions) significantly better (Tukey-Kramer P < .05) than cells treated with VRT-532 or dimethyl sulfoxide vehicle control (mean [SD] fold change over baseline, genistein, 1.63 [0.06]; UCCF-152, 1.56 [0.06]; VRT-532, 1.38 [0.08]; control, 1.27 [0.02]). Activation of CBF was blunted in F508del/F508del HSNE cultures.
CONCLUSIONS AND RELEVANCE
The degree of CBF stimulation was not dependent on the magnitude of Cl− secretion, suggesting that different mechanisms of action may underlie MCC activation by these small molecule potentiators. Agents that activate both CFTR-dependent ISC and CBF are particularly attractive as therapeutics because they may address 2 independent pathways that contribute to deficient MCC in chronic rhinosinusitis.
Functional sinonasal respiratory epithelium plays a critical role in the prevention of chronic rhinosinusitis. Inflammation of sinonasal respiratory epithelium and decreased mucociliary clearance (MCC) predispose patients to upper and lower respiratory bacterial infections and chronic sinus disease.1 Physiologic MCC is dependent on many factors, including ciliary beat frequency (CBF) and the depth and composition of airway surface liquid (ASL), together with constituents of the mucus and its physical properties.
The ASL depth and composition is regulated by vectorial ion transport of ions, such as chloride (Cl−) and bicarbonate (HCO3−).2–7 Diminished Cl− and HCO3− transport through the cystic fibrosis transmembrane conductance regulator (CFTR) is thought to increase mucus viscosity and dehydrate ASL, leading to stasis and impairment of MCC. Agenetic absence or dysfunction of CFTR (the major apical anion transporter in respiratory epithelia) results in the disease cystic fibrosis (CF). Patients with CF develop thick, dehydrated mucus, resulting in widespread chronic pansinusitis.8
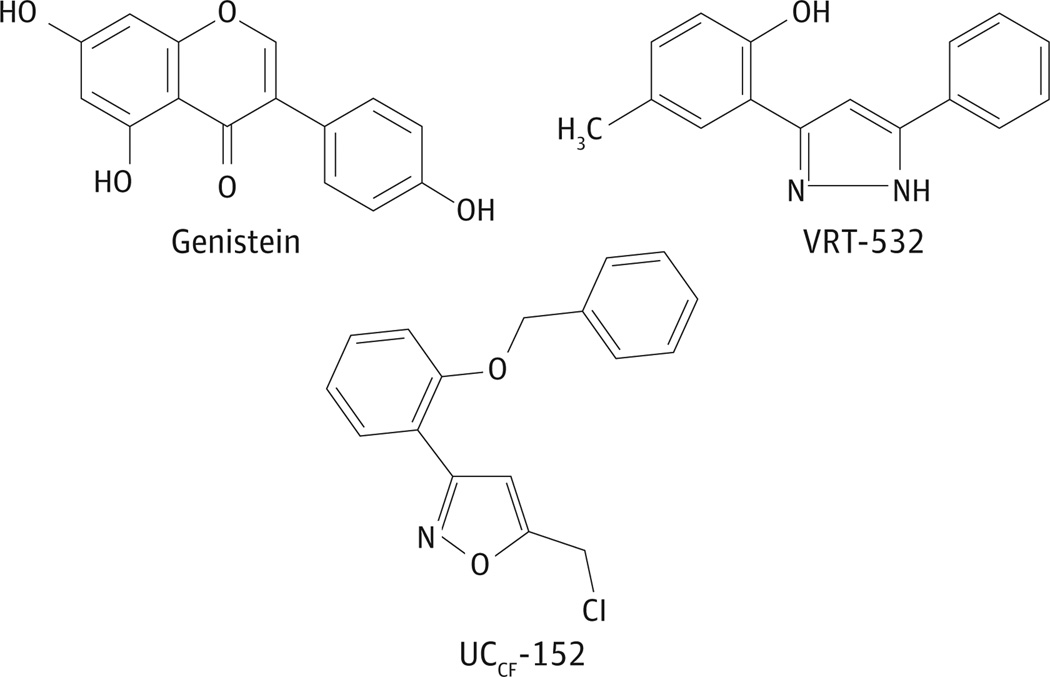
Pharmacologic compounds known to stimulate CFTR enhance Cl− secretion in vitro and in vivo9–14 and have been the subject of a number of research programs directed toward treatment of MCC-related diseases such as CF and chronic obstructive pulmonary disease.15 Prior studies by our laboratory have demonstrated that flavonoids and related compounds enhance Cl− secretion in human sinonasal epithelium (HSNE).9, 10, 12 The isoflavone genistein was shown to potentiate CFTR opening and to increase transepithelial Cl− transport in HSNE.16 The molecule is well studied and has an excellent safety profile.17, 18 In 2006, Vertex Pharmaceuticals used high-throughput screening to identify 4-methyl-2-(5-phenyl-1H-pyrazol-3-yl)phenol, or VRT-532, as a robust potentiator of wild-type CFTR (OMIM 602421), as well as CFTR encoding the common F508del mutation.19 In addition, the isoxazole UCCF-152 [3-(2-benzyloxy-phenyl)-5-chloromethyl-isoxazole] was identified as an activator of CFTR-mediated anion secretion.20 The chemical structures of these small molecules are shown in Figure 1. Because the magnitude of Cl− transport is related to both ASL hydration and ciliary beating,21 the strength of Cl− channel activation specific to human CFTR measured in primary HSNE in vitro may provide a useful means of selecting and optimizing agents worthy of additional clinical study.
Figure 1.
Chemical Structure of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators
Genistein, VRT-532, and UCCF-152 are organic, small molecules (<800 Da) that alter the activity of the CFTR channel protein by either direct effects (eg, potentiation—increasing channel open time) or activation of cell signaling pathways (eg, adenylyl cyclase and intracellular Ca2+).
The present experiments were designed to assess the relative activation of CFTR-mediated Cl− transport and ciliary activity by the modulators genistein, VRT-532, and UCCF-152 in primary HSNE and to guide selection of potential therapeutics directly delivered to the sinus epithelium.
Methods
Institutional review board approval was obtained prior to the initiation of this study.
Tissue Culture
The technique of culturing HSNE at an air-liquid interface has been described previously.22–26 In brief, HSNE was obtained from nasal epithelial tissue removed during endoscopic sinus and skull base surgical procedures. Tissue was also harvested from 1 individual with CF (F508del/F508del) to evaluate CFTR dependence of the agonists. Tissue was grown on Costar 6.5-mm-diameter permeable filter supports (Corning Life Sciences) submerged in culture medium. The medium was removed from the monolayer surface on day 4 after reaching confluence. The cells were then fed via the basal chamber. Differentiation and ciliogenesis occurred in all cultures by 10 to 14 days.
Electrophysiologic Analysis
Solutions and chemicals
The bath solution contained 120mM NaCl, 25mM NaHCO3, 3.3m MKH2PO4, 0.8mMK2HPO4, 1.2mM MgCl2, 1.2mM CaCl2, and 10mM glucose. The pH of this solution is 7.3 to 7.4 when gassed with a mixture of 95% O2 and 5% CO2 at 37°C. Chemicals were obtained from Sigma. The UCCF-152 and VRT-532 were supplied by R. Bridges, PhD (Rosalind Franklin University of Medicine and Science), and Cystic Fibrosis Foundation Therapeutics. Each solution was generated as 1000 × stock and used at 1 × in the Ussing chamber or for CBF analysis. All Ussing chamber experiments were performed with low Cl− concentration (6mM)in the mucosal bath. Pharmacologic working concentrations were as follows: amiloride, 100µM; forskolin, 100nM; genistein, 50µM; VRT-532, 10µM; UCCF-152, 50µM; and CFTRINH-172, 10µM. Optimal working concentrations of genistein, VRT-532, and UCCF-152 were determined after careful dose-response evaluation of CFTR activation in Ussing chambers. All stock solutions were dissolved in a dimethyl sulfoxide (DMSO) vehicle. Amiloride was used to block epithelial Na+ channel activity, ensuring that changes in short-circuit current (ΔISC) during subsequent manipulation were secondary to effects on Cl− transport. Forskolin activates CFTR via a cyclic adenosine monophosphate (cAMP)-dependent mechanism. INH-172 is a specific inhibitor5 of CFTR that allowed the relative contribution of CFTR (vs other anion transport pathways) to be determined in Ussing chamber systems.
Short-circuit (ISC) measurements
Transwell inserts (Co-star) were mounted in vertical Ussing chambers and continuously short-circuited after fluid resistance compensation using automatic voltage clamps (VCC 600; Physiologic Instruments). Inserts were tested in bath solutions warmed to 37°C and continuously gas-lifted with a 95% O2 /5% CO2 mixture. The ISC was measured at 1 sample per second. By convention, a positive deflection in ISC is defined as the net movement of anions in the serosal-to-mucosal direction.
CBF Analysis
Images were visualized using a 20× objective lens on an inverted microscope (Fisher Scientific).Data were captured using a model A602f-2 Basler area scan high-speed monochromatic digital video camera(Basler AG) at a sampling rate of 100 frames per second and a resolution of 640 × 480 pixels. Video images were analyzed using the Sisson-Ammons Video Analysis (SAVA) system, version 2.1.6. For each experiment, a large area of beating cilia on air-liquid interface cultures was identified with the inverted microscope and the digital image signal was routed from the camera directly into an acquisition board (National Instruments)within a Dell Workstation running the Windows XP Professional operating system. Images were analyzed with virtual instrumentation software for CBF analysis. All recordings were made at original magnification ×200.
Experiments were performed at ambient temperature (23°C).A baseline recording of CBF was conducted for each cell monolayer prior to apical administration of test solution because apical fluid addition, by itself, can increase CBF. Additional fluid depth overlying the respiratory epithelial cell surface stimulates CBF as a result of improved hydration and optimal fluid dynamics. Thus, all experiments compared the effects of agonists to that of a DMSO vehicle control solution. Whole field analysis was conducted with each point measured representing 1 cilium. The reported frequencies describe arithmetic means of these values, followed by standard deviations. Each analysis was normalized to fold-change over baseline.
Statistical Analysis
Statistical analyses were performed using analysis of variance followed by post hoc evaluation with the Tukey-Kramer method for multiple comparisons. All statistical tests were assessed at a 5% significance level (ie, α = .05).
Results
A total of 70 primary HSNE (harvested from 3 individuals) and 24 F508del/F508del HSNE cultures were used in the completion of these studies. Only cultures with transepithelial resistance (Rt) of greater than 500 Ω cm2 and ciliary differentiation were evaluated in Ussing chambers and stimulated for CBF analysis. A minimum of 5 wells were used per condition.
ISC Measurements in HSNE
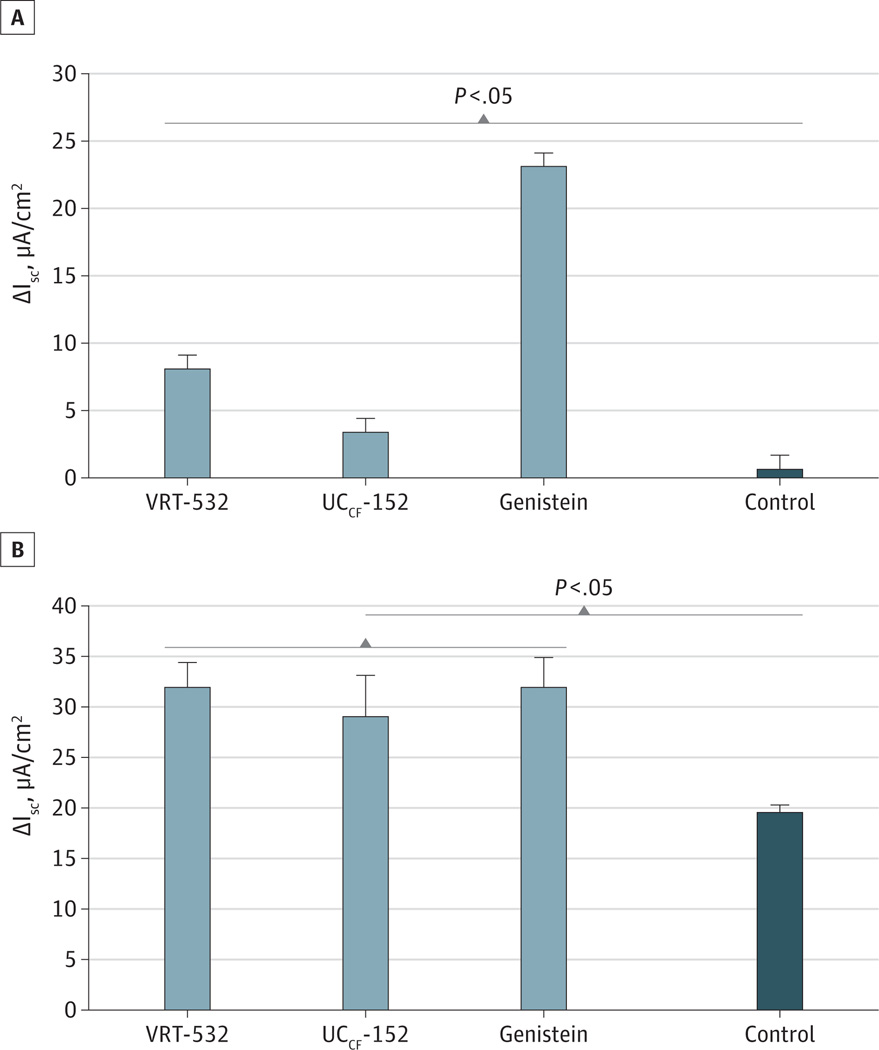
CFTR-mediated anion transport (mean [SD] change in short-circuit current [ΔISC]) was measured using HSNE cultures in Ussing chambers (Figure 2). Activation of transepithelial Cl− transport to significantly different degrees was noted among agents (n ≥ 7 per condition; genistein, 23.1 [1.8] µA/cm2 > VRT-532, 8.1 [1.0] µA/cm2 > UCCF-152, 3.4 [1.4] µA/cm2 > control, 0.7 [0.2] µA/cm2; analysis of variance P < .001; Tukey-Kramer P < .05) (Figure 3A). Importantly, supplementary stimulation of CFTR was measured by means of addition of forskolin and was similar among the 3 modulator groups (genistein, 32.1 [2.9] µA/cm2; VRT-532, 32.1 [2.4] µA/cm2; UCCF-152, 29.2 [4.0] µA/cm2), suggesting that each agent is additive to forskolin and that anion transport phenotypes were similar among cultured HSNE cells (Figure 3B). Stimulation of anion transport was significantly greater with agonist + forskolin vs DMSO control vehicle + forskolin (19.7 [0.6] µA/cm2; Tukey-Kramer P < .05). None of the 3 drugs tested activated CFTR-dependent Cl− secretion in F508del/F508del HSNE (data not shown), indicating that stimulation of anion transport is completely dependent on the presence of functional CFTR.
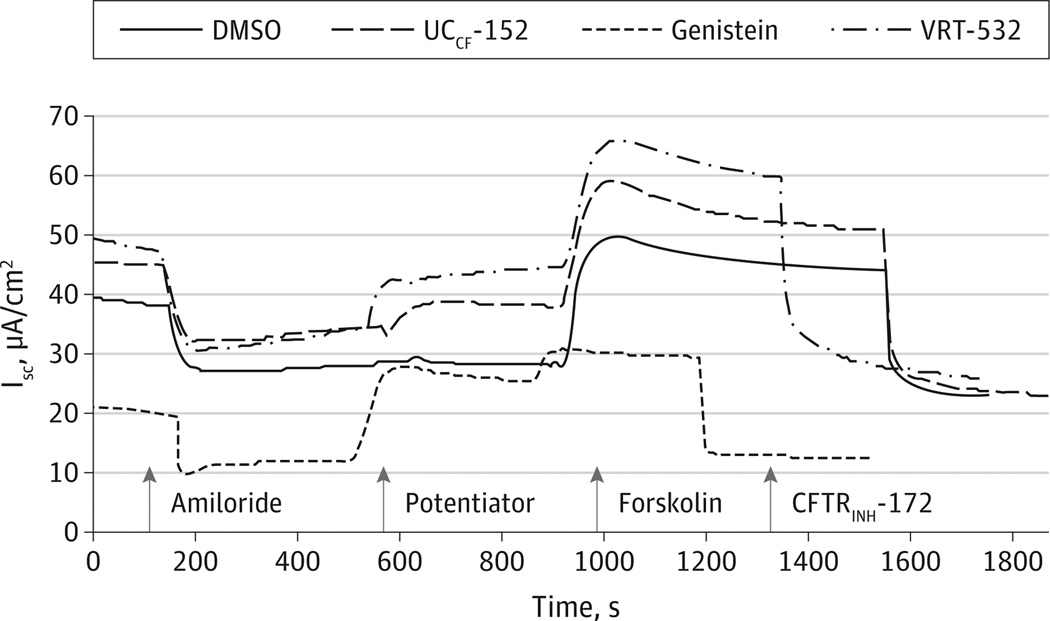
Figure 2.
Representative Ussing Chamber Tracings of Modulator Cl− Transport Activation in Human Sinonasal Epithelium (HSNE)
Wild-type HSNE cells grown on Transwell permeable supports were mounted in modified Ussing chambers under short-circuit conditions and sequentially exposed to amiloride (100µM), modulators, for skolin (100nM), and CFTRINH-172 (10µM). By convention, a positive deflection in the tracing (ΔISC) represents movement of an anion (ie, Cl−) in the serosal to mucosal direction. DMSO, dimethyl sulfoxide.
Figure 3.
Change in Short-Circuit Current (ΔISC) by Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators in Human Sinonasal Epithelium
Genistein was a more robust activator of CFTR-mediated anion transport in the absence of forskolin (A) than the potentiators VRT-532 and UCCF-152 (Tukey-Kramer P < .05). Cl− transport (measured by short-circuit current [ΔISC]) was significantly greater for each modulator compared with dimethyl sulfoxide vehicle control (Tukey-Kramer P < .05). However, total activation of CFTR (plus forskolin 100nM) was similar between drugs (B). All agonists significantly increased total CFTR-mediated transport compared with forskolin alone (Tukey-Kramer P < .05).
CBF Measurements
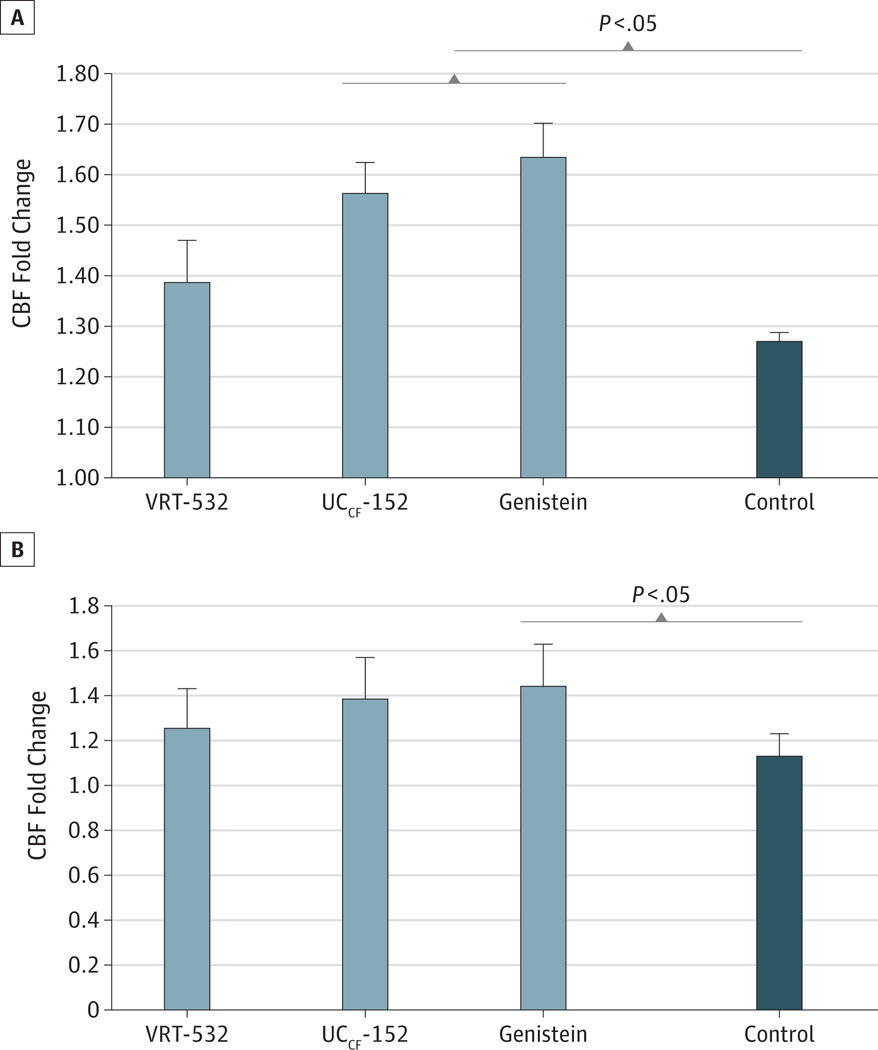
Genistein and UCCF-152 robustly stimulated CBF (mean [SD] fold change over baseline, genistein, 1.63 [0.06];UCCF-152, 1.56 [0.06]) under submerged conditions when compared with DMSO control (1.27 [0.02]; Tukey-Kramer P < .05) (Figure 4A). In contrast, only mild stimulation of CBF was noted with the addition of VRT-532 (mean [SD] fold change, 1.38 [0.08]) that was not significantly different from controls. This suggests minimal direct effects of the agent on the ciliary beat apparatus. Activation of CBF was diminished in F508del/F508del HSNE, signifying that the presence of functional CFTR is crucial to overall effects on mucociliary transport (Figure 4B). However, despite abrogated stimulation with the addition of the 3 drugs, genistein significantly augmented CBF when compared with DMSO control (Tukey-Kramer P < .05).
Figure 4.
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Effects on Ciliary Beat Frequency (CBF)
Potentiators were administered apically to wild-type (A) or F508del/F508del (B) human sinonasal epithelium for 15 minutes. Genistein and UCCF-152 increased CBF compared with baseline, and CBF was significantly greater than in cells treated with dimethyl sulfoxide vehicle control (Tukey-Kramer P < .05) in wild-type HSNE. Ciliary beat frequency was not significantly increased with VRT-532 despite significantly greater stimulation of Cl− secretion when compared with UCCF-152. Activation of CBF was diminished in cells that lack functional CFTR, but genistein significantly increased CBF compared with controls (Tukey-Kramer P < .05).
Discussion
Flavonoids and isoflavonoids make up a group of secondary plant metabolites derived from the common flavones 2-phenyl-1,4-benzopyrone and 3-phenyl-1,4-benzopyrone. In recent years, many of these compounds have been studied for their potential beneficial effects on health, including their antioxidant properties and anticancer activity. Genistein is an isoflavonoid that has also been identified as a robust activator of in vivo CFTR Cl− conductance in human airway epithelial cells.16, 27 Furthermore, some flavonoids, such as quercetin and hesperidin, strongly activate CFTR-mediated anion transport in HSNE.28 These agents amplify the frequency and duration of phosphorylated CFTR Cl− channel openings, leading to increased luminal Cl− concentration.29, 30 In addition, drug discovery efforts using high-throughput screening techniques have identified novel small molecules that activate CFTR. VRT-532 and UCCF-152 are 2 such compounds that stimulate CFTR-mediated anion transport and have been proposed as potential CF therapeutics.19, 20 VRT-532 potentiates CFTR by augmenting cAMP-dependent regulation of the Cl− channel.19 Subsequent investigations have indicated that UCCF-152 activates CFTR by inducing protein kinase A–dependent phosphorylation of the regulatory domain of CFTR.20
In the present study, we evaluated the in vitro effects of genistein, VRT-532, and UCCF-152 on CFTR-mediated Cl− conductance and CBF in primary cultures of HSNE. Our findings indicate that, among the 3 modulators, genistein is the most robust activator of human CFTR. We also established that genistein and UCCF-152 stimulate CBF—a finding that, to our knowledge, has not previously been reported. Whereas anion transport varied across 3 different CFTR modulators, the degree of CBF stimulation did not correlate with magnitude of Cl− secretion. Interestingly, VRT-532 did not demonstrate activation of CBF despite much stronger stimulation of Cl− transport compared with UCCF-152. Activation of cAMP dependent pathways by UCCF-152 is likely responsible for the prominent increase in ciliary beating, on the basis of the observation that CBF is activated, in part, by this cell signaling pathway. Whereas increases in cytosolic Ca2+ concentration through both mechanical (eg, membrane stretch) and paracrine (eg, adenosine triphosphate and uridine triphosphate) mechanisms are also critical factors regulating the velocity of mucociliary transport,4 the lack of Ca2+-activated Cl− channel stimulation in F508del/F508del CF HSNE (data not shown) Ussing chamber tracings indicates that this signaling pathway does not contribute to the measured effects. Abrogation of CBF stimulation in F508del/F508del HSNE demonstrates that the majority of effects are likely attributable to CFTR-dependent fluid secretion and thus illustrates the importance of functional CFTR to the mucociliary apparatus. The composition and/or viscosity of mucus have been suggested to depend on CFTR anion secretion, and robust anion secretion might act in part to augment CBF by altering the physical properties of periciliary mucus. However, genistein significantly increased CBF in the absence of CFTR despite the inability to trigger signaling pathways known to stimulate CBF (cAMP-dependent or Ca2+ signaling). Thus, additional studies-will be required to establish the means by which genistein activates CBF.
Conclusions
Genistein robustly stimulates transepithelial Cl− transport and CBF in primary cultured HSNE, indicating pronounced activity in a model system highly relevant to chronic rhinosinusitis. Other agents tested also promoted CFTR-dependent Cl− secretion but did not necessarily increase CBF, indicating alternative mechanisms of action. These studies establish a basis for clinical testing of CFTR activators on markers of sinonasal MCC, in particular agents that activate both CBF and anion transport through a variety of cell signaling pathways. Our data support the use of genistein and other CFTR modulators as potential therapeutics for diseases of dysfunctional MCC, including non-CF chronic sinus disease.
Acknowledgments
Funding/Support: This research was funded by grants from the Flight Attendant Medical Research Institute Young Clinical Scientist Award (072218) and National Institutes of Health/National Heart, Lung, and Blood Institute (1K08HL107142-03) to B.A.W. and by grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-03) to E. J. S. and (R01HL105487-01 and 1R03DK084110-01) to S.M. R.
Footnotes
Author Contributions: Drs Conger and Zhang, Messrs Skinner and Hicks, and Drs Rowe and Woodworth had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sorscher, Rowe, Woodworth.
Acquisition of data: Zhang, Skinner, Hicks, Woodworth.
Analysis and interpretation of data: Conger, Zhang, Skinner, Sorscher, Rowe, Woodworth.
Drafting of the manuscript: Conger, Skinner, Sorscher.
Critical revision of the manuscript for important intellectual content: Conger, Zhang, Hicks, Sorscher, Rowe, Woodworth.
Statistical analysis: Zhang, Skinner, Hicks, Woodworth.
Obtained funding: Sorscher, Woodworth.
Administrative, technical, or material support: Conger, Zhang, Sorscher, Rowe, Woodworth.
Study supervision: Sorscher, Woodworth.
Conflict of Interest Disclosures: Dr Woodworth is a consultant for ArthroCare ENT, Olympus, and Cook Medical. Drs Woodworth, Sorscher, and Rowe are inventors on a patent pending regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c)) in the United States Patent and Trademark Office.
Previous Presentation: This study was presented at the Triological Society Combined Sectional Meeting; January 27, 2012; Miami, Florida.
Correction: This article was corrected August 28, 2013, for an error in the OMIM gene database number.
REFERENCES
- 1.Möller W, Häussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274(2, pt 1):L258–L263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 3.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope. 2011;121(9):1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120(7):1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23(2):149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 6.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119(11):2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 7.Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol. 2009;94(2):171–174. doi: 10.1113/expphysiol.2008.042994. [DOI] [PubMed] [Google Scholar]
- 8.Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26(1):70–75. doi: 10.2500/ajra.2012.26.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope. 2011;121(6):1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143(3):397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(5):307–312. doi: 10.2500/ajra.2011.25.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120(5):1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 13.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer H, Fukuda N, Barbry P, Illek B, Sartori C, Matthay MA. Partial restoration of defective chloride conductance in DeltaF508 CF mice by trimethylamine oxide. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L52–L57. doi: 10.1152/ajplung.2001.281.1.L52. [DOI] [PubMed] [Google Scholar]
- 15.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122(6):1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mall M, Wissner A, Seydewitz HH, et al. Effect of genistein on native epithelial tissue from normal individuals and CF patients and on ion channels expressed in Xenopus oocytes. Br J Pharmacol. 2000;130(8):1884–1892. doi: 10.1038/sj.bjp.0703520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marini H, Minutoli L, Polito F, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23(5):715–720. doi: 10.1359/jbmr.080201. [DOI] [PubMed] [Google Scholar]
- 18.Bitto A, Polito F, Atteritano M, et al. Genistein aglycone does not affect thyroid function: results from a three-year, randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2010;95(6):3067–3072. doi: 10.1210/jc.2009-2779. [DOI] [PubMed] [Google Scholar]
- 19.Van Goor F, Straley KS, Cao D, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 20.Pyle LC, Ehrhardt A, Mitchell LH, et al. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L587–L597. doi: 10.1152/ajplung.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22(3):235–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 23.Khalid AN, Woodworth BA, Prince A, et al. Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg. 2008;139(5):695–701. doi: 10.1016/j.otohns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22(1):7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 25.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43(2):195–196. 198, 200. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 26.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol. 2007;21(5):533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 27.Sears CL, Firoozmand F, Mellander A, et al. Genistein and tyrphostin 47 stimulate CFTR-mediated Cl− secretion in T84 cell monolayers. Am J Physiol. 1995;269(6, pt 1):G874–G882. doi: 10.1152/ajpgi.1995.269.6.G874. [DOI] [PubMed] [Google Scholar]
- 28.Pyle LC, Fulton JC, Sloane PA, et al. Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue. Am J Respir Cell Mol Biol. 2010;43(5):607–616. doi: 10.1165/rcmb.2009-0281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Zeltwanger S, Yang IC, Nairn AC, Hwang TC. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating: evidence for two binding sites with opposite effects. J Gen Physiol. 1998;111(3):477–490. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Nakkash L, Hwang TC. Activation of wild-type and deltaF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflugers Arch. 1999;437(4):553–561. doi: 10.1007/s004240050817. [DOI] [PubMed] [Google Scholar]