Abstract
The bacterial pathogen Campylobacter jejuni is the leading cause of foodborne gastroenteritis in the developed world, with the organism being transmitted by ingestion of contaminated and undercooked poultry. Exposure to acid is an inevitable stressor for C. jejuni during gastric passage, yet the effect of low pH on C. jejuni virulence is still poorly understood. Here, we investigate the effect of acid-shock on C. jejuni viability, gene expression and host-cell invasion. C. jejuni strain NCTC 11168 survived acid exposure at pH 3.5 and above for up to 30 min without a drop in viability, and this exposure induced the expression of flagellar genes transcribed from σ54-dependent promoters. Furthermore, acid-shock resulted in increased C. jejuni invasion of m-ICcl2 mouse small intestine crypt cells grown on transwells, but not when the cells were grown on flat-bottomed wells. This suggests that C. jejuni might be invading intestinal epithelial cells at the basolateral side, possibly after paracellular passage. We hypothesize that acid-shock prior to intestinal entry may serve as a signal that primes C. jejuni to express its virulence gene repertoire including flagellar motility genes, but this requires further study in the context of an appropriate colonization or disease model.
Keywords: acid resistance, Campylobacter, invasion, motility
Introduction
The bacterial pathogen Campylobacter jejuni is a major cause of foodborne gastroenteritis in the developed world, with infection often associated with the consumption of undercooked poultry products [1]. The UK Department for Environment, Food and Rural Affaires (DEFRA) reported over 55,000 cases in the UK in 2008 alone, although this is thought to be a substantial underestimation of the true incidence, due to under-reporting by the community [2]. Clinical symptoms of C. jejuni infection are acute (2–7 days) and are illustrated by watery or bloody diarrhea, nausea, fever and abdominal pains, although the disease is usually self-limiting in humans [1]. Secondary complications of C. jejuni infection include the autoimmune diseases Guillain–Barré and Miller Fisher syndrome that result in paralysis, and also reactive arthritis and inflammatory bowel disease [1, 3, 4].
Ingestion of C. jejuni, either by humans or in the avian host, is inevitably followed by bacterial passage though the stomach before entering the small intestine and causing disease. In the human stomach, the gastric pH can range from approximately 2 to 7 depending on the state of the stomach (empty or full), gastrin production and stomach contents [5]. In addition, food is retained in the stomach for 30–60 min before the stomach begins to empty [6]. Thus, C. jejuni must have the means to cope with sudden and variable exposures to acidic conditions. However, C. jejuni responses to acid stress have not been as extensively characterized as those in the well-studied enteric pathogens Escherichia coli or Salmonella Typhimurium [7].
Many bacteria have specific acid-resistance systems allowing direct protection against acidic pH, like the well characterised glutamate decarboxylase system in E. coli, which metabolizes glutamate to increase internal pH [7]. This and other acid-resistance systems dependent on growth media are regulated by complex networks and are also shared with S. Typhimurium [8]. However, while widespread in the Enterobacteriaceae, these specific systems are absent in C. jejuni. C. jejuni is known to up-regulate stress-response genes and down-regulate capsular polysaccharide biosynthesis genes in response to acid-shock [9, 10]. As well as these changes in gene expression, it has also been suggested that the alternative sigma factor σ54 (encoded by the rpoN gene), might play a role in acid resistance in C. jejuni [11]. C. jejuni has been reported to mount an adaptive tolerance response (ATR) during the stationary growth phase, where, in the case of acid adaptation, the bacteria are sensitized by mild acid-shock (e.g. pH 5), upon which they display increased survival at low pH [12]. It has been suggested that C. jejuni has mechanisms enabling ATRs, which are initiated by the release of extracellular proteins [12, 13].
Exposure to low pH can induce genes involved in virulence phenotypes, as was shown in the related human pathogen Helicobacter pylori, where acid-shock increases the expression of genes for the acid-resistance factor urease and the expression of motility-associated genes [14]. This enables the bacteria to survive in acidic conditions and may aid taxis responses during initial colonization of the gastric mucosa [14]. Similarly, E. coli displayed increased expression of flagellar genes and motility upon acid-shock, as well as an up-regulation in other stress responses [15, 16].
In this, study, we have investigated the effect of acid-shock on the gene expression and survival of C. jejuni. We show that C. jejuni can survive acid exposure at pH 3.5 and above for up to 30 min without a drop in viability, and that this exposure induces the expression of motility genes transcribed from σ54-dependent promoters. Furthermore, we demonstrate that acid-shock increases invasion of C. jejuni in mouse small intestine crypt cells when grown on transwells but not when grown on flat-bottomed wells.
Materials and methods
Bacterial strains and growth conditions
The motile C. jejuni strain NCTC 11168 [17] was used for all experiments. C. jejuni was cultured in a MACS-MG-1000 controlled atmosphere cabinet (Don Whitley Scientific) under microaerobic conditions (85% N2, 5% O2, 10% CO2) at 37 °C. For growth on plates, strains were grown on Blood Agar Base 2 with Skirrow supplements (10 μg ml–1 vancomycin, 5 μg ml–1 trimethoprim, 2.5 IU polymyxin-B). Broth culture was carried out in Brucella broth (Becton, Dickinson & Company).
Acid-shock and viability assays
Acid-shock was effected by growing C. jejuni to mid-exponential phase, and resuspending cells in Brucella broth adjusted with HCl to pH values from 2.0 to 7.0. Viability assays following acid-shock were performed by determining the number of colony forming units (cfu) after incubation for 10 and 30 min at pH 2.0, 3.0, 3.25, 3.5, 3.75, 4.0, 5.0, 6.0 and 7.0 under microaerobic conditions at 37 °C. C. jejuni was incubated with non-adjusted Brucella broth as a control. Serial 10-fold dilutions were made, 5 pil of each dilution was spotted onto Brucella-agar plates and incubated under microaerobic conditions for 2 days at 37 °C. Three independent assays were performed for each pH value, and survival was expressed as the percentage of surviving bacteria relative to the control.
Nucleic acid isolation for microarray
For analysis of C. jejuni gene expression after acid-shock, log-phase C. jejuni cells were incubated at pH 3.6 and 5.0 for 10 and 30 min as described previously. Cell suspensions were subsequently mixed with 0.1 volume of ice-cold 5% phenol in ethanol, and cells were harvested by centrifugation at 3220 g for 15 min at 4 °C. RNA was isolated as described previously [18]. Chromosomal DNA was isolated from wild-type C. jejuni cultures grown to mid-exponential phase using Qiagen genomic-tip 100/G gravity columns according to the manufacturers’ protocol. Concentration and quality of chromosomal DNA was determined by Nanodrop instrument (Thermo Scientific), and quality of RNA was determined by an Agilent 2100 Bioanalyser (Agilent Technologies).
Transcriptomic analysis
A DNA microarray was constructed as described by Holmes et al. [18]. Three micrograms of chromosomal DNA was labelled with Cy3-conjugated dCTP using the BioPrime labelling kit (Invitrogen), with labelling reactions performed overnight at 37 °C. Labelled cDNA was prepared from 15 mg RNA using Stratascript Reverse Transcriptase (Stratagene) for direct incorporation of Cy5-conjugated dCTPs (Amersham), with labelling reactions performed for approximately 16 h at 42 °C. Labelled nucleic acids were cleaned with the Qiaquick purification kit (Qiagen) and dried before being re-suspended in water and prepared for hybridization. Samples were boiled for 2 min, cooled at 18–25 °C for 5 min and centrifuged at maximum speed in a microfuge for 2 min to remove any solid particles from the hybridization mixture. This mixture was spotted onto microarray slides and incubated at 60 °C for approximately 16 h. Details of the labelling and hybridizations are available on http://www.ifr.ac.uk/safety/microarrays/protocols.html. The slides were washed and dried as described by Holmes et al. [18]. Microarrays were scanned and analysed as described in Lucchini et al. [19], and data were obtained for 1608 genes. For each condition, three independent RNA isolations were hybridized.
Cell culture
The murine intestinal crypt-like cell line m-ICcl2 [20] was cultured in m-IC media consisting of Dulbecco’s modified Eagle Medium/Ham’s F-12 12 g l–1 (1:1 v/v; Gibco), 20 mM D-glucose (Sigma), 10 ng ml–1 mouse epidermal growth factor (Merck), 5 μg ml–1 insulin (Sigma,), 60 nM selenium (Sigma), 5 μg ml–1 human Apo-transferrin (Sigma), 1 nM triiodothyronine (Merck), 20 mM HEPES (Sigma), 2% fetal calf serum (Gibco), 50 nM dexamethasone and 2 mM L-alanyl-L-glutamine (Sigma) at 37 °C in a 5% CO2 atmosphere. Cells of passage 9–15 were used for experiments.
Invasion assays
The m-ICcl2 cells were grown until confluent on transwells with 8 mm pores (Corning) or on flat-bottomed wells in plastic 24-well plates (Sarstedt) at 37 °C in a 5% CO2 atmosphere. For growth on transwells, the trans-epithelial electrical resistance of the monolayer was monitored by the EVOM2 epithelial voltohmmeter (World Precision Instruments) to measure confluence. To compare the effect of acid-shock on invasion, C. jejuni cultures were incubated for 30 min at pH 5.0 (acid-shock) or 7.0 (control) as described previously. Both transwell and flat-bottomed well models were infected with C. jejuni in m-IC media to MOI (multiplicity of infection) 1000 for 2 h at 37 °C and 5% CO2 to allow bacterial invasion. To remove adherent C. jejuni, m-ICcl2 cells were washed twice with phosphate buffered saline (PBS) and incubated in fresh m-ICcl2 media containing 500 mg ml–1 gentamicin for 1 h [21]. To quantify bacterial invasion, the infected m-ICcl2 cells were washed twice with PBS and then lysed with 1% Triton X-100. Serial 10-fold dilutions were made, 5 μl of each dilution was spotted onto Brucella-agar plates and incubated under microaerobic conditions for 2 days at 37 °C. For transwell experiments, the numbers of bacteria in the basal compartment (translocated bacteria) were also counted. Results were expressed as percentage of invasive or translocated bacteria relative to the inoculum. Transwell experiments comprised 10 technical replicates. Flat-bottomed well experiments comprised three independent experiments, each containing six technical replicates. Statistical analyses were performed using IBM SPSS Statistics 19.
Results
The lowest pH that C. jejuni is able to survive is pH 3.5
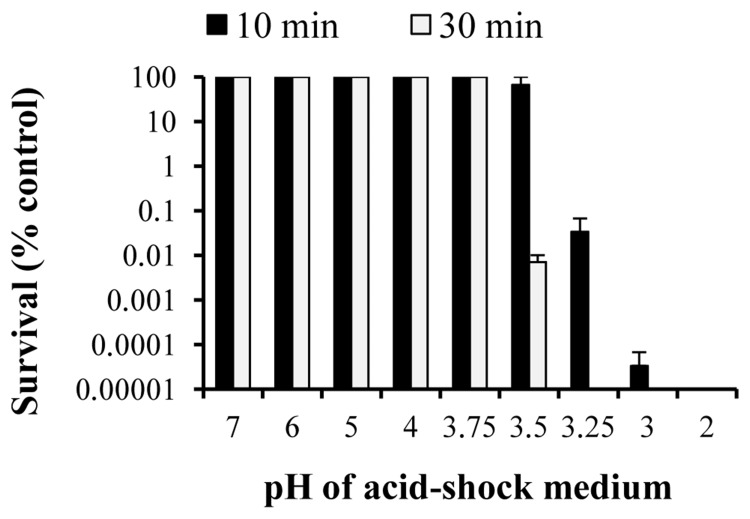
To determine whether C. jejuni can survive the acidic conditions present in the stomach, we incubated bacteria grown to exponential phase in Brucella broth adjusted with HCl to different pH values ranging from 2.0 to 7.0, with survival being assessed by viability counts. There was no significant loss in viability when C. jejuni was incubated for 10 min at pH values of 3.5 and higher, but below pH 3.5 there was a rapid loss of viability, with less than 0.1% of viable cells recovered after acid-shock at pH 3.25 (Fig. 1). When the duration of acid-shock was increased to 30 min, the lowest pH at which C. jejuni showed no decrease of viability was pH 3.75, where less than 0.01% of viable cells were recovered at pH 3.5 (Fig. 1). Acid ATR was assayed by pre-incubating exponential-phase cells of C. jejuni at pH 5.0 and 7.0 prior to acid-shock at pH 2.75. There were no viable cells recovered at pH 2.75 (data not shown), suggesting that C. jejuni lacks an ATR at this growth phase as previously observed [22].
Fig. 1.
The lowest pH that C. jejuni NCTC 11168 is able to survive is 3.5. Motile C. jejuni was grown to exponential phase and incubated for 10 and 30 min in Brucella broth adjusted to pH 2.0–7.0. C. jejuni was incubated with non-adjusted Brucella broth as a control. Survival was assessed by cfu after 10 min (solid bars) and 30 min (white bars). Results were expressed as a percentage of surviving bacteria relative to the control. Error bars denote standard error of the mean, and the results shown are the average of three independent experiments.
Acid-shock increases the expression of σ54-dependent flagellar biosynthesis genes
To assess the effect of acid-shock on gene transcription in C. jejuni, we performed transcriptomic analyses using C. jejuni microarrays to compare transcriptional profiles at pH 7.0 and at acidic pH (pH 5.0 and 3.6, for 10 and 30 min). Levels of RNA were expressed as the ratio of the acidic pH/pH 7.0, and genes were considered differentially expressed if transcript levels were more than twofold different and if the false discovery rate (FDR) was below 0.1. The transcript level of 232 genes was increased more than twofold and that of 294 genes was decreased more than twofold upon acid shock in one of the four tested conditions, with an FDR <0.1. Of the genes with increased transcript levels, 137 showed a significant change in one of the four conditions, whereas 95 showed increased transcript levels in two or more of the tested conditions. Of the genes with decreased transcript levels upon acid-shock, 151 were identified in one of the four conditions, whereas 143 showed decreased transcript levels in two or more of the tested conditions.
Down-regulated genes, as a result of acid-shock, included those encoding 50S and 30S ribosomal proteins (rpm, rpl and rps), which were consistently down-regulated across two or more conditions. Also, genes encoding the F0F1 ATPase subunits (atpDFH) were down-regulated after 30 min shock at both pH 5.0 and 3.6, and this was also the case for sec protein-export genes (secAFY). Genes encoding leucine biosynthesis enzymes (leuABC) were down-regulated at the acid-shock of pH 5.0 only, as were genes for glycosylation enzymes (pglABC). Other pgl locus genes, including pglHIJKI, were down-regulated after 30 min acid-shock at pH 5.0 only. Overall, these changes suggest that the cells shut down protein synthesis and modification in response to acid stress, as a result of the change from exponential growth to survival mode.
Up-regulated genes, as a result of acid-shock, included the catalase gene (katA) and the heat shock proteins encoded by clpB, dnaK, hrcA and htrA, which were up-regulated after 10 minutes acid-shock at both pH 5.0 and 3.6. Genes for respiratory functions, such as succinyl-CoA synthesis (sucCD) and proline metabolism (putA), were up-regulated under all conditions, and the oxidoreductase genes (oorABCD) were up-regulated after 10 min acid-shock. Gluconate dehydrogenase genes (gndAB) were up-regulated after 10 min acid-shock at pH 5.0 and 3.6, and NADH (nicotinamide adenine dinucleotide hydride) dehydrogenase (nuoBGHJK) genes were up-regulated after 10 min acid-shock at pH 5.0 only. Also, the lactate oxidase operon cj0075c-0073c [23] was up-regulated across all acid conditions, suggesting a general stress response upon acid exposure.
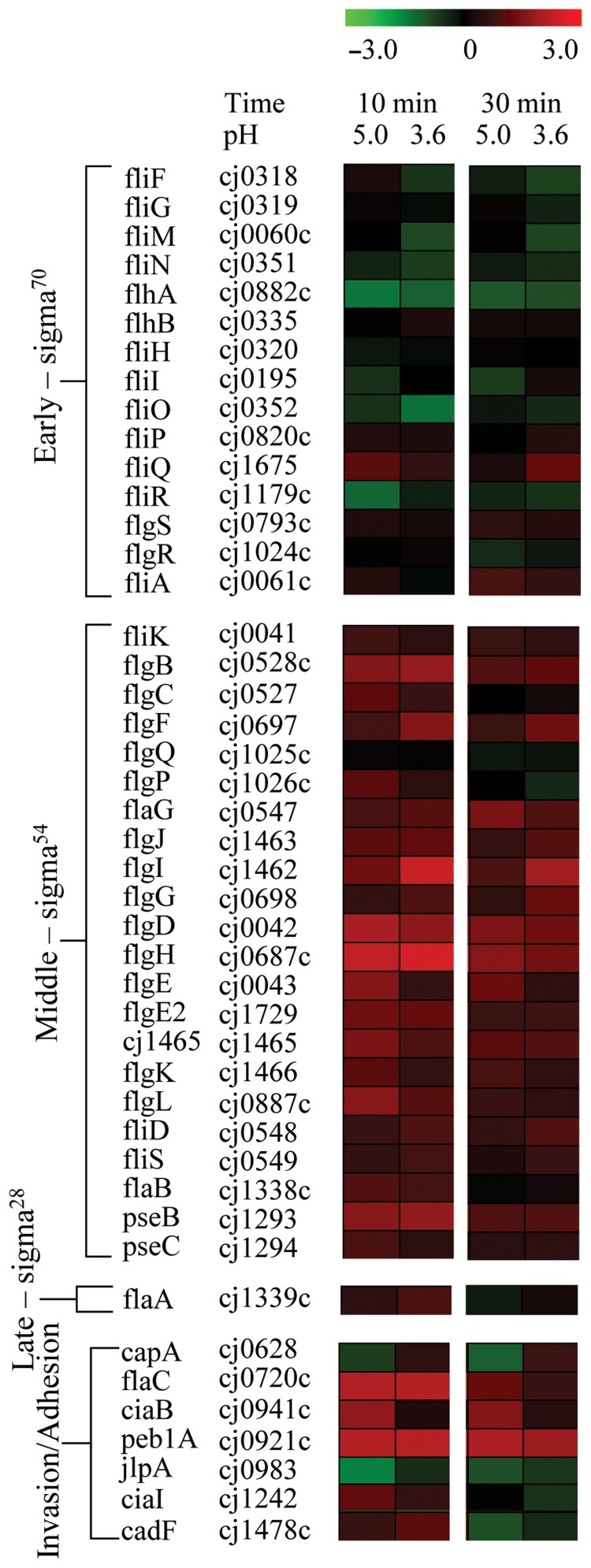
Flagellar genes that are transcribed from σ54-dependent promoters during the middle phase of flagellar assembly [24, 25] were up-regulated after acid-shock at pH 5.0 and 3.6 (Fig. 2). These genes included components of the basal body, hook, junction proteins and associated outer membrane proteins, as shown in Fig. 3. Genes expressed at the early and late phases of flagellar assembly remained mostly unaffected by acid-shock except for fliQ expression, which was significantly up-regulated in response to 10 min acid-shock at pH 5.0. The flaC and ciaB invasion and peb1A adhesion determinants were also significantly up-regulated under one or more acid conditions (Fig. 2).
Fig. 2.
Acid-shock at pH 5.0 and 3.6 increases the expression of a subset of flagellar biosynthesis genes and, in particular, those transcribed by σ54 during the middle phase of flagellar assembly. RNA levels of log phase C. jejuni NCTC 11168 cells incubated at pH 7.0 were compared with those of cells incubated at pH 5.0 and 3.6 for 10 and 30 min. Results are shown for a subset of genes, including flagellar genes and previously identified invasion and adhesion determinants [40, 41]. Flagellar genes are listed and grouped in the order of flagellar assembly: early phase genes are controlled by σ70, middle-phase genes by σ54, and late-phase genes by σ28; invasion and adhesion determinants are grouped separately. Up-regulated genes are shown in red, and down-regulated genes are shown in green. Maximum colour output represents a threefold change in expression. Results shown are averages of three independent experiments.
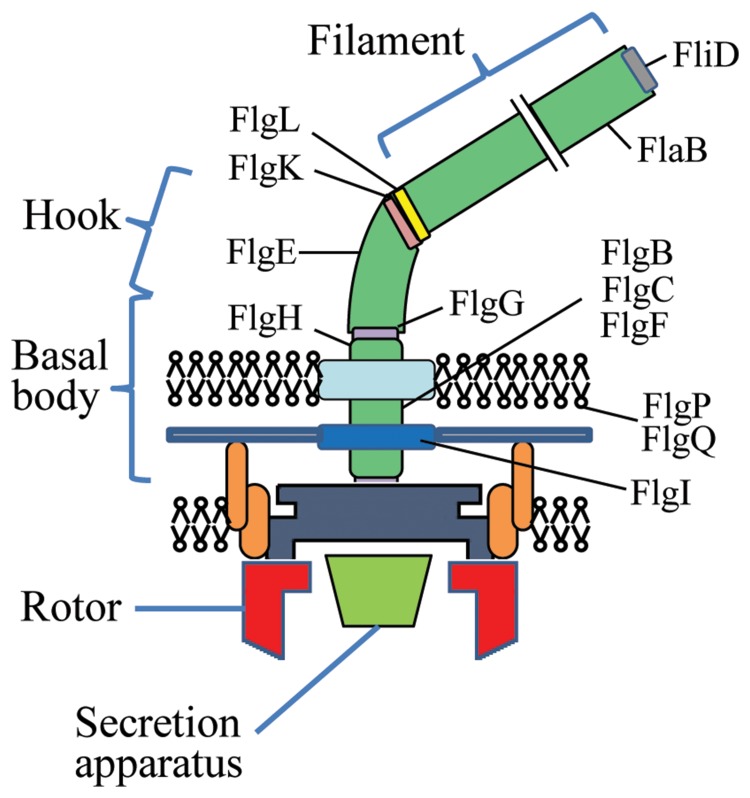
Fig. 3.
Diagram showing the main structural components of the C. jejuni flagellum modified from Wösten et al. [24]. Only the gene names and locations of the flagellar gene products that are up-regulated after acid-shock at pH 5.0 for 10 min are shown.
Acid-shock increases invasion of C. jejuni into mouse intestinal crypt (m-ICcl2) cells grown on transwell inserts, but not when m-ICcl2 cells are grown in flat-bottomed wells
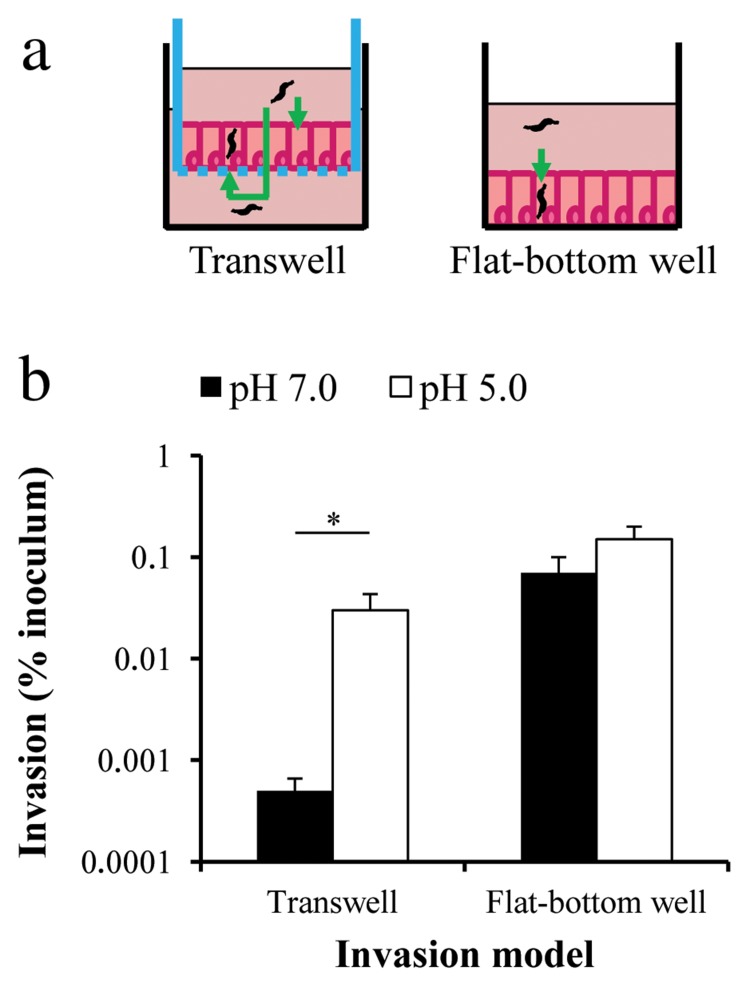
To investigate the effect of acid-shock and the associated increase in flagellar gene expression on C. jejuni virulence phenotypes, we performed invasion assays with a mouse intestinal crypt cell line (m-ICcl2) [20]. This cell line represents the small intestine and was therefore used in preference to colon-derived cell lines such as CaCo-2. Confluent layers of m-ICcl2 cells were grown either on transwell inserts or on flat-bottomed wells (Fig. 4a), and subsequently incubated with C. jejuni at MOI 1000. The C. jejuni cultures had either been incubated at pH 5.0 for 30 min, or at pH 7.0 as a control. Following the commonly used gentamicin killing approach [21], m-ICcl2 cells were lysed, and invasive intracellular C. jejuni were enumerated as cfu.
Fig. 4.
Acid-shock increases C. jejuni NCTC 11168 invasion of mouse intestinal crypt (m-ICcl2) cells grown on transwell inserts, but not on flat-bottomed wells. Increased invasion is seen when m-ICcl2 cells are grown on flat-bottomed wells. (a) Schematic representations of the invasion models highlighting possible routes (green arrows) of C. jejuni invasion: apical and basolateral when m-ICcl2 cells are grown on transwell inserts; and apical only when grown on flat-bottomed wells. Cells were grown until confluent on transwell inserts or flat-bottomed wells prior to exposure to motile C. jejuni (MOI 1000) that had been acid-shocked at pH 5.0 or incubated at pH 7.0 for 30 min. Following a killing wash with gentamicin, m-ICcl2 cells were lysed and viable C. jejuni cfu determined. (b) Results were expressed as percentage of invasive or translocated bacteria relative to the inoculum. C. jejuni incubated at pH 7.0 is represented by solid bars and C. jejuni acid-shocked at pH 5.0 is represented by open bars. Error bars denote standard error of the mean. Transwell experiments comprised 10 technical replicates. Flat-bottomed well experiments comprised three independent experiments of six technical replicates. Astersk indicates P ≤ 0.05 (Mann–Whitney U test).
The transwell model assessed bacterial invasion from both the apical and basolateral sides (after translocation through the m-ICcl2 cell layer), whereas the flat-bottomed well model tested for apical invasion only (Fig. 4a). When comparing control C. jejuni cultures, higher invasion levels were seen in the flat-bottomed well model than in the transwell model (Fig. 4b). In the flat-bottomed well model, there was no significant effect of acid-shock on the number of C. jejuni recovered from m-ICcl2 cells (Fig. 4b). However, in the transwell model, acid-shock increased the levels of C. jejuni invasion by 1–2 log compared to the control, so invasion levels became comparable to those observed in the flat-bottomed well model (Fig. 4b). The number of translocated C. jejuni (recovered from the compartment below the transwell insert) was increased up to 2 log after acid-shock, although cfu recovery was very variable across technical replicates (data not shown).
Discussion
When C. jejuni colonizes a new host, the fecal–oral route of infection will inevitably include exposure to the acidic environments of the stomach in mammals as well as the acidic proventriculus (glandular stomach) in birds [26]. Enteric pathogens require acid-resistance mechanisms for successful transmission to the intestine. In this study, we have shown that C. jejuni can survive mild and strong acid-shock conditions, and that this is linked with increased transcription of a subset of flagellar biosynthetic genes and stress responses, as well as a down-regulation in genes involved in cell division and metabolism. Furthermore, acid-shock increases C. jejuni invasion of intestinal epithelial cells in a transwell model, and mouse small intestinal crypt (m-ICcl2) cells can be used as a C. jejuni invasion system representing the mammalian small intestine.
Previous work describing the response of C. jejuni to acid exposure reported that C. jejuni could not be cultured, but remained viable after prolonged exposure to pH 4.0 [27]. C. jejuni was reported to survive for no more than 4 min exposure at pH 3.0, but that high numbers of C. jejuni were recovered from the pig stomach, which has a pH range of 3.8–4.2, suggesting that pH alone cannot explain this increased survival [9]. The aims of this study were to ascertain the lowest pH that C. jejuni could survive under defined experimental conditions (Brucella broth adjusted to different pH values using HCl), and to use microarrays to access the response of C. jejuni to nonlethal acid-shock. In this study, the threshold for survival of C. jejuni was pH 3.5 after 10 min acid-shock and pH 3.75 after 30 min acid-shock (Fig. 1), implying that C. jejuni can survive the physiological acidic conditions of the stomach. Using this threshold, C. jejuni gene expression in response to acid-shock was analysed at the lower threshold of pH 3.6, and with the mildly acidic condition of pH 5.0, at both 10 and 30 min of incubation. We also investigated whether C. jejuni induced an ATR to acid. Exponential-phase cultures did not show an ATR to lethal acid-shock at pH 2.75 after 2 h pre-incubation with pH 5.0. This is in agreement with the results from Murphy et al., although their pre-incubation step was longer [22].
Transcriptomic analysis of acid-shocked C. jejuni showed that exposure to acidic conditions resulted in increased expression of a subset of flagellar biosynthetic genes. Flagellar gene expression is tightly regulated by a hierarchy of σ factors and begins with σ70-dependent transcription of the inner membrane ring and secretion apparatus [25]. Middle-phase expression of genes coding for the minor flagellin, basal body and junction proteins is σ54 dependent, and the expression of the major flagellin gene is dependent on σ28 during late phase [25]. The flagellar genes that were up-regulated are transcribed by σ54 during the middle phase of flagellar assembly. This finding is consistent with a recent report in which a C. jejuni rpoN mutant, which lacks the σ54 factor, showed reduced survival at pH 5.0 [11], and is comparable with more recent studies on the response of C. jejuni to acid-shock, which was reported to result in transient expression of many of the flagellar biosynthetic genes that we report as up-regulated [9, 10]. Perhaps, as suggested [9, 10], a change in expression of flagellar genes may be part of a general stress response. Indeed, a down-regulation of genes encoding the cell cycle ATPases and ribosomal proteins was observed, which indicates a cessation of cell division and replication as well as an up-regulation of oxidative stress and heat shock proteins. Although C. jejuni did not directly encounter these stressors, specific stress response proteins may also have roles in general stress responses as shown with mutants lacking some of the heat shock proteins [9, 10]. However, alongside the reported overlap in responses, there were also a large number of differences compared with previously published datasets [9, 10], which may be a consequence of experimental design and analysis, bacterial growth and the natural variations between strains.
Flagellar genes are linked with acid responses in other bacterial pathogens, including E. coli and H. pylori. In E. coli, flagellar genes are strongly induced in acidic conditions, but few flagellar regulators are up-regulated [16]. Increased levels of flagellar gene transcription have also been observed in E. coli responses to long-term acid exposure, but not after short-term exposure to acidic conditions [15]. In H. pylori, acid-shock resulted in increased expression of σ54-dependent flagellar genes, and this correlated with an increase in the number of motile cells and speed of motility [14]. Thus, exposure to a range of acidic conditions seems to correlate with increased flagellar gene expression among gastrointestinal bacterial pathogens.
How many bacteria sense low pH is currently not well understood, especially in the Epsilon subdivision of the Proteobacteria, such as C. jejuni. In C. jejuni and H. pylori, FlgS is a cytoplasmic histidine kinase that regulates flagella assembly [28, 29]. In H. pylori, FlgS senses low pH and activates urease expression, which contributes to bacterial survival, although this occurs independently of the two-component response regulator partner FlgR [29]. In C. jejuni, our transcriptomic analysis showed no change in flgR regulation, making it possible that the up-regulation of flagellar genes in C. jejuni is mediated by an alternative regulatory pathway.
One possible explanation for the observed acid-induced increase in transcript levels of flagellar genes could be that acid-shock prepares C. jejuni for invasion or colonization of the small intestine. Therefore, in vitro invasion experiments were performed with acid-shocked C. jejuni cultures compared to a pH 7.0 control, which resulted in increased invasion of m-ICcl2 cells by C. jejuni when the m-ICcl2 cells were grown in a transwell model (Fig. 4b). Interestingly, this phenomenon was not observed when m-ICcl2 cells were grown on flat-bottomed wells. This could be due to the elimination of the basolateral route of invasion in cells grown on flat-bottomed wells. Since C. jejuni can translocate through epithelial cell monolayers [30–32], this may be an important route of invasion for more motile acid-shocked bacteria. Acid-shock may therefore increase the numbers of C. jejuni with flagella, which are required for translocation [32], enabling more bacteria to translocate the epithelial cell layer and invade cells. However, the numbers of translocated C. jejuni varied between individual wells, reflecting perhaps differences in invasion mechanisms or responses to host-secreted factors within the bacterial population. Quantification or visualization of translocated bacteria at different time points after infection would address this.
Another difference was that lower invasion levels were seen in the transwell model than in the flat-bottomed well model when comparing non-shocked control C. jejuni cultures (Fig. 4b). Translocated C. jejuni that did not subsequently invade m-ICcl2 cells may account for this difference. Acid-shock then increased invasion levels because C. jejuni were more able to invade m-ICcl2 cells or were forced to escape acid-shock. Alternatively, the transwell invasion model was more physiologically relevant due to greater m-ICcl2 cell polarization, and so invasion levels were, in fact, more realistic than those in the flat-bottomed well model.
This is the first report of the use of m-ICcl2 cells in C. jejuni invasion experiments. These cells can be used to complement in vivo mouse studies [33–35]. There is, however, no perfect invasion model for studying C. jejuni pathogenesis, making the choice of model to use for both in vitro and in vivo work difficult. Currently, the majority of in vitro studies use Caco-2, Int407, Hep-2 and HeLa cell lines [21], which have greatly advanced our knowledge of the molecular mechanisms of C. jejuni pathogenesis. However, as C. jejuni initially colonizes the small intestine, which is inflamed and damaged in C. jejuni infected patients [36], we propose that m-ICcl2 cells may be a suitable addition to the cell line “toolbox” for investigating host cell invasion, as they are morphologically and functionally similar to small intestinal crypts [20].
This is the first attempt to characterize the capacity of acid-shock to induce C. jejuni invasion of intestinal epithelial cells. Acidic conditions may trigger the activation of C. jejuni virulence phenotypes in preparation for host cell invasion. One study investigating co-incubation of C. jejuni with amoebae demonstrated that incubation of C. jejuni under mildly acidic conditions increased its adherence to and invasion of amoebae [37]. The same study also reported that bacterial survival increased after long-term acid exposure and that incubation of C. jejuni with low pH for 1 h increased motility on a swarm plate. However, this was not observed in an earlier study, where C. jejuni motility was reduced when inoculated onto swarm plates adjusted to different pH values [38]. The effect of acidic conditions on C. jejuni motility is therefore not clear and needs to be investigated further, as motility is required for host cell invasion, which is important for virulence [39].
In summary, this work shows that C. jejuni responds to acid-shock by down-regulating genes involved in cell division and replication and by up-regulating flagellar and stress response genes. Acid-shock increases C. jejuni invasion of intestinal epithelial cells when the basolateral invasion route is available. More work is now needed to extend these observations using virulence models. Also, the mechanisms that C. jejuni employs to sense acid-shock need to be elucidated to enhance our understanding of C. jejuni survival and response to acidic conditions, which is relevant to both the food industry and C. jejuni pathogenesis.
Acknowledgements
This work is supported by the Core Institute Strategic Programme funding and the Doctoral Training Grant [number BB/F016816/1] from the Biotechnology and Biological Sciences Research Council (BBSRC) UK to the Institute of Food Research (IFR). The authors acknowledge Sacha Lucchini for help with microarray procedures and analyses, and the members of the Campylobacter research group at the IFR for helpful comments and suggestions.
Contributor Information
M. T. Le, 1Institute of Food Research, Norwich, UK.
I. Porcelli, 1Institute of Food Research, Norwich, UK.
C. M. Weight, 1Institute of Food Research, Norwich, UK.
D. J. H. Gaskin, 1Institute of Food Research, Norwich, UK.
S. R. Carding, 1Institute of Food Research, Norwich, UK; 2School of Medicine, Health Policy and Practice, University of East Anglia, UK.
A. H. M. van Vliet, 1Institute of Food Research, Norwich, UK.
References
- 1.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008 Jul;21(3):505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DEFRA (2010) Zoonoses report. United Kingdom: 2008. [Google Scholar]
- 3.Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol. 2011 Oct 25;8(12):669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N. Campylobacter sialyltransferase gene polymorphism directs clinical features of Guillain-Barré syndrome. J Neurochem. 2007 Nov;103(Suppl 1):150–158. doi: 10.1111/j.1471-4159.2007.04707.x. [DOI] [PubMed] [Google Scholar]
- 5.Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, Jarvenpaa KM. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990 Jul;7(7):756–761. doi: 10.1023/a:1015827908309. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JA, Urbain JL, Adler LP, Charkes ND, Maurer AH, Krevsky B, Knight LC, Fisher RS, Malmud LS. Biphasic nature of gastric emptying. Gut. 1988 Jan;29(1):85–89. doi: 10.1136/gut.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audia JP, Webb CC, Foster JW. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int J Med Microbiol. 2001 May;291(2):97–106. doi: 10.1078/1438-4221-00106. [DOI] [PubMed] [Google Scholar]
- 8.Burton NA, Johnson MD, Antczak P, Robinson A, Lund PA. Novel aspects of the acid response network of E. coli K-12 are revealed by a study of transcriptional dynamics. J Mol Biol. 2010 Sep 3;401(5):726–742. doi: 10.1016/j.jmb.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Reid AN, Pandey R, Palyada K, Naikare H, Stintzi A. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Appl Environ Microbiol. 2008 Mar;74(5):1583–1597. doi: 10.1128/AEM.01507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid AN, Pandey R, Palyada K, Whitworth L, Doukhanine E, Stintzi A. Identification of Campylobacter jejuni genes contributing to acid adaptation by transcriptional profiling and genome-wide mutagenesis. Appl Environ Microbiol. 2008 Mar;74(5):1598–1612. doi: 10.1128/AEM.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang S, Jeon B, Yun J, Ryu S. Roles of RpoN in the resistance of Campylobacter jejuni under various stress conditions. BMC Microbiol. 2011 Sep 22;11:207. doi: 10.1186/1471-2180-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Irene H, Slavik M. Stress-induced adaptive tolerance response and virulence gene expression in Campylobacter jejuni. J Food Safety. 2009;29(4):126–143. [Google Scholar]
- 13.Murphy C, Carroll C, Jordan KN. Identification of a novel stress resistance mechanism in Campylobacter jejuni. J Appl Microbiol. 2003;95(4):704–708. doi: 10.1046/j.1365-2672.2003.02029.x. [DOI] [PubMed] [Google Scholar]
- 14.Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003 Jun;71(6):3529–3539. doi: 10.1128/IAI.71.6.3529-3539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polen T, Rittmann D, Wendisch VF, Sahm H. DNA microarray analyses of the long-term adaptive response of Escherichia coli to acetate and propionate. Appl Environ Microbiol. 2003 Mar;69(3):1759–1774. doi: 10.1128/AEM.69.3.1759-1774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J Bacteriol. 2005 Jan;187(1):304–319. doi: 10.1128/JB.187.1.304-319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000 Feb 10;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 18.Holmes K, Mulholland F, Pearson BM, Pin C, McNicholl-Kennedy J, Ketley JM, Wells JM. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology. 2005 Jan;151(Pt 1):243–257. doi: 10.1099/mic.0.27412-0. [DOI] [PubMed] [Google Scholar]
- 19.Lucchini S, McDermott P, Thompson A, Hinton JC. The H-NS-like protein StpA represses the RpoS (sigma 38) regulon during exponential growth of Salmonella Typhimurium. Mol Microbiol. 2009 Dec;74(5):1169–1186. doi: 10.1111/j.1365-2958.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- 20.Bens M, Bogdanova A, Cluzeaud F, Miquerol L, Kerneis S, Kraehenbuhl JP, Kahn A, Pringault E, Vandewalle A. Trans-immortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am J Physiol. 1996;270:C1666–C1674. doi: 10.1152/ajpcell.1996.270.6.C1666. [DOI] [PubMed] [Google Scholar]
- 21.Friis LM, Pin C, Pearson BM, Wells JM. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J Microbiol Methods. 2005 May;61(2):145–160. doi: 10.1016/j.mimet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Murphy C, Carroll C, Jordan KN. Induction of an adaptive tolerance response in the foodborne pathogen, Campylobacter jejuni. FEMS Microbiol Lett. 2003 Jun 6;223(1):89–93. doi: 10.1016/S0378-1097(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MT, Shepherd M, Poole RK, van Vliet AH, Kelly DJ, Pearson BM. Two respiratory enzyme systems in Campylobacter jejuni NCTC 11168 contribute to growth on L-lactate. Environ Microbiol. 2011 Jan;13(1):48–61. doi: 10.1111/j.1462-2920.2010.02307.x. [DOI] [PubMed] [Google Scholar]
- 24.Wösten MM, Wagenaar JA, van Putten JP. The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J Biol Chem. 2004 Apr 16;279(16):16214–16222. doi: 10.1074/jbc.M400357200. [DOI] [PubMed] [Google Scholar]
- 25.Smith TG, Hoover TR. Deciphering bacterial flagellar gene regulatory networks in the genomic era. Adv Appl Microbiol. 2009;67:257–295. doi: 10.1016/S0065-2164(08)01008-3. [DOI] [PubMed] [Google Scholar]
- 26.Avila RE, Semar ME, de Fabro SP. Ultrastructural differentiation of glandular stomach (proventriculus) in chick embryo. Folia Histochem Cytobiol. 1986;24(3):227–231. [PubMed] [Google Scholar]
- 27.Chaveerach P, ter Huurne AA, Lipman LJ, van Knapen F. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl Environ Microbiol. 2003 Jan;69(1):711–714. doi: 10.1128/AEM.69.1.711-714.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joslin SN, Hendrixson DR. Activation of the Campylobacter jejuni FlgSR two-component system is linked to the flagellar export apparatus. J Bacteriol. 2009 Apr;191(8):2656–2667. doi: 10.1128/JB.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Y, Feng J, Scott DR, Marcus EA, Sachs G. The pH-responsive regulon of HP0244 (FlgS), the cytoplasmic histidine kinase of Helicobacter pylori. J Bacteriol. 2009 Jan;191(2):449–460. doi: 10.1128/JB.01219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Alphen LB, Bleumink-Pluym NM, Rochat KD, van Balkom BW, Wösten MM, van Putten JP. Active migration into the subcellular space precedes Campylobacter jejuni invasion of epithelial cells. Cell Microbiol. 2008 Jan;10(1):53–66. doi: 10.1111/j.1462-5822.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Tall BD, Curtis SK, Kopecko DJ. Enhanced microscopic definition of Campylobacter jejuni 81-176 adherence to, invasion of, translocation across, and exocytosis from polarized human intestinal Caco-2 cells. Infect Immun. 2008 Nov;76(11):5294–5304. doi: 10.1128/IAI.01408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant CC, Konkel ME, Cieplak W, Jr., Tompkins LS. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993 May;61(5):1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson RO, Novik V, Hofreuter D, Lara-Tejero M, Galán JE. A MyD88-deficient mouse model reveals a role for Nramp1 in Campylobacter jejuni infection. Infect Immun. 2007 Apr;75(4):1994–2003. doi: 10.1128/IAI.01216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006 Sep;74(9):5261–5271. doi: 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konkel ME, Monteville MR, Rivera-Amill V, Joens LA. The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr Issues Intest Microbiol. 2001 Sep;2(2):55–71. [PubMed] [Google Scholar]
- 37.Axelsson-Olsson D, Svensson L, Olofsson J, Salomon P, Waldenström J, Ellström P, Olsen B. Increase in acid tolerance of Campylobacter jejuni through coincubation with amoebae. Appl Environ Microbiol. 2010 Jul;76(13):4194–4200. doi: 10.1128/AEM.01219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szymanski CM, King M, Haardt M, Armstrong GD. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect Immun. 1995 Nov;63(11):4295–4300. doi: 10.1128/iai.63.11.4295-4300.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007 Oct;15(10):456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007 Sep;5(9):665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 41.Buelow DR, Christensen JE, Neal-McKinney JM, Konkel ME. Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol Microbiol. 2011 Jun;80(5):1296–1312. doi: 10.1111/j.1365-2958.2011.07645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]