Abstract
In certain conditions Campylobacter jejuni cells are capable of changing their cell shape from a typically spiral to a coccoid form (CF). By similarity to other bacteria, the latter was initially considered to be a viable but non-culturable form capable of survival in unfavourable conditions. However, subsequent studies with C. jejuni and closely related bacteria Helicobacter pylori suggested that CF represents a non-viable, degenerative form. Until now, the issue on whether the CF of C. jejuni is viable and infective is highly controversial. Despite some preliminary experiments on characterization of CF cells, neither biochemical mechanisms nor genetic determinants involved in C. jejuni cell shape changes have been characterized. In this review, we highlight known molecular mechanisms and genes involved in CF formation in other bacteria. Since orthologous genes are also present in C. jejuni, we suggest that CF formation in these bacteria is also a regulated and genetically determined process. A possible significance of CF in the lifestyle of this important bacterial pathogen is discussed.
Keywords: Campylobacter jejuni, cell shape, cell wall, coccoid form, peptidoglycan, stress response, viability, viable but not culturable form
Introduction
Classification of bacteria is traditionally based on their cell shape. The names of bacterial species such as Streptococcus, Staphylococcus, Vibrio, etc. are based on the usual appearance of the cells under a microscope. However, bacterial cell morphology is not static. Under certain conditions such as nutrient deficiency, alleviated temperature and other stress factors, the cell shape may undergo dramatic changes.
One remarkable change is transformation from a rod or spiral form into a spherical or coccoid form (CF). CF cells of some bacteria represent dormant viable but non-culturable (VBNC) forms able to resuscitate and convert to culturable and fully infective forms. Characteristically, spiral cells of Campylobacter spp. (from “campylos” meaning “spiral” in Greek) are also known to be able to convert into CF (Fig. 1). Whether the latter form of Campylobacter can be resuscitated and cause a disease is a controversial issue.
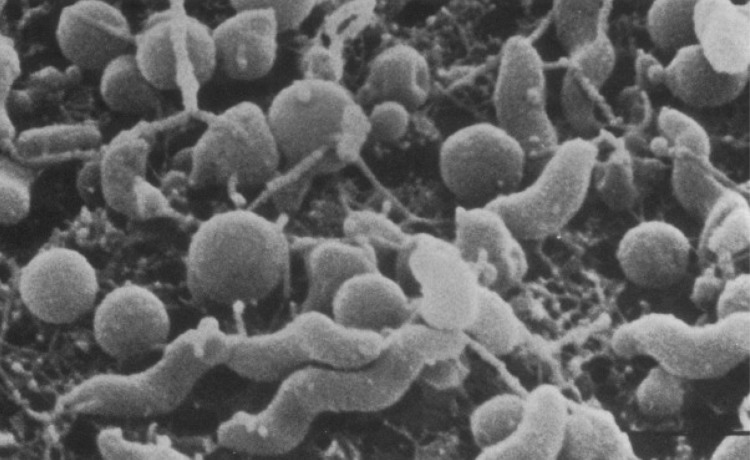
Fig. 1.
Scanning electron micrographs of spiral and coccoid cells of C. jejuni. Reproduced from Ref. [14] (with kind permission from the publisher, American Society of Microbiology)
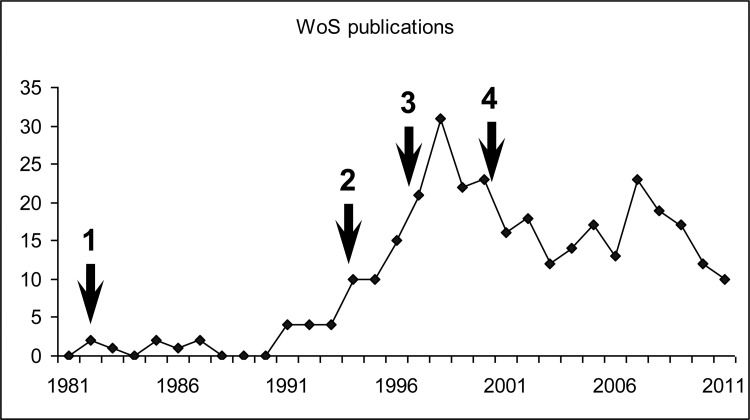
Following publication of some reports suggesting that CF of Campylobacter and that of the closely related bacteria Helicobacter pylori are just degenerative forms of these bacteria, there has been a remarkable decline in interest in this phenomenon. Figure 2 demonstrates significant initial interest to this phenomenon as shown by a hike in a number of publications in year 1994, when it was suggested that CF represents VBNC cells with a potential to “hide and strike”. Publications suggesting that CF of Campylobacter jejuni and H. pylori are simply degenerative/dead cells led to a loss of interest in such studies, and even publication of the first C. jejuni sequence failed to reverse the trend (Fig. 2).
Fig. 2.
Important milestones in studies of CF in Campylobacter and Helicobacter and the annual rate of relevant publications. The keyword combination “coccoid AND (Campylobacter OR Helicobacter)” was used for search of Web of Science publication database. The following time-points are marked by arrows: (1) the first mentioning of CF of Camplobacter spp. [11]; (2) the interest to studies of CF in these and related species received a burst after a publication, suggesting that CF are dormant but viable (potentially infective) cells [13]; (3) publication of an article suggesting that CF of Helicobacter is “morphologic manifestation of cell death” [47], which stimulated decline in these studies; (4) availability of the first complete Campylobacter genome sequence [66] had little impact on the rate of publications mentioning CF formation in these and related bacteria, with very little progress on investigation of the molecular mechanisms of this process, the biological role of which remains a mystery
This review will provide a critical analysis of the current state of studies on CF of C. jejuni. One aim of this review is “resuscitation” of interest to a remarkable phenomenon of CF formation in Campylobacter by highlighting possible biochemical and genetic mechanisms of this process, and its possible role in the life style of this important pathogen.
Campylobacter: an overview
Campylobacter bacteria are known as spiral cells between 0.5 and 5 µm long and 0.2 and 0.8 µm wide, which can be uni- or bi-flagellated, with some spp. being multi-flagellated (C. showae) or non-motile (C. gracilius) [1, 2].
C. jejuni is a microaerophilic, capnophilic, thermophilic subspecies with optimal growth temperature varying from 37 °C to 42 °C. Despite fastidious growth requirements in laboratory environment, C. jejuni is the greatest cause of bacterial foodborne illnesses to humans in the world, more so than Shigella and Salmonella combined [3, 4]. The pathogen is transmitted by avian species including chickens, in which it is commonly present as a part of commensal microbial flora. It is not therefore surprising that infection often results from consumption of undercooked poultry products [5].
C. jejuni is the aetiological agent of Campylobacteriosis, characterized by watery or bloody diarrhoea, vomiting, nausea and fever, with reports of an infectious dose being as low as 500 organisms [6, 7]. However, due to its self-limiting nature, it is only fatal in the immuno-compromised, the very young, or the elderly. This infection can also lead to such complications as reactive arthritis, inflammatory bowel syndrome and Guillian–Barré syndrome (GBS) [8].
The rates of reported cases of Campylobacter, according to HPA (Health Protection Agency, United Kingdom), are increasing every year, becoming a public health concern as well as an economic burden. Despite increased public awareness of the disease and preventative measures, the rate of reported Campylobacter infections in England and Wales in 2010 was 62 684, corresponding to an 8.5% annual rise [9]. In 1995, the costs of Campylobacter-associated diseases was estimated to cost the US economy $1.5–8 billion [10]. Because of a particular nature of the disease (usually a short-term acute form followed by a quick recovery), there is likely to be a number of under-reported cases, and so the actual economic impact of these infections is likely to be much higher than estimated.
Properties of coccoid forms
The first Campylobacter CF-related report available on Web of Science reference database appeared in 1982 [11]. However, it may have been known long before that, because of changes in bacterial names. For example, in 1964, CF cells were described in Vibrio fetus [12]. According to changes in nomenclature and bacterial classification in 1994, these bacteria were later renamed into a subspecies of Campylobacteriaceae [13].
In addition to a CF, Campylobacter cells may also be seen as filaments, doughnuts and straight rods [14]. For the purpose of this review, we use RF (rod form) both for rod-shaped and spiral bacteria. The RF of C. jejuni is considered the usual viable form found at the exponential phase of growth, whilst filaments and CF are associated with the stationary phase of growth [15]. It was suggested that transition from RF to CF occurs via an intermediate shape that resembles a “doughnut” as the cells curl to become spherical [14]. Another intermediate shape that has been seen is the “club” shape, which is characterized by localized expansions of the cell [16, 17]. However, this structure is rarely seen in C. jejuni electron micrographs, suggesting that the form is very short lived and may be an artefact.
Formation of the CF of Campylobacter is stimulated by stress conditions, which include suboptimal temperature, starvation and osmotic stress [18–20]. Coccoid cells have other characteristics besides the obvious feature of their shape. It was revealed that, despite the presence of flagella, coccoid cells of C. jejuni are non-motile [17, 21]. The lack of motility could be a repercussion of the morphology. The corkscrew shape allows for smoother movement within mucous membrane of the gastrointestinal tract [22, 23]. It was suggested that the non-motile nature of the CF is the result of inability of these cells to provide the energy required for flagella movement [24].
Electron microscopy studies revealed that the size of the CF cells can also vary to a greater extent than that of the spiral cells [25]. Because of changes in lysozyme and ethylenediaminetetraacetic acid (EDTA) sensitivity, it was suggested that CF transition involves changes in cell wall peptidoglycan (PG) [17]. This was also supported by difference in Gram staining of CF and RF cells. Whilst the rods and spiral cells were stained typically as Gram-negative bacteria, the coccoid cells were unable to retain as much counterstain (safranin or carbol fuchsin), indicating some changes in the cell wall structure [17, 26].
The lack of structural integrity of the membranes of CF and their “leakage” were also confirmed by reduced levels of nucleic acids, as well as polypeptides, such as superoxide dismutase, when compared to the RF [17, 21]. The CF cells were found to undergo autolysis, suggesting that they may represent a degenerative form of the bacteria [18]. However, this finding contradicted with electron microscopy studies, which demonstrated no signs of autolysis of cells in CF [27].
The conflicting results may be due to different types of CF cells formed under different stress conditions. For example, CF formed under high temperature stress reveal much higher degradation of the cell wall as compared to CF induced under other conditions (described in more details in section “Temperature stress”) [28]
CF and VBNC
Morphological changes to CF are coincident with the decrease in colony-forming unit (cfu) counts and were initially associated with the VBNC state [29, 30]. The VBNC state is defined as “a state of dormancy where growth ceases on bacteriological media normally used for culture of the organism yet the bacteria retain vitality with minimal activity” [26].
Formation of the VBNC cells allows bacterial survival in a dormant state in harsh conditions and is considered to be of great importance for many bacteria [31]. A link between CF and the VBNC form was noticed in a variety of microorganisms, for example, Actinomyces radicidentis [32], V. vulnificus [33], V. cholera [34], Mycobacterium smegmatis [35] and Salmonella typhimurium [36]. Association of CF with the VBNC state of C. jejuni would help to explain the incidences of infections by Campylobacter with no discernable environmental source [37].
According to one study, CF retained viability as judged by cell membrane integrity [29]. Despite a decrease in culturability, the amount of adenosine triphosphate (ATP) within the cells was constant for 3 weeks, which could be indicative of potential viability [29]. However, the relationship between CF and VBNC states for these bacteria has become more ambiguous after a discovery of non-coccoid VBNC cells [38]. Formation of spiral VBNC cells has been confirmed at lower temperatures [30, 39]. It was also reported that killed cells with damaged membranes retained spiral morphology, whereas cells in CF retained membrane integrity [40]. However, the issue of “viability” is controversial, as different assays and criteria for bacterial viability are employed in different labs.
There have been reports of reversion of VBNC forms into culturable forms after acid treatment [41]. In addition, according to some studies using animal models of infection, the VBNC CF of C. jejuni was suggested to be able to revert into fully infectious forms [42–44]. However, it was noticed that some of these results were also controversial due to irreproducibility of the data [45].
CF formation in C. jejuni was accompanied by a significant reduction in the level of protein synthesis, thus supporting the argument in favour of degeneracy [21, 30, 46]. In addition, CF formation in the closely related bacteria H. pylori was considered as a sign of programmed cell death (PCD) [47–49]. However, specific changes in the protein profile of H. pylori concomitant with accumulation of specific proteins during conversion into CF suggested biochemical processes different from a simple decay-like “degenerative” mechanism [49].
The VBNC state and CFF (CF formation) are clearly distinct though related events in the lifestyle of Campylobacter and other bacteria. It is possible that the VBNC state may induce CF formation. However, being in the CF state may not necessarily be an indication of a VBNC state. Conflicting results regarding viability of CF and RF may depend on the different stages and conditions under which these forms are generated and observed, as well as on the methods used in these studies [46]
Stimuli in the induction of coccoid form formation
As stated previously, transformation into CF is stimulated by various stress-inducing factors. The respective mechanisms of CF formation my also be different, and thus the nature and properties of CFs observed under these conditions may also vary, which partly explains the reason for contradictory results obtained with CF of C. jejuni in different labs.
Temperature stress
Depending on temperature, both the rate of CF formation and the type of coccoid cells formed may be remarkably different. In particular, fatty acid composition of CF cells formed at 4 and 12 °C was different from that obtained at 25 °C [46]. There is also variation in the level of biochemical activity at temperatures below 30 °C [50]. The rate of transition to CF in both C. coli and C. jejuni was higher at 37 °C compared to that at 10, 20 and 4 °C [30, 51, 52].
The effect of low temperatures on bacterial viability and the transition to CF was also found to be strain dependent [39, 53]. Types of CF formed at different temperatures may have physiological importance taking into account the conditions C. jejuni encounters during its life cycle, such as 37 °C within the human host compared to lower temperatures bacteria may encounter in the environment or at 4 °C used for storage of poultry and other potential food reservoirs [51].
Oxidative stress
Exposure to oxygen is a well-known factor inducting CFF in Campylobacter [12, 17, 21, 54]. Oxidative stress results from the effect of reactive oxygen species (ROS), such as superoxides and hydrogen peroxide. C. jejuni CF cells formed as a result of prolonged exposure to atmospheric oxygen retained membrane integrity, suggesting their potential viability [40]. Conversely, other studies demonstrated high level of cell membrane damage in CF formed in these conditions [55].
In certain conditions, C. jejuni was shown to be able to grow at ambient atmosphere. This is thought to be a result of either adaptation of the bacterium to aerobic environment or to growth media containing oxygen scavengers, for example, blood and pyruvate [3, 52, 56, 57]. The presence of these oxygen scavengers can also have an effect on the transformation to CF [52]. CF induced at 37 °C under anaerobic condition appeared uniformly spherical unlike the irregular shaped coccoid cells formed under microaerophilic and aerobic conditions [58].
In a biofilm, bacteria form layers differentially exposed to atmospheric oxygen, with the bacteria in the outer layer mostly affected and those in the inner layer protected from adverse effect of oxidative stress. The monospecies biofilms of C. jejuni have been shown to increase resistance to environmental stresses [59]. Bacterial cells in biofilms formed by Campylobacter are heterogeneous in morphology, with approximately equal ratio of CF and RF in C. jejuni, but predominately CF or RF in C. mucosalis and C. curvus, respectively [60]. It was suggested that CFs in biofilms of C. jejuni may have a supportive role by forming a layer of coccoid cells as a means of protecting the viable spiral form from the hostile environment [61].
The extracellular matrix of C. jejuni biofilms was found to contain DNA [62]. However, how the DNA becomes a part of the biofilm is still questioned, with two options being active DNA secretion or its passive release from cells with damaged membranes. In Pseudomonas aeruginosa, DNA is released into the biofilm matrix via small vesicles without cell lysis [63, 64]. However, in C. jejuni, it is possible that the exogenous DNA for the biofilm matrix could be contributed by CF forms as opposed to vesicles. The presence of DNA in biofilms along with the various states of permeability CF formation membranes could provide great insight into the lifestyle of Campylobacter and reveal the potential biological role of the CF formation.
Starvation and stationary phase
Entry into the stationary phase in many bacteria is accompanied by biochemical and morphological changes enabling the cells to increase resistance to inhospitable environments [65]. In Campylobacter, transition of RF to CF is a common observation in the stationary phase of growth due to stress caused by the reduction in nutrients and increase in toxic products [25, 37]. Stress response in these bacteria does not involve the “traditional” RpoS-mediated stationary phase response found in most other Gram-negative bacteria [22, 66, 67].
Programmed cell death and stringent response
The theory of bacterial PCD is a relatively new concept [68]. A link between CF and PCD in H. pylori has been postulated, as morphology change was coincident with a decline in viability and loss of membrane integrity [48]. A particular way of induction of bacterial PCD is via toxin–antitoxin system modules, also known as “addiction modules”. An example of this is the mazEF gene pair in Escherichia coli [69]. The toxin–antitoxin systems consist of a stable toxin and unstable antitoxin that prevents the action of a toxin. The toxin–antitoxin system can be triggered by DNA damage or other stress effects, resulting in the unstable antitoxin being degraded at a faster rate compared to the stable toxin. Accumulation of the latter would result in cell death. Systems similar to the MazEF module of E. coli were also described in other organisms [70].
Although no Campylobacter “toxin–antitoxin” systems have been described, expression of E. coli mazF/mazE genes was found to be regulated by 3´,5´-bispyrophosphate (ppGpp) [69], which also plays a role in CF formation in Campylobacter (see below). This could also lead to speculation of the potential of PCD within C. jejuni biofilms. If the biological role of CF within biofilm is to maintain and create a microenvironment providing protection from external stresses and enabling the survival of a subpopulation of viable cells, PCD would likely be necessary.
Stringent response is defined as “a global stress response that alters gene expression pathways to allow bacterial survival under a multitude of unfavourable conditions and is typically activated by environmental stresses such as nutrient deprivation” [71]. In other bacteria, the stringent response is controlled by the genes spoT and relA. The respective gene products share sequence similarity and are involved in maintenance of a global stress response regulator, guanosine tetra- and pentaphosphates [(p)ppGpp] [72].
Although both spoT and relA genes are found in many bacteria including E. coli, only spoT gene is found in Campylobacter and Helicobacter. The spoT gene is important for bacterial survival inside epithelial cells, and its mutation in C. jejuni was shown to have a pleiotropic effect [71]. A link between spoT expression and CFF in other bacteria has been reported. For example, ppGpp was accumulated during CF formation of M. smegmatis [35]. Moreover, over-expression of ppGpp via introduction of an
E. coli copy of relA gene induced CF production by these bacteria [35]. In the closely related bacteria H. pylori, spoT mutation resulted in accelerated rate of CF formation and decreased resistance to aerobic shock and acid stress [73]. A similar observation was reported for the spoT mutant of C. jejuni [71].
Factors involved in bacterial cell shape maintenance
The main structural component involved in the maintenance of a bacterial cell shape is PG. It is the major element of the cell wall in Gram-positive bacteria, whilst Gram-negative bacteria also have additional structures outside this layer that can add to strength and rigidity.
The PG of E. coli, in which it was studied in detail, is composed of disaccharide-pentapeptide units containing two amino sugars N-acetylglucosamine and N-acetyl-muramic acid, which are connected by a β-1,4 glycosidic bond. PG in other bacteria may have some difference compared to that in E. coli. In particular, PG of H. pylori was found to have very different composition of muropeptides [74]. Because of the overall close genetic, biochemical and morphological relationship between C. jejuni and H. pylori, their PGs are likely to be more similar in structure between each other that to that of E. coli.
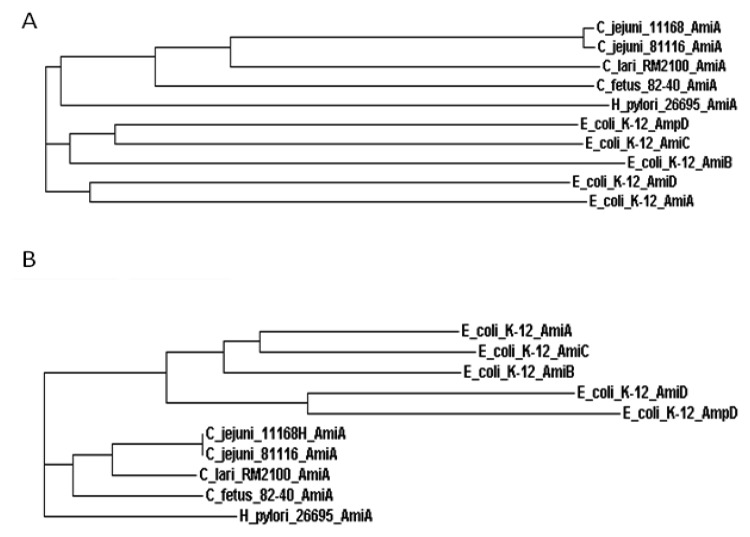
The amount of PG can be indicative of the proportion of spiral bacteria present. It was found that the yield of PG extracted from CF cells of C. coli, C. jejuni and C. fetus was much lower than that from RF cells. [75]. Remarkably, PG was always obtainable from C. fetus, coincident with inability of this subspecies to form CF. It was therefore suggested that transformation of C. jejuni and C. coli into CF may be induced by partial degradation of PG. Among a number of factors involved in the biosynthesis of PG in a model organism E. coli are PG hydrolases that are also required for bacterial cell division. A major class of enzymes involved in the hydrolysis of PG is N-acetylmuramoyl-L-alanine amidases. In case of E. coli, there are five known N-acetylmuramoyl-L-alanine amidases: AmiA, AmiB, AmiC, AmiD and AmpD [76, 77] (Fig. 3).
Fig. 3.
Phylogenetic relationship between selected N-acetylmuramoyl-L-alanine amidases. A and B are phylograms based on the N- and C-terminal regions of the proteins, respectively. ClustalW software was used for multiples alignments and generation of phylogenetic trees. The domains were identified using Pfam database
AmiA, AmiB and AmiC amidases, belonging to family 3 of amidases, have specificity for the amide bond between the sugar backbone of PG and the L-alanine residue of the peptide chain [77]. However, these enzymes do not hydrolyse PG units containing the anhydro-MurNAc group [78]. The enzymes of this family play an important role in the hydrolysis of PG during cell division [79]. Inactivation of genes amiA and amiC individually, but not of the amiB gene, inhibited cell division and resulted in the formation of long chains of cells [79].
Amidase AmpD belongs to family 2 of amidases and is specific for PG units with the anhydro-MurNAc. In contrast to other PG amidases, AmpD is found in cytoplasm, as it is necessary for PG recycling [80]. The function of an outer-membrane-located lipoprotein amidase AmiD is not fully studied [78, 81].
Owing to some overlap in their functions, some of N-acetylmuramoyl-L-alanine amidases of E. coli may partially substitute each other. Despite the seeming redundancy in the number of amidases in E. coli, each of them appears to have a specific role in bacterial lifestyle [79]. In particular, AmiC was found to be localized around the septal ring, whereas AmiA appeared to be distributed throughout the periplasm [82].
In contrast to E. coli, only one amidase-encoding gene is present in the genomes of C. jejuni and the closely related bacteria H. pylori. Sharing sequence similarity with other amidases found in E. coli, the orthologous gene products in these bacteria are annotated as AmiA, and so may be involved in PG biogenesis. As H. pylori PG undergoes substantial modifications upon bacterial entry into CF [74], it was suggested that AmiA protein may play a role in the process [83].
Indeed, the amiA mutation in H. pylori resulted in a profound effect on the bacterial ability to form spherical cells. Unexpectedly, transition to CF was characterized by the accumulation of N-acetyl-D-glucosaminyl-β(1,4)-N-acetylmuramyl-L-Ala-D-Glu (GM-dipeptide), which may indicate an AmiA function separate from amidase activity. Although the function of AmiA in C. jejuni has not been elucidated, amino acid sequence analysis also suggests a bifunctional nature of this protein. According to Pfam domain analysis, all PG amidases are characterized by high conservation in the C-terminal regions containing an “amidase” motif and high variability in the N-terminal regions. Despite close similarities in C-terminal domains of AmiA proteins of C. jejuni and H. pylori (indicating a common PG amidase-related function), the N-terminal moieties of these proteins appear to vary significantly suggesting a difference in their functions.
The discovery of the role of PG in CF formation and of some genes/products in this morphological change suggests that this is a genetically regulated process.
Although the bacterial cytoskeleton plays an important role in control of cell shape [84], some bacteria have a different shape despite similarity in their wall PG structures [85, 86]. A number of non-PG cytoskeletal components and factors involved in bacterial shape maintenance have been identified. One of them, MreB, was found to be important for cell shape maintenance [87]. The mreB gene is usually found in the dcw (for division/cell wall) gene clusters involved in defining bacterial cell shape in various bacteria [88]. Remarkably, MreB contributes nearly as much to the stiffness of a cell as the PG [89–92]. MreB-like proteins are actin-related homologues required for the maintenance of bacterial cell shape by forming helical filaments underneath the cell membrane [93–95]. In E. coli, this protein interacts with an outer penicillin-binding protein 2 (PBP2) [96, 97]). Inactivation of mreB genes in E. coli, Bacillus subtilis and Caulobacter crescentus resulted in CF formation [90, 91, 98]. Transcriptional down-regulation of the mreB and mreC genes Helicobacter hepaticus also induced the formation of spherical cells [99]. It may be suggested that the mreB-like gene present in the genome of C. jejuni NCTC11168 plays a similar role in the maintenance of the bacterial cell shape. It should be mentioned that MreB is not the only known non-PG bacterial cytoskeletal element. For example, a filament-like structural protein crescentin is an essential factor required for maintaining the curved rod shape of C. crescentus [100].
Two other mutations, rodA and pbp2, were shown to stimulate production of spherical, osmotically resistant cells in E. coli [101–104]. Pbp2 gene encodes one of the penicillin-binding proteins (PBPs) necessary for the maintenance and composition of the PG [96]. RodA, which is also integral for the synthesis of PG in E. coli, shares sequence similarity to the FtsW protein involved in the recruitment of PBP2 into the mid-cell region [105, 106].
Although mreB, pbp2, and rodA-like genes are present in Campylobacter genomes, their role in CF formation in C. jejuni remains to be elucidated.
Conclusion
Despite extensive studies on the molecular mechanisms and factors involved in morphological changes in other bacteria, there has been a decline in interest into the investigation of CF in both Campylobacter and Helicobacter, with most of the studies focused on other aspects of biology and epidemiology of these pathogens. This could be explained by a general consensus that in these bacteria CFs are not in a VBNC state, and so unlikely to impose any health risk. However, even considered as degenerative forms of bacteria, CF appears to play a part in a bacterial lifestyle, and so the biological role of this process deserves further investigation. In particular, preliminary data suggest a possible role of CF in biofilm formation. In addition to a possible role in the protection of a subpopulation of bacteria in a biofilm from adverse conditions, disintegration of CF cell membranes may result in leakage of genomic DNA, which is a known component of a biofilm matrix formed by Campylobacter. As a result of bacterial response to stress, CF formation seems to play a role in bacterial adaptation to changing environmental conditions, and may therefore be a regulated and genetically determined process, as in the case of other bacteria. Further studies focusing on deciphering the genetic and biochemical mechanisms involved in CF formation in C. jejuni are essential for understanding of the role of morphological changes in bacterial survival in the environment and mechanisms of transmission of this pathogen from various sources of infection. Clarification of a possible role it may play in pathogenesis and/or in resistance to stress response may ultimately assist in the development of novel antibacterial drugs.
Contributor Information
N. Ikeda, School of Life Sciences, Faculty of Science, Engineering and Computing, Kingston University, Penrhyn Road, Kingston-upon Thames, KT1 2EE, UK
A. V. Karlyshev, School of Life Sciences, Faculty of Science, Engineering and Computing, Kingston University, Penrhyn Road, Kingston-upon Thames, KT1 2EE, UK.
References
- 1.Debruyne L, Gevers D, et al. Taxonomy of the family Campylobacteraceae. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3. Washington, DC: ASM Press; 2008. pp. 3–25. [Google Scholar]
- 2.Vandamme P, Deley J. Proposal for a new family, Campylobacteraceae. Int J Syst Bacteriol. 1991;41:451–455. [Google Scholar]
- 3.Verhoeff-Bakkenes L, Hazeleger WC, de Jonge R, Zwietering MH. Campylobacter jejuni: a study on environmental conditions affecting culturability and in vitro adhesion/invasion. J Appl Microbiol. 2009 Mar;106(3):924–931. doi: 10.1111/j.1365-2672.2008.04072.x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy C, Carroll C, Jordan KN. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl Microbiol. 2006 Apr;100(4):623–632. doi: 10.1111/j.1365-2672.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs-Reitsma W, Lyhs U, Wagenaar J. Campylobacter in the Food Supply. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3. Washington, DC: ASM Press; 2008. pp. 627–644. [Google Scholar]
- 6.Kothary M, Babu U. Infective dose of foodborne pathogens in volunteers: A review. J Food Saf. 2001;21:49–73. [Google Scholar]
- 7.Robinson DA. Infective dose of Campylobacter jejuni in milk. Br Med J (Clin Res Ed) 1981 May 16;282(6276):1584. doi: 10.1136/bmj.282.6276.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes KM, Tattersfield AE. Guillain-Barre syndrome associated with Campylobacter infection. Br Med J (Clin Res Ed) 1982 Jul 17;285(6336):173–174. doi: 10.1136/bmj.285.6336.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Protection Agency (HPA) Campylobacter infections per year in England and Wales, 2000–2010.
- 10.Buzby JC, Allos BM, Roberts T. The economic burden of Campylobacter-associated Guillain-Barré syndrome. J Infect Dis. 1997 Dec;176(Suppl 2):S192–S197. doi: 10.1086/513785. [DOI] [PubMed] [Google Scholar]
- 11.Koike Y, Shimazaki Y. The coccoid form of Campylobacter spp.: fluctuation in cellular morphology. Bulletin of the Nippon Veterinary and Animal Science University. 1982;31:136–142. [Google Scholar]
- 12.Ogg JE. Studies on the coccoid form of ovine Vibrio fetus I. Cultural and serologic investigations. Am J Vet Res. 1962 Mar;23:354–358. [PubMed] [Google Scholar]
- 13.Ursing JB, Lior H, Owen RJ. Proposal of minimal standards for describing new species of the family Campylobacteraceae. Int J Syst Bacteriol. 1994 Oct;44(4):842–845. doi: 10.1099/00207713-44-4-842. [DOI] [PubMed] [Google Scholar]
- 14.NG LK, Sherburne R, Taylor DE, Stiles ME. Morphological forms and viability of Campylobacter species studied by electron microscopy. J Bacteriol. 1985 Oct;164(1):338–343. doi: 10.1128/jb.164.1.338-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths PL. Morphological changes of Campylobacter jejuni growing in liquid culture. Lett Appl Microbiol. 1993 Oct;17(4):152–155. doi: 10.1111/j.1472-765x.1993.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Hill DJ, Mabey M. Morphological changes of synchronized Campylobacter jejuni populations during growth in single phase liquid culture. Lett Appl Microbiol. 1999 Mar;28(3):194–198. doi: 10.1046/j.1365-2672.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 17.Moran AP, Upton ME. A comparative study of the rod and coccoid forms of Campylobacter jejuni ATCC 29428. J Appl Bacteriol. 1986 Feb;60(2):103–110. doi: 10.1111/j.1365-2672.1986.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 18.Buck GE, Parshall KA, Davis CP. Electron microscopy of the coccoid form of Campylobacter jejuni. J Clin Microbiol. 1983 Aug;18(2):420–421. doi: 10.1128/jcm.18.2.420-421.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roszak D, Colwell RR. Viable but non-culturable bacteria in the aquatic environment. J Appl Bacteriol. 1985;59:R9. [Google Scholar]
- 20.Reezal A, McNeil B, Anderson JG. Effect of low-osmolality nutrient media on growth and culturability of Campylobacter species. Appl Environ Microbiol. 1998 Dec;64(12):4643–4649. doi: 10.1128/aem.64.12.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boucher SN, Slater ER, Chamberlain AH, Adams MR. Production and viability of coccoid forms of Campylobacter jejuni. J Appl Bacteriol. 1994 Sep;77(3):303–307. doi: 10.1111/j.1365-2672.1994.tb03078.x. [DOI] [PubMed] [Google Scholar]
- 22.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002 Apr 5;74(3):177–188. doi: 10.1016/s0168-1605(01)00678-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrero RL, Lee A. Motility of Campylobacter jejuni in a viscous environment: comparison with conventional rod-shaped bacteria. J Gen Microbiol. 1988 Jan;134(1):53–59. doi: 10.1099/00221287-134-1-53. [DOI] [PubMed] [Google Scholar]
- 24.Moore JE. Bacterial dormancy in Campylobacter, abstract theory or cause for concern? Int J Food Science Technol. 2001;36:593–600. [Google Scholar]
- 25.Rollins DM, Colwell RR. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson SL, Frirdich E, et al. Survival strategies of Campylobacter jejuni: Stress responses, the viable but non-culturable state, and biofilms. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3. Washington, DC: ASM Press; 2008. pp. 571–590. [Google Scholar]
- 27.Merrell BR, Walker RI, et al. Campylobacter fetus ss jejuni, a newly recognized enteric pathogen – morphology and intestinal colonization. Scan Electron Microsc. 1981:125–132. [PubMed] [Google Scholar]
- 28.Hazeleger WC, Wouters JA, Rombouts FM, Abee T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol. 1998 Oct;64(10):3917–3922. doi: 10.1128/aem.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beumer RR, de Vries J, Rombouts FM. Campylobacter jejuni non-culturable coccoid cells. Int J Food Microbiol. 1992 Jan-Feb;15(1-2):153–163. doi: 10.1016/0168-1605(92)90144-r. [DOI] [PubMed] [Google Scholar]
- 30.Hudock JF, Borger AC, Kaspar CW. Temperature-dependent genome degradation in the coccoid form of Campylobacter jejuni. Curr Microbiol. 2005 Feb;50(2):110–113. doi: 10.1007/s00284-004-4400-x. [DOI] [PubMed] [Google Scholar]
- 31.Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005 Feb;43(Spec No):93–100. [PubMed] [Google Scholar]
- 32.Nair PN, Brundin M, Sundqvist G, Sjögren U. Building biofilms in vital host tissues: a survival strategy of Actinomyces radicidentis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Oct;106(4):595–603. doi: 10.1016/j.tripleo.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Oliver JD, Nilsson L, Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991 Sep;57(9):2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB, Sack DA, Ahmed KU, Sadique A, Watanabe H, Grim CJ, Huq A, Colwell RR. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci U S A. 2007 Nov 6;104(45):17801–17806. doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojha AK, Mukherjee TK, Chatterji D. High intracellular level of guanosine tetraphosphate in Mycobacterium smegmatis changes the morphology of the bacterium. Infect Immun. 2000 Jul;68(7):4084–4091. doi: 10.1128/iai.68.7.4084-4091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakhrouf A, Ben Abdallah F, et al. Morphological changes of starved Salmonella enterica serovar Agona cells in soil after resuscitation. Ann Microbiol. 2008;58:521–525. [Google Scholar]
- 37.Bovill RA, Mackey BM. Resuscitation of 'non-culturable' cells from aged cultures of Campylobacter jejuni. Microbiology. 1997 May;143(Pt 5):1575–1581. doi: 10.1099/00221287-143-5-1575. [DOI] [PubMed] [Google Scholar]
- 38.Federighi M, Tholozan JL, et al. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998;15:539–550. [Google Scholar]
- 39.Lázaro B, Cárcamo J, Audícana A, Perales I, Fernández-Astorga A. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl Environ Microbiol. 1999 Oct;65(10):4677–4681. doi: 10.1128/aem.65.10.4677-4681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Chen CY. Quantitative analysis of viable, stressed and dead cells of Campylobacter jejuni strain 81-176. Food Microbiol. 2010 Jun;27(4):439–446. doi: 10.1016/j.fm.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Chaveerach P, ter Huurne AA, Lipman LJ, van Knapen F. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl Environ Microbiol. 2003 Jan;69(1):711–714. doi: 10.1128/AEM.69.1.711-714.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones DM, Sutcliffe EM, Curry A. Recovery of viable but non-culturable Campylobacter jejuni. J Gen Microbiol. 1991 Oct;137(10):2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- 43.Saha SK, Saha S, Sanyal SC. Recovery of injured Campylobacter jejuni cells after animal passage. Appl Environ Microbiol. 1991 Nov;57(11):3388–3389. doi: 10.1128/aem.57.11.3388-3389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tholozan JL, Cappelier JM, Tissier JP, Delattre G, Federighi M. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl Environ Microbiol. 1999 Mar;65(3):1110–1116. doi: 10.1128/aem.65.3.1110-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medema GJ, Schets FM, van de Giessen AW, Havelaar AH. Lack of colonization of 1 day old chicks by viable, non-culturable Campylobacter jejuni. J Appl Bacteriol. 1992 Jun;72(6):512–516. doi: 10.1111/j.1365-2672.1992.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 46.Hazeleger WC, Janse JD, Koenraad PM, Beumer RR, Rombouts FM, Abee T. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl Environ Microbiol. 1995 Jul;61(7):2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997 Sep;65(9):3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cellini L, Robuffo I, Maraldi NM, Donelli G. Searching the point of no return in Helicobacter pylori life: necrosis and/or programmed death? J Appl Microbiol. 2001 May;90(5):727–732. doi: 10.1046/j.1365-2672.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 49.Benaissa M, Babin P, Quellard N, Pezennec L, Cenatiempo Y, Fauchère JL. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect Immun. 1996 Jun;64(6):2331–2335. doi: 10.1128/iai.64.6.2331-2335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazeleger W, Arkesteijn C, Toorop-Bouma A, Beumer R. Detection of the coccoid form of Campylobacter jejuni in chicken products with the use of the polymerase chain reaction. Int J Food Microbiol. 1994 Dec;24(1-2):273–281. doi: 10.1016/0168-1605(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 51.Höller C, Witthuhn D, Janzen-Blunck B. Effect of low temperatures on growth, structure, and metabolism of Campylobacter coli SP10. Appl Environ Microbiol. 1998 Feb;64(2):581–587. doi: 10.1128/aem.64.2.581-587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou SP, Dular R, Kasatiya S. Effect of ferrous sulfate, sodium metabisulfite, and sodium pyruvate on survival of Campylobacter jejuni. J Clin Microbiol. 1983 Oct;18(4):986–987. doi: 10.1128/jcm.18.4.986-987.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan KF, Le Tran H, Kanenaka RY, Kathariou S. Survival of clinical and poultry-derived isolates of Campylobacter jejuni at a low temperature (4 degrees C) Appl Environ Microbiol. 2001 Sep;67(9):4186–4191. doi: 10.1128/AEM.67.9.4186-4191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klancnik A, Guzej B, Jamnik P, Vucković D, Abram M, Mozina SS. Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res Microbiol. 2009 Jun;160(5):345–352. doi: 10.1016/j.resmic.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Harvey P, Leach S. Analysis of coccal cell formation by Campylobacter jejuni using continuous culture techniques, and the importance of oxidative stress. J Appl Microbiol. 1998 Aug;85(2):398–404. doi: 10.1046/j.1365-2672.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 56.Chynoweth RW, Hudson JA, Thom K. Aerobic growth and survival of Campylobacter jejuni in food and stream water. Lett Appl Microbiol. 1998 Dec;27(6):341–344. doi: 10.1046/j.1472-765x.1998.00453.x. [DOI] [PubMed] [Google Scholar]
- 57.Verhoeff-Bakkenes L, Hazeleger WC, Zwietering MH, De Jonge R. Lack of response of INT-407 cells to the presence of non-culturable Campylobacter jejuni. Epidemiol Infect. 2008 Oct;136(10):1401–1406. doi: 10.1017/S0950268807000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimomura H, Hayashi S, Yokota K, Oguma K, Hirai Y. Alteration in the composition of cholesteryl glucosides and other lipids in Helicobacter pylori undergoing morphological change from spiral to coccoid form. FEMS Microbiol Lett. 2004 Aug 15;237(2):407–413. doi: 10.1016/j.femsle.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Joshua GW, Guthrie-Irons C, Karlyshev AV, Wren BW. Biofilm formation in Campylobacter jejuni. Microbiology. 2006 Feb;152(Pt 2):387–396. doi: 10.1099/mic.0.28358-0. [DOI] [PubMed] [Google Scholar]
- 60.Gunther NW, 4th., Chen CY. The biofilm forming potential of bacterial species in the genus Campylobacter. Food Microbiol. 2009 Feb;26(1):44–51. doi: 10.1016/j.fm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Karlyshev AV, Wren BW. Development and application of an insertional system for gene delivery and expression in Campylobacter jejuni. Appl Environ Microbiol. 2005 Jul;71(7):4004–4013. doi: 10.1128/AEM.71.7.4004-4013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svensson SL, Davis LM, MacKichan JK, Allan BJ, Pajaniappan M, Thompson SA, Gaynor EC. The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol Microbiol. 2009 Jan;71(1):253–272. doi: 10.1111/j.1365-2958.2008.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002 Feb 22;295(5559):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 64.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995 Jul;177(14):3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolter R, Siegele DA, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 66.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000 Feb 10;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 67.Kelly AF, Park SF, Bovill R, Mackey BM. Survival of Campylobacter jejuni during stationary phase: evidence for the absence of a phenotypic stationary-phase response. Appl Environ Microbiol. 2001 May;67(5):2248–2254. doi: 10.1128/AEM.67.5.2248-2254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006 Oct;2(10):e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal "addiction module" regulated by guanosine [corrected] 3',5'-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pellegrini O, Mathy N, Gogos A, Shapiro L, Condon C. The Bacillus subtilis ydcDE operon encodes an endoribonuclease of the MazF/PemK family and its inhibitor. Mol Microbiol. 2005 Jun;56(5):1139–1148. doi: 10.1111/j.1365-2958.2005.04606.x. [DOI] [PubMed] [Google Scholar]
- 71.Gaynor EC, Wells DH, MacKichan JK, Falkow S. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol Microbiol. 2005 Apr;56(1):8–27. doi: 10.1111/j.1365-2958.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- 72.Mittenhuber G. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins) J Mol Microbiol Biotechnol. 2001 Oct;3(4):585–600. [PubMed] [Google Scholar]
- 73.Mouery K, Rader BA, Gaynor EC, Guillemin K. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. J Bacteriol. 2006 Aug;188(15):5494–5500. doi: 10.1128/JB.00366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa K, Bacher G, Allmaier G, Dominguez-Bello MG, Engstrand L, Falk P, de Pedro MA, García-del Portillo F. The morphological transition of Helicobacter pylori cells from spiral to coccoid is preceded by a substantial modification of the cell wall. J Bacteriol. 1999 Jun;181(12):3710–3715. doi: 10.1128/jb.181.12.3710-3715.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amano K, Shibata Y. Structural studies of peptidoglycans in Campylobacter species. Microbiol Immunol. 1992;36(9):961–967. doi: 10.1111/j.1348-0421.1992.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 76.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008 Mar;32(2):259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 77.Kerff F, Petrella S, Mercier F, Sauvage E, Herman R, Pennartz A, Zervosen A, Luxen A, Frère JM, Joris B, Charlier P. Specific structural features of the N-acetylmuramoyl-L-alanine amidase AmiD from Escherichia coli and mechanistic implications for enzymes of this family. J Mol Biol. 2010 Mar 19;397(1):249–259. doi: 10.1016/j.jmb.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 78.Uehara T, Park JT. An anhydro-N-acetylmuramyl-L-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J Bacteriol. 2007 Aug;189(15):5634–5641. doi: 10.1128/JB.00446-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heidrich C, Templin MF, Ursinus A, Merdanovic M, Berger J, Schwarz H, de Pedro MA, Höltje JV. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol Microbiol. 2001 Jul;41(1):167–178. doi: 10.1046/j.1365-2958.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994 Oct 3;13(19):4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pennartz A, Généreux C, Parquet C, Mengin-Lecreulx D, Joris B. Substrate-induced inactivation of the Escherichia coli AmiD N-acetylmuramoyl-L-alanine amidase highlights a new strategy to inhibit this class of enzyme. Antimicrob Agents Chemother. 2009 Jul;53(7):2991–2997. doi: 10.1128/AAC.01520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernhardt TG, de Boer PA. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol Microbiol. 2003 Jun;48(5):1171–1182. doi: 10.1046/j.1365-2958.2003.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaput C, Ecobichon C, Cayet N, Girardin SE, Werts C, Guadagnini S, Prévost MC, Mengin-Lecreulx D, Labigne A, Boneca IG. Role of AmiA in the morphological transition of Helicobacter pylori and in immune escape. PLoS Pathog. 2006 Sep;2(9):e97. doi: 10.1371/journal.ppat.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cabeen MT, Jacobs-Wagner C. Skin and bones: the bacterial cytoskeleton, cell wall, and cell morphogenesis. J Cell Biol. 2007 Nov 5;179(3):381–387. doi: 10.1083/jcb.200708001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young KD. Bacterial shape. Mol Microbiol. 2003 Aug;49(3):571–580. doi: 10.1046/j.1365-2958.2003.03607.x. [DOI] [PubMed] [Google Scholar]
- 86.Young KD. The selective value of bacterial shape. Microbiol Mol Biol Rev. 2006 Sep;70(3):660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamames J, González-Moreno M, Mingorance J, Valencia A, Vicente M. Bringing gene order into bacterial shape. Trends Genet. 2001 Mar;17(3):124–126. doi: 10.1016/s0168-9525(00)02212-5. [DOI] [PubMed] [Google Scholar]
- 89.Wang S, Arellano-Santoyo H, Combs PA, Shaevitz JW. Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9182–9185. doi: 10.1073/pnas.0911517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004 Mar;51(5):1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- 91.Kawai Y, Asai K, Errington J. Partial functional redundancy of MreB isoforms, MreB, Mbl and MreBH, in cell morphogenesis of Bacillus subtilis. Mol Microbiol. 2009 Aug;73(4):719–731. doi: 10.1111/j.1365-2958.2009.06805.x. [DOI] [PubMed] [Google Scholar]
- 92.Divakaruni AV, Baida C, White CL, Gober JW. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Mol Microbiol. 2007 Oct;66(1):174–188. doi: 10.1111/j.1365-2958.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 93.Graumann PL. Cytoskeletal elements in bacteria. Annu Rev Microbiol. 2007;61:589–618. doi: 10.1146/annurev.micro.61.080706.093236. [DOI] [PubMed] [Google Scholar]
- 94.van den Ent F, Amos LA, Löwe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001 Sep 6;413(6851):39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- 95.Daniel RA, Errington J. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell. 2003 Jun 13;113(6):767–776. doi: 10.1016/s0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 96.Popham DL, Young KD. Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr Opin Microbiol. 2003 Dec;6(6):594–599. doi: 10.1016/j.mib.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Young KD. Approaching the physiological functions of penicillin-binding proteins in Escherichia coli. Biochimie. 2001 Jan;83(1):99–102. doi: 10.1016/s0300-9084(00)01205-0. [DOI] [PubMed] [Google Scholar]
- 98.Bendezú FO, de Boer PA. Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli. J Bacteriol. 2008 Mar;190(5):1792–1811. doi: 10.1128/JB.01322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okoli AS, Wilkins MR, Raftery MJ, Mendz GL. Response of Helicobacter hepaticus to bovine bile. J Proteome Res. 2010 Mar 5;9(3):1374–1384. doi: 10.1021/pr900915f. [DOI] [PubMed] [Google Scholar]
- 100.Ausmees N, Kuhn JR, Jacobs-Wagner C. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell. 2003 Dec 12;115(6):705–713. doi: 10.1016/s0092-8674(03)00935-8. [DOI] [PubMed] [Google Scholar]
- 101.Matsuzawa H, Asoh S, Kunai K, Muraiso K, Takasuga A, Ohta T. Nucleotide sequence of the rodA gene, responsible for the rod shape of Escherichia coli: rodA and the pbpA gene, encoding penicillin-binding protein 2, constitute the rodA operon. J Bacteriol. 1989 Jan;171(1):558–560. doi: 10.1128/jb.171.1.558-560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vinella D, Joseleau-Petit D, Thévenet D, Bouloc P, D'Ari R. Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J Bacteriol. 1993 Oct;175(20):6704–6710. doi: 10.1128/jb.175.20.6704-6710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsuzawa H, Hayakawa K, Sato T, Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henriques AO, Glaser P, Piggot PJ, Moran CP., Jr. Control of cell shape and elongation by the rodA gene in Bacillus subtilis. Mol Microbiol. 1998 Apr;28(2):235–247. doi: 10.1046/j.1365-2958.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- 105.Margolin W. Sculpting the bacterial cell. Curr Biol. 2009 Sep 15;19(17):R812–R822. doi: 10.1016/j.cub.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishino F, Park W, Tomioka S, Tamaki S, Takase I, Kunugita K, Matsuzawa H, Asoh S, Ohta T, Spratt BG. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986 May 25;261(15):7024–7031. [PubMed] [Google Scholar]