Abstract
Campylobacter (C.) jejuni is among the leading bacterial agents causing enterocolitis worldwide. Despite the high prevalence of C. jejuni infections and its significant medical and economical consequences, intestinal pathogenesis is poorly understood. This is mainly due to the lack of appropriate animal models. In the age of 3 months, adult mice display strong colonization resistance (CR) against C. jejuni. Previous studies underlined the substantial role of the murine intestinal microbiota in maintaining CR. Due to the fact that the host-specific gut flora establishes after weaning, we investigated CR against C. jejuni in 3-week-old mice and studied intestinal and extra-intestinal immunopathogenesis as well as age dependent differences of the murine colon microbiota. In infant animals infected orally immediately after weaning C. jejuni strain B2 could stably colonize the gastrointestinal tract for more than 100 days. Within six days following infection, infant mice developed acute enterocolitis as indicated by bloody diarrhea, colonic shortening, and increased apoptotic cell numbers in the colon mucosa. Similar to human campylobacteriosis clinical disease manifestations were self-limited and disappeared within two weeks. Interestingly, long-term C. jejuni infection was accompanied by distinct intestinal immune and inflammatory responses as indicated by increased numbers of T- and B-lymphocytes, regulatory T-cells, neutrophils, as well as apoptotic cells in the colon mucosa. Strikingly, C. jejuni infection also induced a pronounced influx of immune cells into extra-intestinal sites such as liver, lung, and kidney. Furthermore, C. jejuni susceptible weaned mice harbored a different microbiota as compared to resistant adult animals. These results support the essential role of the microflora composition in CR against C. jejuni and demonstrate that infant mouse models resemble C. jejuni mediated immunopathogenesis including the characteristic self-limited enterocolitis in human campylobacteriosis. Furthermore, potential clinical and immunological sequelae of chronic C. jejuni carriers in humans can be further elucidated by investigation of long-term infected infant mice. The observed extraintestinal disease manifestations might help to unravel the mechanisms causing complications such as reactive arthritis or Guillain–Barré syndrome.
Keywords: Campylobacter jejuni, colonization resistance, Escherichia coli, extra-intestinal manifestations, gut microbiota, host–pathogen interaction, infant mice, innate immunity, long-term C. jejuni infection, susceptibility
Introduction
Campylobacter (C.) jejuni infection is a leading cause of bacterial enterocolitis. The Gram-negative, spiral-shaped rod is a zoonotic mucosal pathogen that exists as a commensal in most wild and domestic animals [1, 2]. Human infection mostly arises following consumption of contaminated chicken products [3]. Infected patients present with abdominal pain, fever, myalgia, and watery or bloody diarrhea. The acute stage of C. jejuni induced enterocolitis is further characterized by crypt abscesses, ulcerations, and accumulation of immune cells in the colon [4–7]. In the vast majority of cases the disease is self-limited. However, post-infectious complications such as the demy-elinating disorders Guillain–Barre syndrome and Miller Fisher syndrome as well as Reiter’s syndrome and reactive polyarthropathy might arise [4, 8]. Despite the high prevalence of C. jejuni infections, its significant medical and economical consequences and the fact that the entire C. jejuni genome has been sequenced, the molecular and cellular mechanisms of pathogenesis are poorly understood [1, 9]. This paucity of knowledge is mainly due to the need for a suitable animal model mimicking characteristics of human campylobacteriosis. Mice provide a convenient model for the study of colonization capacity of several pathogenic bacteria, but murine C. jejuni infection models have the disadvantage of sporadic colonization without clinical signs or intestinal pathology [4, 9]. This is mainly due to the “colonization resistance” (CR) against C. jejuni displayed by immunocompetent mice harboring a conventional commensal gut microbiota. Even though this phenomenon has been known for decades, the mechanistic basis of CR and how it can be overcome by commensal or pathogenic bacteria is still incompletely defined [10–12]. Studies with isolator raised germfree (GF) mice underlined the substantial role of the murine microbiota in CR against C. jejuni. Following infection, C. jejuni was not only shown to colonize the entire gastrointestinal (GI) tract of these animals, but also induced clinical signs of disease including granulocyte infiltrates and bloody diarrhea, as well as humoral immune responses [13–17]. Despite the increased susceptibility to C. jejuni infection, the use of GF mouse models is hampered by limitations caused by the absence of an intact immune system. Therefore, GF mice do not represent a suitable experimental model of C. jejuni infections in humans. Previous work from our group revealed that gnotobiotic (GB) mice generated by quintuple antibiotic treatment as well as with human flora associated (hfa) GB mice are suited for dissecting the interplay of C. jejuni, the gut microbiota, and immune responses. Following peroral infection, GB and hfa mice developed C. jejuni mediated apoptosis and inflammatory responses in the colon in situ [18]. Furthermore, very recent work demonstrated that the host-specific diet plays a crucial role in affecting CR and modulating susceptibility to C. jejuni infection. Infection of GB mice fed on human cafeteria diet (CAF) resulted in the development of characteristic immune responses similar to those seen in humans [19]. Most importantly, weaned mice were shown earlier to be susceptible to C. jejuni infection. The corresponding models have been used successfully to study the impact of C. jejuni flagella, bismuth subsalicylate, and endotoxin in intestinal C. jejuni colonization, including potential vaccination strategies [20–27]. However, neither intestinal and extra-intestinal disease manifestation, nor specific immune responses and intestinal microbiota composition have been investigated in C. jejuni infected weaned mice so far. Results of the present study demonstrate that weaned mice infected perorally with C. jejuni strain B2 developed significant, but self-limited intestinal disease manifestations mimicking human campylobacteriosis. Remarkably, despite the fact that infected mice recovered completely from intestinal disease, C. jejuni stably colonized the intestines for more than 100 days. Even though long-term infected mice were clinically asymptomatic, distinct pro-inflammatory immune responses in the colonic mucosa could be observed. Most strikingly, pro-inflammatory immunopathology was not restricted to the GI tract but also affected extra-intestinal organs such as liver, lung, and kidney. Finally, cultural and molecular analyses of the intestinal flora revealed significant differences in the microbiota composition in infant as compared to adult mice. Taken together, weaned mice harboring their conventional gut microbiota provide a valuable murine C. jejuni infection model, characterized not only by stable colonization, but also by significant clinical immunopathology mimicking human campylobacteriosis.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice
All animals were bred and maintained in the facilities of the “Forschungsinstitut für Experimentelle Medizin” (FEM, Charité – Universitätsmedizin, Berlin, Germany), under specific pathogen-free (SPF) conditions. C57BL/6 (C6) mice were used in the experiments. Age matched female mice 3 weeks (right after weaning) and 3 months of age were used.
C. jejuni infection of mice
Mice were infected with approximately 109 viable CFU of C. jejuni strain B2 (kindly provided by Prof. Dr. Uwe Groß, University of Göttingen, Germany) by gavage in a total volume of 0.3 ml PBS (phosphate buffered saline) on three consecutive days as described earlier in detail [18].
Sampling procedures, determination of colon length and histopathology
Mice were sacrificed by isofluran treatment (Abbott, Germany). GI samples from each mouse were collected in parallel for histological, microbiological, immunobiological, and molecular analyses. Tissue samples from liver, lungs, kidneys, and GI tract (stomach, duodenum, ileum, colon) were removed under sterile conditions. Histopathological changes were determined in colon and organ samples immediately fixed in 5% formalin and embedded in paraffin. Sections (5 m) were stained with hematoxylin and eosin (HE) and examined by light microscopy (magnification × 100 and × 400).
Immunohistochemistry
In situ immunohistochemical analysis of colon paraffin sections was performed as described previously [18]. Primary antibodies against CD3 (#N1580, Dako, Denmark, dilution 1:10), FOXP-3 (FJK-16s, eBioscience, 1:100), B220 (eBioscience, San Diego, CA, USA, 1:200), myeloperoxidase-7 (MPO-7, # A0398, Dako, 1:10,000), and cleaved caspase-3 (Asp175, Cell Signaling, USA, 1:200) were used. For each animal the average number of positively stained cells within at least six representative high power fields (HPF, × 400 magnification) was determined microscopically by two independent double-blinded investigators.
Analysis of the intestinal microflora
Cultural analyses, biochemical identification, and molecular detection of luminal bacterial communities from stomach, duodenum, ileum, and colon were performed as previously described [18, 28–30].
Detection of blood in stool
The occurrence of blood in stool of infected mice was assessed by the Guajak method using Haemoccult™ (Beckman Coulter/PCD, Krefeld, Germany) [30].
Statistical analysis
Mean values, medians, standard deviations, and levels of significance were determined using appropriate tests as indicated (two-tailed Student’s t-test, Mann–Whitney-U test). Two-sided probability (P) values <0.05 were considered significant. All experiments were repeated at least twice.
Results
Kinetic analysis of intestinal pathogen loads following C. jejuni infection of infant mice
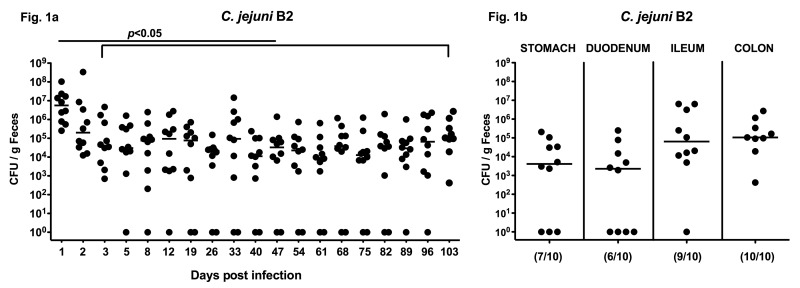
Given that microbial colonization of the GI tract takes place right after birth and the gut microbiota composition changes during lifetime with respect to complexity and predominance of distinct bacterial species, we were interested in finding whether mice display CR already right after weaning. Therefore, we assessed the infection capacity of C. jejuni in infant mice harboring a conventional gut microbiota. Following infection right after weaning, C. jejuni colonized the distal GI tract at high concentrations of approximately 107 colony forming units (CFU) per gram feces at day 1 post infection (p.i.) slightly decreasing by 2 orders of magnitude thereafter until day 3 p.i. Long-term kinetic analyses revealed that C. jejuni could stably infect the intestines of infant mice for more than 100 days p.i. with median loads between 104 and 105 bacteria per gram feces (Fig. 1a). In addition, we assessed numbers of live C. jejuni within distinct compartments of the GI tract of infected infant mice at the end of the long-term kinetic analysis. Following infection of weaned mice, C. jejuni colonized the stomach, duodenum, ileum, and colon with the highest loads of approximately 105 CFU per gram luminal content in the distal GI tract (ileum and colon) at day 103 p.i. (Fig. 1b).
Fig. 1.
Long-term C. jejuni B2 infection of infant mice. Infant mice 3 weeks of age were infected right after weaning. (a) Following oral infection with C. jejuni B2, kinetic analyses of the pathogen loads were performed in feces samples until day 103 p.i. by culture (see section Materials and methods; CFU, colony forming units). (b) At day 103 p.i., C. jejuni B2 densities were determined in distinct compartments of the gastrointestinal tract by quantification of C. jejuni in luminal samples taken from stomach, duodenum, ileum, and colon. Numbers of animals harboring C. jejuni B2 out of the total number of analyzed animals are given in parentheses. Days post infection (on the X-axis), medians (black bars) and significance levels (P-values) determined by the Mann–Whitney-U test are indicated
C. jejuni B2 induced intestinal inflammation in infant mice
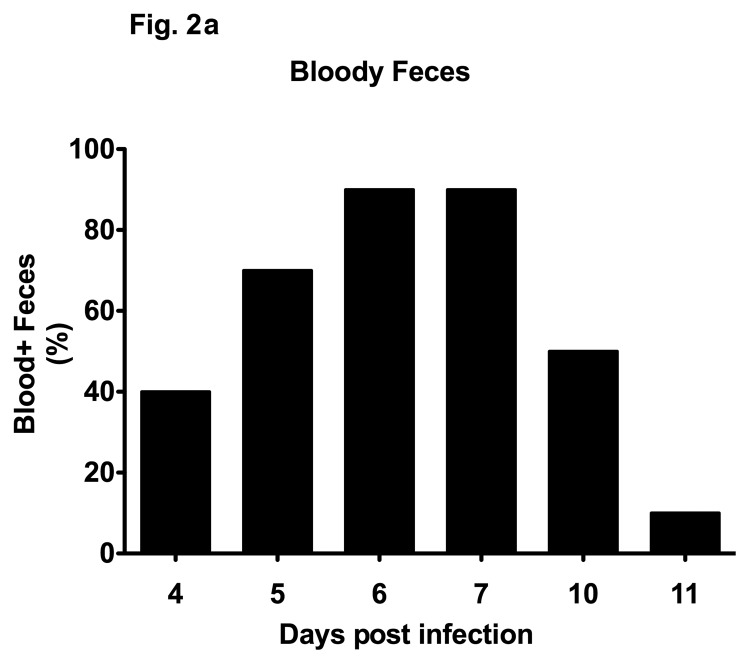
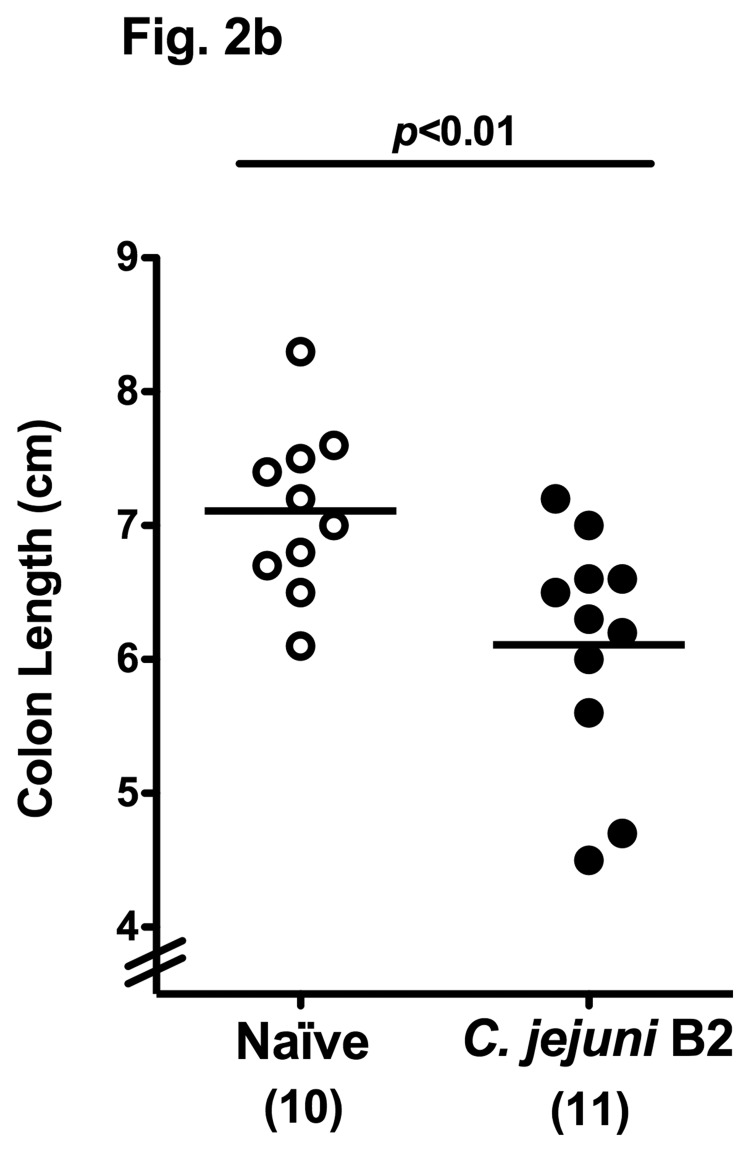
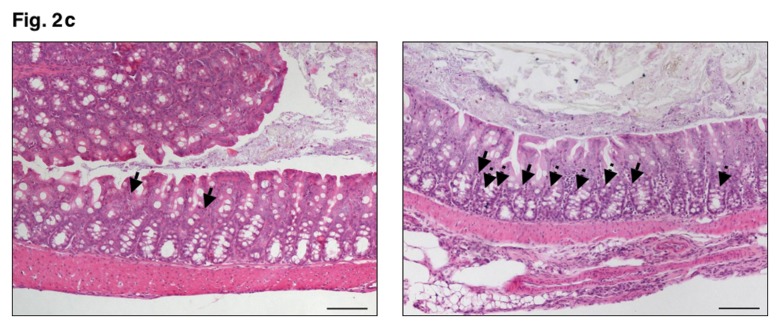
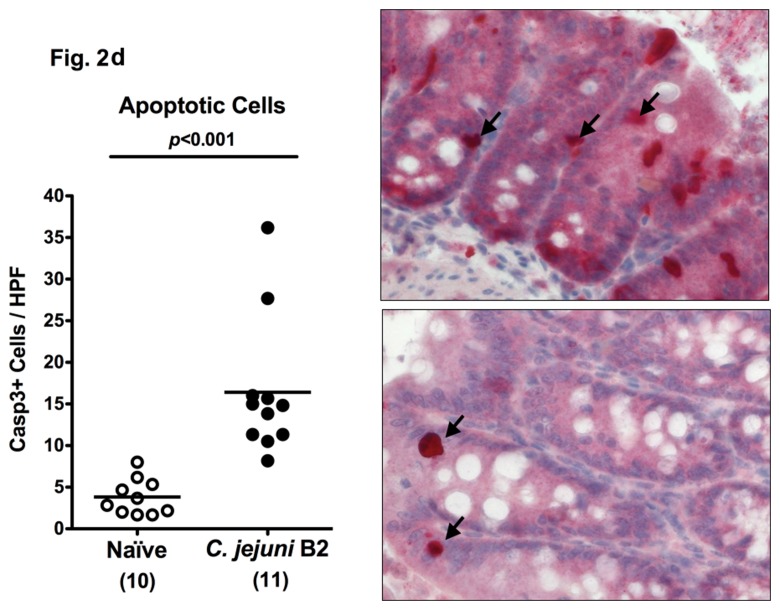
In other murine C. jejuni infection models including GB mice and mice harboring a human flora, clinical symptoms such as bloody diarrhea (pronounced in severe human campylobacteriosis) were rather subtle. Following oral C. jejuni B2 infection, up to 90% of 3-week-old infant mice developed blood-positive feces samples by day 6 and 7 p.i. whereas until day 10 p.i. one half of infected mice still excreted blood-positive feces with a progressive decline thereafter (Fig. 2a). Thus, our infant mouse model mimics clinical key features of human campylo-bacteriosis in a corresponding kinetic and self-limited fashion. Given that due to intestinal inflammation a significant shortening of the intestine can be observed, we next assessed the colon lengths in infected infant mice at the time-point of highest blood-positivity in stool samples of C. jejuni infected mice. At day 6 p.i., infant mice displayed an approximately 15% shorter colon as compared to age- and sex-matched naïve control animals (Fig. 2b) underlining the macroscopic aspects seen following C. jejuni B2 infection. Given that C. jejuni infection is accompanied by an influx of immune cells such as lymphocytes further contributing to mucosal damage, we next assessed histopathological changes in the colonic mucosa. At day 6 p.i., C. jejuni B2 infected infant mice displayed an influx of immune cells into the mucosal layer as well as more mitotic and apoptotic cells in HE-stained colon section versus uninfected infant controls (Fig. 2c). This was further confirmed by specific immunohistochemical staining of colon sections against the apoptosis marker caspase-3: Remarkably, C. jejuni infection induced a more than fourfold increase in apoptotic cells within the colon mucosa as compared to uninfected control animals (Fig. 2d).
Fig. 2.
Clinical signs of enterocolitis following C. jejuni infection of infant mice. Infant mice 3 weeks of age were infected with C. jejuni B2 right after weaning (see section Materials and methods). (a) Kinetic analyses of the occurrence of blood in feces samples were performed in C. jejuni B2 infected mice (n = 10). Black bars indicate the relative number of blood positive feces samples (in %). Days post infection are indicated on the X-axis. (b) Colon lengths were determined in C. jejuni B2 infected infant mice at day 6 p.i. (solid circles) and compared to naïve controls (Naïve, open circles). Numbers of animals are given in parentheses. (c) Paraffin sections of colon samples were obtained from C. jejuni B2 infected infant mice at day 6 p.i. and HE-stained (right). Uninfected age-matched mice served as negative controls (left). Solid arrows indicate apoptotic cells and dotted arrows indicate mitotic cells. Representative photomicrographs (magnification ×100; scale bar 100 pm) from three independent experiments are shown. (d) Paraffin sections of colon samples from C. jejuni B2 infected (day 6 p.i.; solid circles) and naïve (naïve, open circles) infant mice were stained with cleaved caspase-3 antibody (see section Materials and methods). The average numbers of apoptotic cells (positive for caspase-3, left panel) from at least six high power fields (HPF, ×400 magnification) per animal were determined microscopically in immunohistochemically stained colon sections. Representative photomicrographs (right panel) show more caspase3+ cells in infected mice (arrows) at day 6 p.i. (upper right) as compared to control mice (lower right). Numbers of animals are given in parentheses. Medians (black bars) and significance levels (P-values) as determined by Student’s t-test are indicated
Long-term C. jejuni induced intestinal immunopathology in infant mice
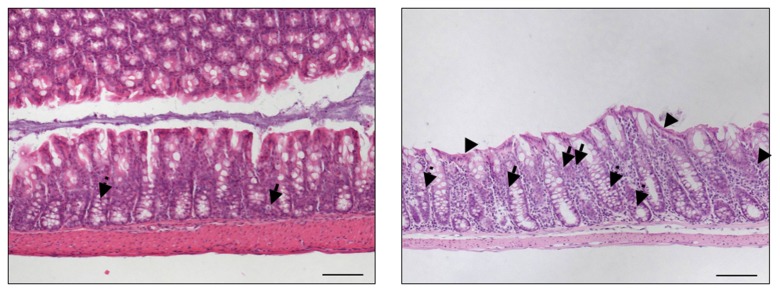
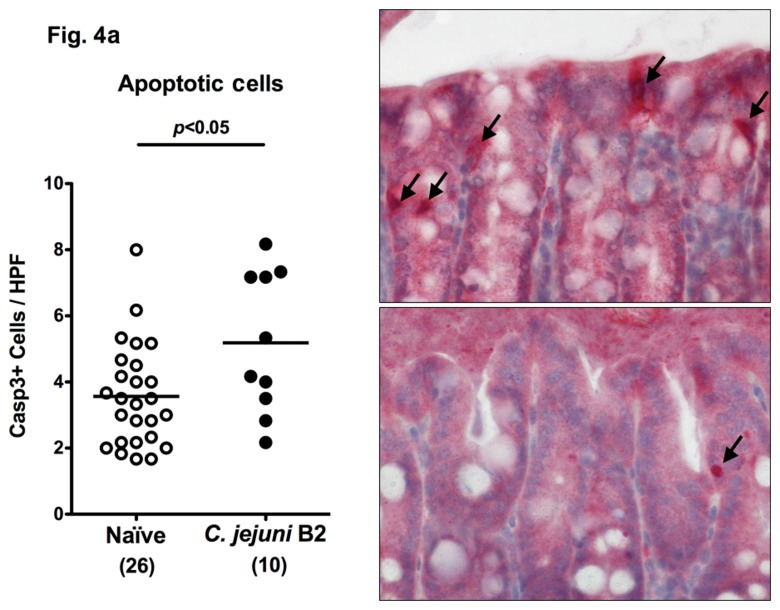
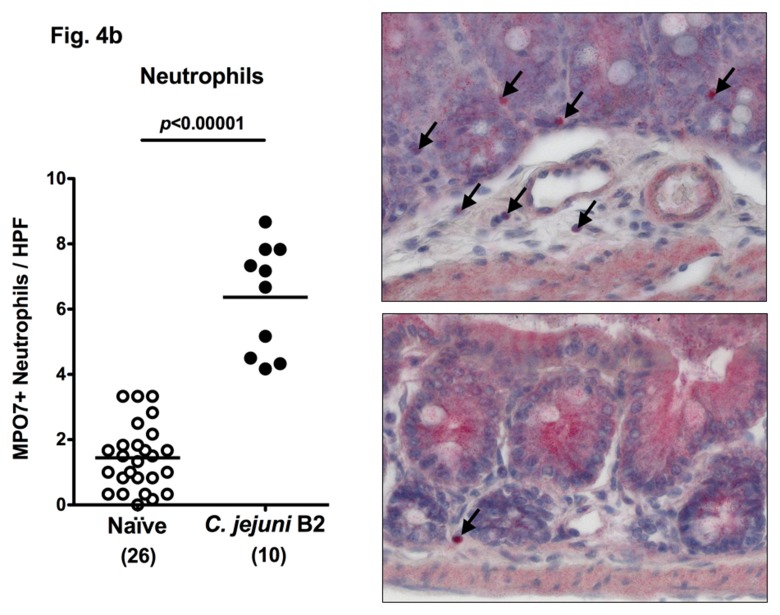
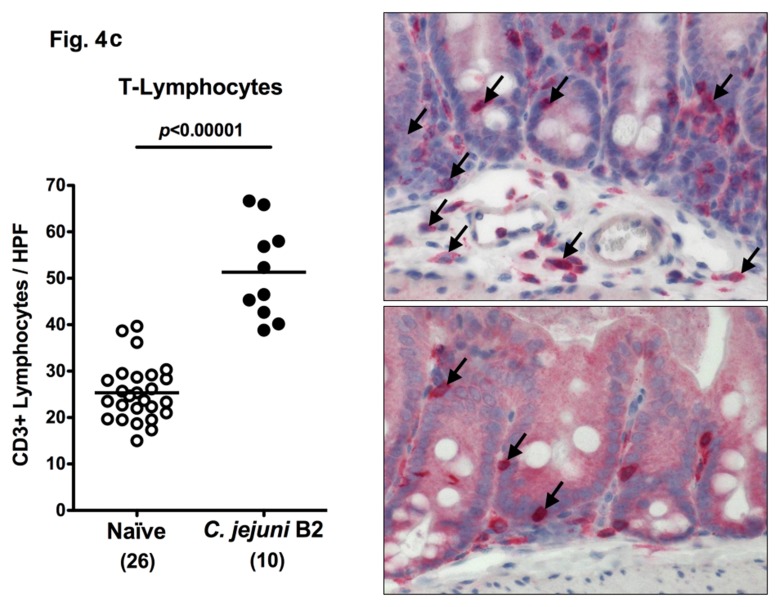
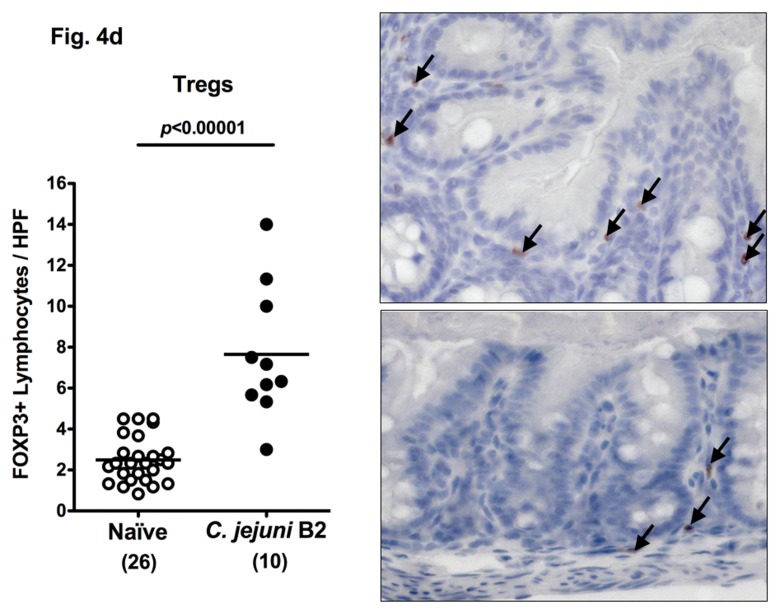
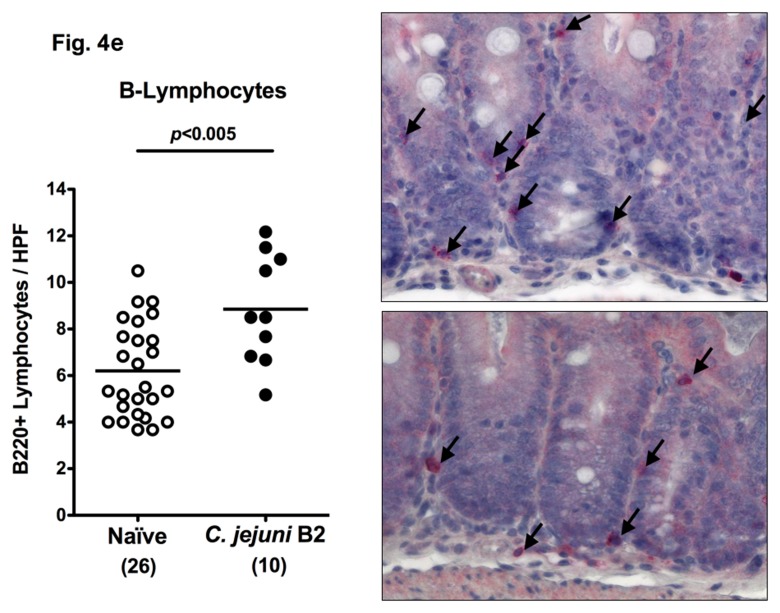
Following infection of infant mice, clinical disease manifestations were self-limited and disappeared within two weeks after infection. We were next interested whether long-term C. jejuni infection might result in intestinal immunopathology in situ despite the absence of clinical symptoms two weeks following infection. Surprisingly, increased numbers of mitotic and apoptotic cells besides infiltrating immune cells could be detected in HE-stained colon sections of infected mice at day 103 p.i. (Fig. 3). Given that C. jejuni induces the recruitment of pro-inflammatory immune cell populations to sites of intestinal inflammation in humans, we further analyzed inflammatory cells by immunohistochemical staining of colon paraffin sections with antibodies against cas-pase-3 (apoptotic cells), MPO7 (neutrophils), CD3 (T-lymphocytes), FOXP3 (regulatory T-cells, Tregs), and B220 (B-lymphocytes). At day 103 following oral C. jejuni B2 infection of infant mice, numbers of apoptotic cells in colon sections were significantly higher as compared to naïve infant mice (Fig. 4a), whereas numbers of neutrophils, T-, B-lymphocytes, and Tregs increased multifold as compared to uninfected controls (Fig. 4b–e). Thus, stable C. jejuni B2 infection results in longterm local (i.e. intestinal) pro-inflammatory immune responses in susceptible infant mice whereas clinical symptoms had been absent.
Fig. 3.
Histopathological changes in the colons of C. jejuni B2 infected infant mice at day 103 p.i. Infant mice 3 weeks of age were infected with C. jejuni B2 right after weaning. Paraffin sections of ex vivo colon biopsies at day 103 p.i. (right panel) were HE-stained as described in the section Materials and Methods. Uninfected infant mice (left panel) served as negative controls. Representative photomicrographs from three independent experiments are shown (100× magnification, scale bar 100 mm). Solid arrows indicate apoptotic cells, and dotted arrows indicate mitotic cells. Arrow heads indicate flattened epithelium
Fig. 4.
Immune cell response in colon sections of C. jejuni B2 infected infant mice at day 103 p.i. Infant mice 3 weeks of age were infected with C. jejuni B2 right after weaning, and paraffin sections of colon samples obtained at day 103 p.i. (solid circles). Uninfected infant mice (naïve, open circles) served as negative controls. The average numbers of apoptotic cells (positive for caspase-3, panel A), neutrophilic granulocytes (neutrophils, positive for MPO-7, panel B), T-lymphocytes (positive for CD3, panel C), regulatory T-cells (positive for FOXP3, Tregs, panel D), and B-lymphocytes (positive for B220, panel E) from at least six high power fields (HPF, ×400 magnification) per animal were determined microscopically in immunohistochemically stained colon sections (left panel). Representative photomicrographs of positively stained cells in colon section (right panel; solid arrows) at day 103 p.i. (upper panel) versus naïve controls (lower panel) from three independent experiments are shown (HPF, ×400 magnification). Data were pooled from at least three independent experiments. Numbers of animals are given in parentheses. Means (black bars) and levels of significance (P-values) as determined by the Student’s t-test are indicated
Long-term C. jejuni induced extra-intestinal immunopathology in infant mice
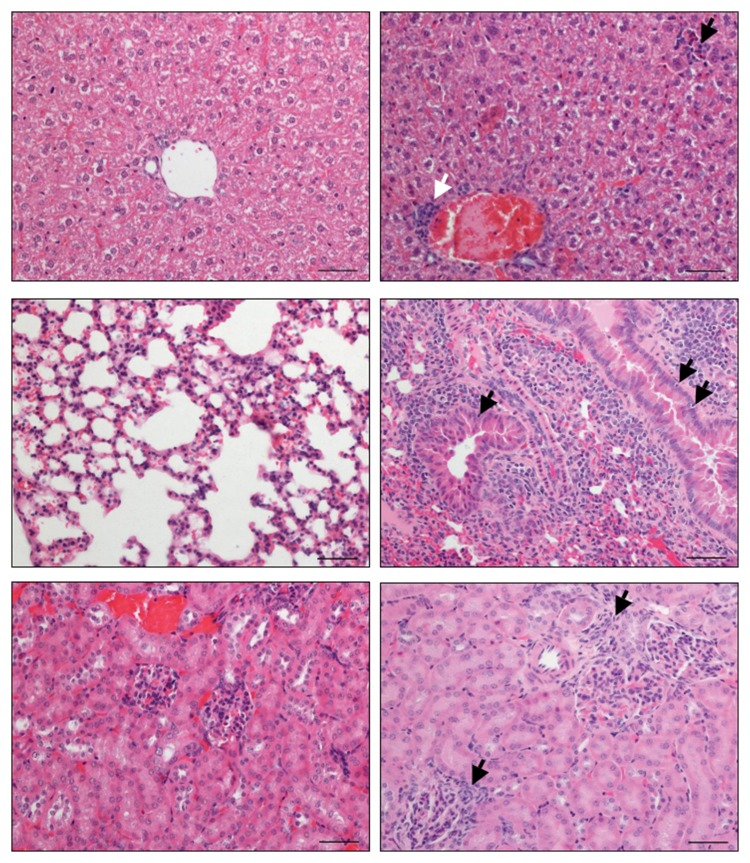
C. jejuni infection of humans is known to result in postinfectious complications affecting peripheral nerves and joints for instance. The analysis of extra-intestinal disease manifestations in long-term infected infant mice revealed a significant influx of immune cells into livers (periportal as well as parenchymal localization), lungs (perivascular, peribronchial, and interstitial) as well as into kidneys (peri-glomerular) at day 103 p.i. (Fig. 5). These findings illustrate that long-term C. jejuni infection not only affects the GI tract but also leads to extra-intestinal inflammatory responses.
Fig. 5.
Long-term extra-intestinal inflammatory responses in C. jejuni B2 infected infant mice. Infant mice 3 weeks of age were infected with C. jejuni B2 right after weaning. Paraffin sections of ex vivo biopsies taken from livers (upper panel), lungs (middle panel), and kidneys (lower panel) at day 103 p.i. (right panel) were HE-stained as described in the section Materials and methods. Uninfected infant mice (left panel) served as negative controls. Representative photomicrographs from three independent experiments are shown (×200 magnification, scale bar 50 μm). Lymphocytes infiltrating the periportal field (solid white arrow, upper right), the liver parenchyma (solid black arrow, upper right), and the glomerular kidney tubuli (solid black arrows, lower right) are indicated. Solid black arrows in the lung (middle right) point toward apoptotic cells
Differences of intestinal microbiota composition in infant versus adult mice
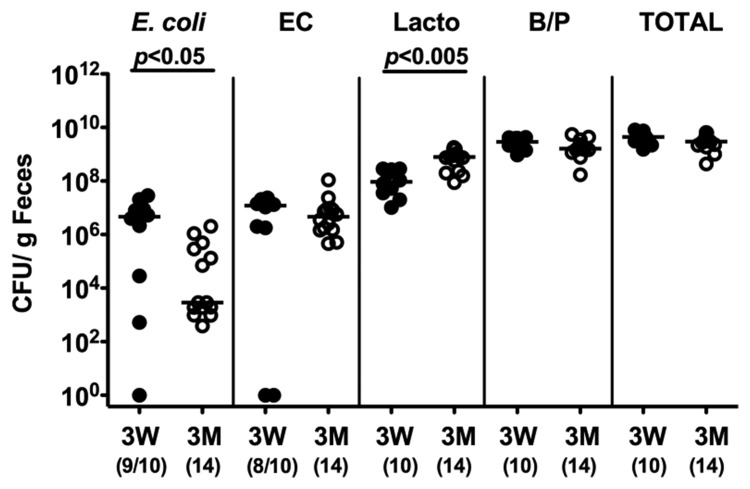
Given that the host gut microbiota composition determines CR or susceptibility to C. jejuni infection, we next performed a quantitative survey of the main bacterial species in the colon lumen derived from susceptible 3-week-old weaned mice and resistant 3-month-old adult animals. Interestingly, infant mice harbored significantly higher E. coli loads (more than 2.5 orders of magnitude) in their large intestines as compared to adult mice whereas Lactobacillus spp. loads were lower (approximately 1.5 order of magnitude; Fig. 6). Enterococci, Bacteroides/Prevotella spp. and total bacterial counts were comparable. Thus, infant mice harbored enterobacteria loads comparable to those in (also C. jejuni susceptible) “humanized” mice and GB animals fed a Western diet.
Fig. 6.
Microflora analysis of infant versus adult mice. Main gut bacterial groups of naïve infant and adult mice were quantified by culture (see section Materials and methods). Total bacterial counts (TOTAL) as well as numbers of E. coli, Enterococcus spp. (EC), Lactobacillus spp. (Lacto), and Bacterioides/Prevotella spp. (B/P) were determined in feces samples of naïve mice 3 weeks (3W, solid circles) and 3 months (3M, open circles) of age, respectively, by detection of colony forming units (CFU) per gram feces on appropriate culture media. Numbers of animals harboring the respective bacterial species are given in parentheses. Medians and significance levels (P-values) determined by Mann–Whitney-U test are indicated.
Long-term E. colicolonization of infant mice following C. jejuni B2 infection
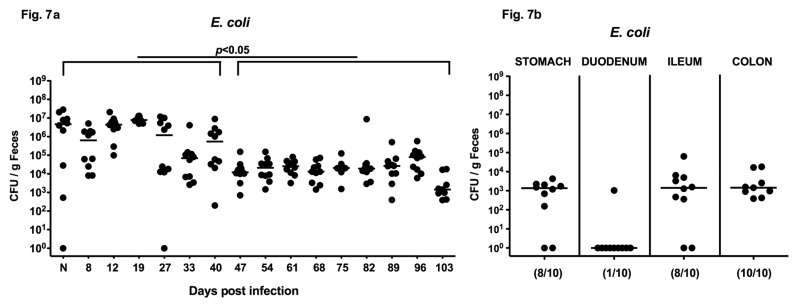
Given that naïve 3-week-old infant mice harbored significant higher E. coli counts in their intestines as compared to conventional adult mice, we next analyzed E. coli colonization densities in a long-term follow up of C. jejuni B2 infection. Kinetic studies revealed that the highest E. coli loads of approximately 107 CFU per gram feces were detected immediately after weaning. Following C. jejuni B2 infection, E. coli loads ranged from 106 to 107 CFU per gram feces until day 40 p.i. followed by a significant drop of about 2 orders of magnitude at day 47 p.i. (Fig. 7a). Until the end of the observation period E. coli loads remained stable between 103 and 105 CFU per g. At day 103 p.i., E. coli could be cultured from stomach, ileum, and colon, but not from the duodenum (103–104 CFU per g; Fig. 7b), Given that C. jejuni infected infant mice harboring higher E.coli loads can be stably infected by C. jejuni and commensal E. coli loads drop around the “adult” age of 7–8 weeks (when mice display CR), it is tempting to speculate that E. coli (among other intestinal bacteria) might be essentially involved in mediating susceptibility to C. jejuni infection.
Fig. 7.
Long-term E. coli colonization of infant mice following C. jejuni B2 infection. Infant mice 3 weeks of age were infected with C. jejuni B2 right after weaning. (a) Before (N, naïve) and until 103 days following C. jejuni B2 infection, E. coli loads in feces samples were performed by culture (as described in section Materials and methods; CFU, colony forming units). (b) At day 103 p.i., E. coli densities in distinct compartments of the gastrointestinal tract were determined by quantification of E. coli in luminal samples taken from stomach, duodenum, ileum, and colon. Numbers of animals harboring E. coli out of the total number of analyzed animals are given in parentheses. Days post infection (on the X-axis), medians (black bars), and significance levels (P-values) determined by Mann–Whitney-U test are indicated.
Changes in microbiota composition during long-term C. jejuni B2 infection
Because CR of mice against C. jejuni is due to their host specific microbiota composition, we investigated potential changes of the gut microbiota composition of infant mice over time. To address this question, we performed a quantitative molecular analysis of the main gut bacterial groups in fecal samples taken before and 103 days following oral C. jejuni B2 infection. Interestingly, over the period of stable C. jejuni colonization, a significant decrease of the Gram-negative enterobacteria (such as E. coli) and Bacteroides/Prevotella spp. as well as Clostridium coccoides could be determined whereas the total bacterial loads including lactobacilli, bifidobacteria, and Clostridium leptum members increased (Fig. 8).
Fig. 8.
Molecular intestinal microflora analysis in long-term C. jejuni B2 infected infant mice. Main gut bacterial groups were quantified by molecular analysis of fecal samples before (N, naïve, open circles; n = 10) and after C. jejuni B2 infection of infant mice (d103 p.i., solid circles; n = 10). Quantitative real-time-PCR (polymerase chain reaction) analyses amplified bacterial 16S rRNA variable regions and 16S rRNA gene numbers per nanogram DNA. From feces the following bacterial groups were determined: Enterobacteriaceae (EB), enterococci (EC), lactic acid bacteria (LB), Bifidobacteria (BB), Bacteroides/Prevotella spp. (BP), Clostridium leptum group (CLEP), Clostridium coccoides group (CLOCC), mouse intestinal bacteroidetes (MIB), and total eubacterial load (TL). Numbers of animals harboring the respective bacterial rRNA are given in parentheses. Medians and significance levels (P-values) determined by Mann–Whitney-U test are indicated. Data shown were pooled from three independent experiments.
In summary, the results presented here show that infant mice harboring a conventional gut microbiota are susceptible to C. jejuni infection and develop significant, but selflimited symptoms of enterocolitis comparable to those in severe human campylobacteriosis. Furthermore, C. jejuni B2 can stably infect the intestines of infant mice in an asymptomatic clinical fashion for more than 14 weeks paralleled by intestinal inflammatory and immune cell responses in the colon mucosa at day 103 p.i. Strikingly, long-term C. jejuni B2 infection was paralleled by extra-intestinal inflammatory responses as shown in liver, lung, and kidney.
Discussion
It is still not well understood how C. jejuni causes inflammation. Investigations of pathogenesis have been restricted by default of an appropriate animal model of disease. Mice are in general useful models to dissect pathogenicity of infectious diseases but display strong CR against C. jejuni and therefore have the disadvantage of sporadic colonization and an absence of disease-defining clinical manifestations. In the past, studies with GF as well as GB mice underlined the substantial role of the intestinal microbiota in maintaining CR [13–18]. The molecular basis of CR and how it can be overcome by pathogens is still incompletely understood. Mechanisms such as direct inhibition of pathogen growth by microbiota-derived substances, nutrient depletion, and microbiota-induced stimulation of innate and adaptive immune responses for example were discussed to contribute to CR elsewhere [12]. Different murine infection models pointed out that the intestinal microbiota composition and complexity play a decisive role in bearing up CR [10, 18–19, 31].
In the study presented here, we focused on susceptibility of infant mice to C. jejuni infection. In the early days of Campylobacter research about three decades ago, neonatal mice had been used for investigating the role of flagella in the colonization of the intestine, the efficacy of bismuth subsalicylate in the prevention of colonization, the bacteriostatic effects of endotoxin and for vaccination strategies [20–27]. However, intestinal and extra-intestinal disease manifestations including clinical symptoms such as bloody diarrhea, histopathological changes, specific immune responses, and the complex microbiota have not been investigated so far. Given that host-specific gut microbiota composition determines CR against C. jejuni infection and due to the fact that colonization of the GI tract sets in right after birth and complexity increases thereafter, we hypothesized that there is a development of CR, starting with high susceptibility against C. jejuni immediately after weaning.
The first week following infection, C. jejuni B2 colonized the intestinal tract of 3-week-old infant mice at high levels between 105 and 108 bacteria per gram feces. The stable infection was paralleled by distinct clinical and histopathological features such as bloody diarrhea in 90% of infected mice, colon shortening as well as increase of apoptotic cells in the colon mucosa in situ during the first week p.i., illustrating the significant C. jejuni induced tissue damage. Of note, the severity as well as the time course of disease manifestations were mimicking “classical” features of human campylobacteriosis. Given that in our previous infection models pro-inflammatory immune responses following C. jejuni infection were overt, but severe symptoms of ulcerative colitis such as bloody diarrhea were missing, we can now provide a robust “human-like” campylobacteriosis model. Furthermore, kinetic analyses revealed that following infection of infant mice, within two weeks clinical symptoms were self-limited despite a stable long-term C. jejuni infection of the GI tract throughout a time period of more than 100 days. The intestinal effect of long-term C. jejuni infection was accompanied by an increase of recruited innate immune and effector cells including T- and B-lymphocytes, Tregs, and neutrophils in the colon at day 103 p.i. Thus, the long-term infection model of infant mice provides a valuable tool to further unravel immunopathological sequelae of chronic C. jejuni carriers in humans. Strikingly, long-term C. jejuni infection not only affected the intestine but was also paralleled by significant cellular immune responses seen in extra-intestinal organs such as liver, lung, and kidney. This phenomenon is well in line with observations in humans: advanced age or a compromised immune status can predispose to extra-intestinal manifestations of C. jejuni infection such as bacteremia and subsequent spread to other organs in rare cases [32]. Comparative cultural analyses of the intraluminal bacterial species composition of the colon of infant mice right after weaning (3 weeks of age) vs. those obtained in naïve adult mice (3 months of age) showed significant differences. Infant mice harbored approximately 2.5 orders of magnitude higher E. coli loads in their large intestines whereas Lactobacillus spp. counts were 1.5 orders lower as compared to adult mice.
Considering the significant age-related differences in the gut microbiota composition comparing 3-week- and 3-month-old mice shown here, it is tempting to speculate that susceptibility to C. jejuni B2 infection might be facilitated by higher E. coli and/or lower Lactobacillus spp. loads. This assumption is consistent with previous work from our group and others. GB mice recolonized with a complex human intestinal flora harboring higher enterobacteria and lower lactobacilli in their intestines as compared to mice with a conventional microbiota were highly susceptible to C. jejuni infection and displayed significant host immune and inflammatory responses (summarized in Ref. [18]). Studies on the enteropathogenic bacteria Citrobacter rodentium and Salmonella Typhimurium also point towards an important role of the host gut microbiota composition in susceptibility to the pathogens [10, 31, 33]. Mice harboring high commensal E. coli densities were more susceptible to Salmonella induced gut inflammation. In addition, increased gut microbiota complexity correlated well with increased resistance to infection. Recently, we could show that nutritional modification plays a crucial role in murine susceptibility to C. jejuni infection [19]. GB mice fed with a human CAF as well as obese (ob/ob) mice served as suitable models for the study of C. jejuni infection due to their susceptibility to the intestinal pathogen. One common feature of these previously described models is a higher enterobacteria (E. Coli) load in the colon as compared to respective controls. Another frequently raised aspect in the discussion of potential predisposing factors for susceptibility to gut pathogens is a breakdown of CR in intestinal inflammation [10–12, 31, 33]. It is easy to imagine that disruption of the epithelial barrier during inflammation results in a breakdown of CR and thereby fosters pathogen invasion and growth. Remarkably, various murine models demonstrated changes in microbiota composition during inflammation such as an accumulation of enterobacteria (E. coli) and a loss of diversity [28–31, 33].
Molecular quantification of main gut bacterial groups of mice infected right after weaning revealed changes in microbiota composition over > 100 days of stable C. jejuni B2 infection. Whereas the total bacterial loads, enterobacteria (E. Coli), Bacteroides/Prevotella spp. and Clostridium coccoides group decreased, lactobacilli, bifidobacteria and Clostridium leptum group members increased until d103 p.i. Taking these complex molecular flora analyses together with the above-mentioned age-related differences in microbiota composition obtained by culture into consideration, the murine microbiota established immediately after weaning might not be capable of outcompeting the pathogen C. jejuni. Even though infected infant mice harbored a different microbiota as compared to adult mice, it is inappropriate to simply attribute CR to absolute loads or relative distribution of single bacterial species alone. Bringing the complexity of the intestinal ecosystem, the impact of the physiological intraluminal milieu, the complexity of the microflora as well as so far unknown factors to mind, further studies will be needed to further unravel the mechanisms underlying murine CR against C. jejuni.
In summary, the study presented here demonstrates an age-related development of CR against C. jejuni. Immunocompetent infant mice infected immediately after weaning were highly susceptible for C. jejuni infection and displayed severe enterocolitis which was self-limited after two weeks of infection. Thus, the time course as well as the severity of clinical disease manifestations mimicked human campylobacteriosis. Furthermore, long-term asymptomatic C. jejuni infection not only induced intestinal but also extra-intestinal immune responses in organs such as liver, lung, and kidneys. Thus, by applying the long-term infection model of infant mice immunopathological responses in human chronic C. jejuni carriers can be elucidated. We conclude that our novel murine C. jejuni model for acute enterocolitis serves as a suitable vertebrate model for further dissecting the interactions between gut pathogens, commensal gut bacteria, intestinal intraluminal milieu, and innate as well as host immune responses at intestinal and extra-intestinal sites.
Acknowledgements
This work was supported by grants from the German Research Foundation (DFG) to UBG (GO363/12–1, Campy-Germ; SFB633, TP A7), SB and AF (SFB633, TP A7), AAK (SFB633, TP Z1), MMH (SFB633, TP B6), LMH, BO and UG (SFB633, Immuco) and from the German Federal Ministry of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP 1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Michaela Wattrodt, Ursula Ruschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance and animal breeding. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections.
Glossary
Abbreviations
- GB:
gnotobiotic
- CR:
colonization resistance
- hfa:
human flora associated
- SPF:
special pathogen free
- CAF:
cafeteria diet
- p.i.:
post infection
- CFU:
colony forming units
- GI:
gastrointestinal
Contributor Information
L.-M. Haag, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
B. Otto, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
U. Grundmann, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 2Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
U. B. Göbel, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010 Aug 15;142(1-2):1–13. doi: 10.1016/j.ijfoodmicro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008 Sep;16(9):428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007 Sep;5(9):665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 4.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 5.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985 Sep;26(9):945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997 Jan;143(Pt 1):5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 8.Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. J Infect Dis. 1997 Dec;176(Suppl 2):S125–S128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell N, Wren BW. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr Opin Infect Dis. 2007 Oct;20(5):514–518. doi: 10.1097/QCO.0b013e3282a56b15. [DOI] [PubMed] [Google Scholar]
- 10.Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, Macpherson AJ, Hardt WD. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010 Jan;6(1):e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008 Mar;16(3):107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011 Feb;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Yrios JW, Balish E. Immune response of athymic and euthymic germfree mice to Campylobacter spp. Infect Immun. 1986 Nov;54(2):339–346. doi: 10.1128/iai.54.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yrios JW, Balish E. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect Immun. 1986 Aug;53(2):378–383. doi: 10.1128/iai.53.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yrios JW, Balish E. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect Immun. 1986 Aug;53(2):384–392. doi: 10.1128/iai.53.2.384-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesudason MV, Hentges DJ, Pongpech P. Colonization of mice by Campylobacter jejuni. Infect Immun. 1989 Aug;57(8):2279–2282. doi: 10.1128/iai.57.8.2279-2282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssef M, Corthier G, Goossens H, Tancrede C, Henry-Amar M, Andremont A. Comparative translocation of enteropathogenic Campylobacter spp. and Escherichia coli from the intestinal tract of gnotobiotic mice. Infect Immun. 1987 Apr;55(4):1019–1021. doi: 10.1128/iai.55.4.1019-1021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bereswill S, Plickert R, Fischer A, Kühl AA, Loddenkemper C, Batra A, Siegmund B, Göbel UB, Heimesaat MM. What you eat is what you get: Novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Bereswill S, Plickert R, Fischer A, Kühl AA, Loddenkemper C, Batra A, Siegmund B, Göbel UB, Heimesaat MM. EuJMI. 2011;1(3):237–248. doi: 10.1556/EuJMI.1.2011.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diker KS, Hascelik G, Diker S. Colonization of infant mice with flagellar variants of Campylobacter jejuni. Acta Microbiol Hung. 1992;39(2):133–136. [PubMed] [Google Scholar]
- 21.Hänninen ML. Bismuth subsalicylate in the prevention of colonization of infant mice with Campylobacter jejuni. Epidemiol Infect. 1990 Jun;104(3):397–404. doi: 10.1017/s0950268800047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hänninen ML. Effect of endotoxin on colonisation of Campylobacter jejuni in infant mice. J Med Microbiol. 1989 Nov;30(3):199–206. doi: 10.1099/00222615-30-3-199. [DOI] [PubMed] [Google Scholar]
- 23.Abimiku AG, Dolby JM, Borriello SP. Comparison of different vaccines and induced immune response against Campylobacter jejuni colonization in the infant mouse. Epidemiol Infect. 1989 Apr;102(2):271–280. doi: 10.1017/s0950268800029940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abimiku AG, Dolby JM. The mechanism of protection of infant mice from intestinal colonisation with Campylobacter jejuni. J Med Microbiol. 1987 Jun;23(4):339–344. doi: 10.1099/00222615-23-4-339. [DOI] [PubMed] [Google Scholar]
- 25.Dolby JM, Newell DG. The protection of infant mice from colonization with Campylobacter jejuni by vaccination of the dams. J Hyg (Lond) 1986 Apr;96(2):143–151. doi: 10.1017/s0022172400065918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell DG, McBride H, Dolby JM. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond) 1985 Oct;95(2):217–227. doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field LH, Underwood JL, Pope LM, Berry LJ. Intestinal colonization of neonatal animals by Campylobacter fetus subsp. jejuni. Infect Immun. 1981 Sep;33(3):884–892. doi: 10.1128/iai.33.3.884-892.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 29.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007 Jul 25;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007 Oct;5(10):2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tee W, Mijch A. Campylobacter jejuni bacteremia in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients: comparison of clinical features and review. Clin Infect Dis. 1998 Jan;26(1):91–96. doi: 10.1086/516263. [DOI] [PubMed] [Google Scholar]
- 33.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007 Sep 13;2(3):204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]