Abstract
Pemphigus vulgaris is an autoimmune blistering disease of the skin and mucous membranes. Despite the potentially fatal prognosis, there are currently no FDA-approved treatments specifically for pemphigus. In 2006, the FDA designated orphan drug status to mycophenolate mofetil for the treatment of pemphigus vulgaris indicating both federal and commercial interest in developing therapies for this devastating disease. This review focuses on pemphigus therapies that are currently in preclinical or clinical trials, as well as potential novel therapies based on recent advances in the understanding of the pathophysiology of this disease.
Keywords: Autoantibody, azathioprine, CD154, cholinergic agonist, cyclophosphamide, dapsone, intravenous immunoglobulin, p38 MAPK, rituximab, TNF
Introduction
Pemphigus is a potentially fatal autoimmune disease in which severe blistering of the skin and mucous membranes can lead to malnutrition and sepsis. There are different clinical forms of pemphigus, including pemphigus vulgaris (PV), pemphigus foliaceus and paraneoplastic pemphigus. Statistics on disease prevalence are not available; however, PV is classified as a rare disease by the NIH, indicating a prevalence of < 200,000 in the US. Estimates of disease incidence range from 0.76 to 5 new cases per million per year [1], with rates as high as 32 per million per year reported in the Ashkenazi Jewish population, in which > 90% of PV patients possess the HLA-DR4 haplotype DRB1*0402 [2].
PV is characterized by autoantibodies against desmogleins (Dsgs), cell surface adhesion proteins. Most current treatments for pemphigus induce general immunosuppression to reduce circulating autoantibody titers. Corticosteroids are the mainstay of therapy to achieve rapid disease control; however, given the chronic course of PV, steroid-sparing agents such as azathioprine, dapsone, mycophenolate mofetil (MMF) or cyclophosphamide are typically introduced to allow a reduction in corticosteroid dose. Some patients continue to experience severe disease flares even when treated with maximal therapy that includes corticosteroids and adjunctive immunosuppressives. For these refractory patients, more aggressive treatments, such as plasmapheresis, intravenous Ig and more recently, rituximab, are used to control the disease. Unfortunately, most PV therapies are associated with significant morbidity and even mortality, with osteoporosis, liver and hematological toxicity, fatal infection, and secondary risk of cancer among the potential complications of treatment. With this in consideration, more specific and potentially safer disease-targeted therapies are desirable.
This review focuses on drugs recently or currently undergoing clinical trials for PV [3], with a brief discussion of alternative approaches to therapy based on scientific advances in the field (previously reviewed in detail in reference [4•]). Although this review primarily addresses PV, treatments for pemphigus foliaceus are often identical. One relevant issue for clinical trials in PV is the lack of disease definitions, as well as standardized scoring systems for disease activity [5]. The International Pemphigus Committee published a consensus statement on disease definitions and endpoints in 2008 [6•], and is currently validating an instrument for scoring disease activity, which should help to standardize future clinical trials.
Current PV therapies under investigation
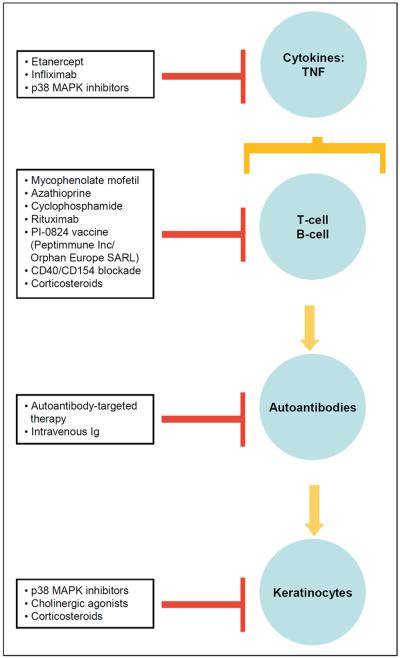
The proposed mechanisms of actions of (Table 1), and drug targets (Figure 1) for pemphigus are shown.
Table 1.
Proposed mechanisms of action for pemphigus therapies.
| Drug | Mechanism | Reference |
|---|---|---|
| Autoantibody-targeted therapy | Specific binding or inhibition of disease-associated anti-Dsg autoantibodies. | [66•] |
| Azathioprine | Prodrug of 6-mercaptopurine, which is metabolized to 6-thioguanine purine analog and 6-thioinosinic acid, a de novo and salvage purine synthesis inhibitor. | [68] |
| CD40/CD154 blockade | Blocks a critical co-stimulatory signal for initiation of the T-cell dependent humoral immune response. | [56•] |
| Cholinergic agonists | Unknown, but may modulate overall keratinocyte cell adhesion. | [61] |
| Corticosteroids | Multiple proposed mechanisms for immunosuppression, including inhibition of NFκB. May upregulate keratinocyte adhesion molecules. | [69,70] |
| Cyclophosphamide | Prodrug metabolized by tissue oxidases to the alkylating agent phosphoramide mustard. | [71] |
| Dapsone | Inhibits leukocytes through multiple proposed mechanisms, including reversible inhibition of myeloperoxidase; mechanism in PV unclear. | [72] |
| Etanercept | Soluble TNFα receptor fusion protein; TNFα may play a role in the pathogenesis of PV. | [38] |
| Infliximab | Anti-TNFα chimeric mAb; TNFα may play a role in the pathogenesis of PV. | [38] |
| IVIg | May saturate serum neonatal Fc receptors, allowing more rapid catabolism of serum IgG; may also prevent keratinocyte apoptosis. | [25,26,27•] |
| MMF | Noncompetitive inhibitor of inosine monophosphate dehydrogenase (de novo nucleotide synthesis inhibitors preferentially target lymphocytes). | [13] |
| p38 MAPK inhibitors | First generation competitive and second generation allosteric inhibitors regulate TNFα production and may have direct effects on keratinocytes. | [47] |
| PI-0824 vaccine (Peptimmune Inc/Orphan Europe SARL) | Synthetic Dsg3 186–204 peptide intended to induce anergy of disease-associated T-cells. | [46] |
| Rituximab | Anti-CD20 chimeric mAb; may deplete autoreactive B-cells, as well as Dsg3-specific CD4+ Th cells. | [M Hertl, personal communication] |
Dsg desmoglein, IVIg Intravenous Ig, MMF mycophenolate mofetil, PV pemphigus vulgaris.
Figure 1.
Drugs and drug targets for PV.
MMF
Several case reports and series have reported that MMF is an effective steroid-sparing agent used in pemphigus [7–9]. MMF has been compared with azathioprine in a clinical trial of pemphigus patients (n = 40) randomized to receive methylprednisolone (2 mg/kg/day) and either azathioprine (2 mg/kg/day) or MMF (2 g/day) [10•]. The majority of patients treated with azathioprine (72%) achieved complete remission (defined as complete re-epithelialization) in a mean of 74 days, compared with 95% of MMF-treated patients achieving complete remission within a mean of 91 days. The average cumulative methylprednisolone doses were 8916 and 9334 mg in the azathioprine and MMF groups, respectively. A population of patients receiving azathioprine (33%) and MMF (19%) experienced grade three or higher adverse effects. None of these differences in results were statistically significant, leading to the conclusion that these two agents demonstrate comparable efficacy and safety in the treatment of pemphigus.
In 2004, a three-year, multicenter, prospective, randomized, double-blind, placebo-controlled phase III trial of PV patients (n = 77) was initiated to assess the safety and efficacy of MMF in achieving remission with reduced corticosteroids [11]. At the time of publication, no results were available for this study. In 2006, the FDA granted orphan drug status to MMF for the treatment of PV, thereby increasing the feasibility of a new drug approval for MMF for the treatment of PV [12].
Despite these promising developments, MMF must be used with caution. Fatal infection and sepsis occurred in 2 to 5% of transplant patients receiving MMF, and pre- and post-marketing surveillance indicates that MMF is associated with an increased risk of infection or reactivation of CMV, herpes zoster, atypical mycobacteria and tuberculosis [13].
Azathioprine
As discussed above, azathioprine (2 mg/kg/day) was reported to demonstrate similar efficacy and safety compared with MMF (2 g/day) [10•]. Another randomized, controlled trial of PV patients (n = 120) compared the efficacy of four different treatment regimens: prednisolone alone or prednisolone plus either azathioprine (2.5 mg/kg/day), MMF (2 g/day) or pulse intravenous cyclophosphamide [14•]. All three immunosuppressives demonstrated comparable safety, although the mean total dose of prednisolone was lower in the group treated with azathioprine compared with MMF, suggesting greater efficacy for azathioprine. Notably, this study used a higher daily dose of azathioprine than the previously mentioned study, and neither study used the maximal dose of MMF (3 g/day). Another phase II clinical trial of prednisone plus azathioprine (2.5 mg/kg/day) has been planned by Tehran University Medical Center to evaluate the efficacy and safety of adjuvant azathioprine therapy in new cases of PV [15]. The study was expected to begin in April 2008.
Patients with genetic polymorphisms in thiopurine methyltransferase (TPMT) that confer low to absent enzyme activity have an increased risk of azathioprine-induced myelotoxicity. This has been estimated to affect approximately 5% of patients [16], although genetic testing for TPMT is not widely commercially available.
Intravenous Ig with or without cyclophosphamide
Intravenous Ig (IVIg) and cyclophosphamide are being investigated in a phase II study to evaluate whether IVIg treatment plus cyclophosphamide results in a more rapid decline in circulating PV antibodies than IVIg alone [17]. Both cyclophosphamide and IVIg have been independently studied in PV. Cyclophosphamide was evaluated in a small case series using a variety of different regimens, including daily oral therapy (1.1 to 2.5 mg/kg/day), daily oral therapy (50 mg) with intermittent high-dose intravenous dexamethasone and cyclophosphamide, and immunoablative intravenous cyclophosphamide [18–22]. All methods were effective in the short-term, although none were curative. Significant side effects, including hematuria, infection and transitional cell carcinoma of the bladder, were observed with higher dose regimens, although one study using a lower daily dose of cyclophosphamide (1.1 to 1.5 mg/kg/day) did not report a significantly different safety profile compared with other immunosuppressive agents. Taken together with the risk of infertility, cyclophosphamide is not generally considered a first-line agent in the treatment of PV, although further studies examining the optimal dosing regimen for safety and efficacy may be warranted.
IVIg has generated clinical and research interest in its efficacy and mechanism of action in treating autoantibody-mediated diseases [23,24], with proposed mechanisms including protection from keratinocyte apoptosis and induction of serum IgG catabolism [25,26]. Studies using a mouse model of PV have suggested that IVIg may saturate neonatal Fc receptors that are responsible for the clearance of serum IgG, providing a molecular mechanism for how circulating serum IgG may be more rapidly degraded [27•]. The benefit of IVIg over immunosuppressive regimens is the relative decrease in disease-causing autoimmune antibodies, since desirable immunne antibodies are present in pooled donor preparations [26]. Despite the reported clinical success of IVIg, patients must be carefully selected given the risk of thrombotic events, acute renal failure, aseptic meningitis and bloodborne diseases [28].
Dapsone
Among the adjunctive immunosuppressants discussed, dapsone is unique in its pregnancy category C designation (MMF, azathioprine and cyclophosphamide all carry a pregnancy category D label). Dapsone was reported in an observational study to be an effective steroid-sparing agent in the maintenance phase of PV treatment [29]. A subsequent phase II clinical trial of PV patients (n = 19) supported modest efficacy for dapsone, with patients receiving dapsone (73%) and placebo (30%) able to reduce daily prednisone dosage to 7.5 mg/day or less [30,31].
Rituximab
Several clinical trials have featured the efficacy of rituximab (an anti-CD20 mAb) in the treatment of PV. One study investigated patients (n = 11) with severe treatment-resistant PV who were dosed with two cycles of rituximab weekly for 3 weeks, followed 1 week later by IVIg (2 g/kg) [32••]. This was followed by a monthly infusion of rituximab and IVIg for 4 consecutive months. The majority of patients (n = 9) responded with clinical remission ranging from 22 to 37 months, with no adverse effects. A second study evaluated the treatment of PV patients (n = 21) with four weekly infusions of rituximab, of whom 18 achieved complete remission within 90 days (20 patients within 360 days) [33••]. Two patients developed pyelonephritis and fatal septicemia. Other reports have noted infectious complications of rituximab therapy in PV, including severe pneumonia and CMV infection [34]. The rationale for concomitant use of IVIg with rituximab is to maintain the humoral immunity otherwise depleted by rituximab; however, given the proposed mechanism of IVIg, concomitant use of IVIg may decrease the therapeutic effect of rituximab by increasing its catabolism. A comparison of rituximab monotherapy to rituximab plus IVIg would be of interest in determining the most effective and safe treatment regimen.
The development of neutralizing human anti-chimera antibodies (HACAs) has been described in a patient with systemic lupus erythematosus treated with rituximab [35]. HACAs have also been observed with the use of other therapeutic chimeric mAbs, such as infliximab, for which pretreatment or concurrent treatment with immunosuppressives, such as azathioprine or methotrexate, has been advocated to avoid HACA development [36]. This complication has not yet been reported in the treatment of PV.
Anti-TNF agents
Interest in the use of anti-TNFα agents in PV was initiated after the finding that TNFα is increased in the blister fluid of patients with PV [37]. Additionally, TNFα receptor-deficient mice exhibit a modestly decreased sensitivity to blister formation after passive transfer of PV serum [38]. Subsequently, several case reports have described the efficacy of infliximab and etanercept in PV [39–41].
Etanercept, a recombinant human soluble TNFα receptor fusion protein that is administered intramuscularly, is being tested in a 16-week, randomized, double-blind, placebo-controlled, phase II trial to evaluate its efficacy as a steroid-sparing agent [42]. Infliximab is a chimeric mAb against TNFα that is administered by intravenous infusion. A randomized, double-blind, placebo-controlled, phase II trial is evaluating infliximab in the treatment of PV, with infusions at weeks 2, 6 and 14 [43]. The primary outcome measurements of this trial are safety and efficacy at week 18, with a total observation period of 26 weeks. Additionally, this trial will evaluate a number of secondary outcomes, including changes in quality-of-life, serum Dsg antibody levels, TNFα and IL-6 levels in serum and skin, and the development of HACA.
PI-0824 vaccine
The HLA-DR4 allele DRB1*0402 confers susceptibility to PV in the Ashkenazi Jewish population [2]. A Dsg3 peptide consisting of amino-acid residues 190–204 was shown to bind specifically to residues within the DRB1*0402 peptide binding pocket, but not to other HLA-DR4 subtypes [44•]. A synthetic Dsg3 peptide consisting of amino-acid residues 186–204 (PI-0824, Peptimmune Inc/Orphan Europe SARL) was subsequently developed to examine whether binding of the peptide in the absence of co-stimulatory signals could selectively suppress the production of anti-Dsg3 antibodies through inactivation and/or deletion of disease-associated CD4 T-cells. In a phase I/II study, patients (n = 17) received two intravenous infusions of the peptide (0.4, 2.0 or 10.0 mg/kg) at 7-day intervals [45,46]. No serious adverse events were observed. Two patients experienced a flare of disease 1 to 5 months post-treatment. There were no significant changes in the levels of anti-Dsg antibodies observed [46]. It was suggested that longer-term studies might be necessary in order to observe changes in antibody levels. Given the pathophysiology, it might be expected that HLA restriction (namely DRB1*0402) would influence patient response, as patients without this haplotype may not present the infused peptide to induce an immunological response. No further clinical trials were being conducted for PI-0824 for PV at the time of publication.
p38 MAPK inhibitors
p38 MAPK was first identified as a potential downstream target in PV through a phosphoprotein screen of radiolabeled human keratinocytes after treatment with PV IgG [47]. Subsequently, activated p38 MAPK was observed in pemphigus patient skin [48], and p38 MAPK inhibition prevented blistering in the murine passive transfer model of PV [49••].
p38 MAPK is a key regulator of inflammatory cytokines, including TNFα. Early clinical studies of p38 MAPK as a target in inflammatory disorders, such as rheumatoid arthritis, Crohn's disease and psoriasis, raised significant safety issues, including liver and CNS effects, infection, skin disorders, and renal impairment [50•,51]. These early compounds targeted the p38 kinase domain through competitive inhibition of ATP binding. Despite sophisticated design strategies to increase their specificity, many second generation p38 allosteric inhibitors bind other cellular kinases, perhaps accounting for their broad toxicity profile [52].
A newer generation allosteric p38 MAPK inhibitor, KC-706 (Kémia Inc), is being evaluated in a phase II multicenter, open-label trial in patients with active, stable PV to evaluate the safety and efficacy of KC-706 (300 mg/day) in achieving remission while maintaining stable doses of corticosteroids and/or immunosuppressants over a 3-month period [53]. At the time of publication, the trial was still recruiting patients. KC-706 has previously demonstrated effectiveness in the passive transfer PV mouse model [DS Rubenstein, personal communication].
Future PV therapies
CD40/CD154 blockade
The interaction of CD40 (expressed by B-cells) and CD154 (expressed on activated T-cells) is an essential costimulatory signal for initiation of the T-cell-dependent humoral immune response [54]. An anti-CD154 mAb has demonstrated promise in the treatment of lupus nephritis [55]. The mAb was tested in an active disease mouse model for PV, in which splenocytes from Dsg3-deficient mice were adoptively transferred to Rag2-deficient mice that express Dsg3 [57•]. Mice treated with negative control IgG prior to adoptive transfer developed anti-Dsg3 antibodies, with patchy hair loss and mucous membrane blistering. In contrast, mice treated with anti-CD154 mAb demonstrated no evidence of the PV phenotype or an anti-Dsg3 serum antibody response through day 70. When anti-CD154 mAbs were infused after adoptive transfer, no protective effect was observed. Although these studies suggest that CD40/CD154 blockade will have little effect on established PV, it is unknown whether treatment would be effective in PV patients in remission to prevent future relapse. Additionally, an endemic form of pemphigus foliaceus with 3.4% prevalence has been described in rural Brazil [57]. It would be of interest to determine if anti-CD154 mAb treatment could prevent disease onset in this susceptible population.
Cholinergic agonists
Anecdotally, PV has been reported to improve with cigarette smoking [58], as well as with the cholinergic agonists pyridostigmine, carbachol and pilocarpine [59•,60]. Studies suggest that activation of cholinergic receptors may regulate signaling pathways modulated by PV IgG, thereby affecting cell adhesion [61]. These results are intriguing given the clinical benefit of nicotine noted in other inflammatory diseases, such as ulcerative colitis [62].
Pathogenic autoantibody-targeted therapy
Pathogenic PV anti-Dsg3 mAbs have been cloned from the active disease mouse model for PV [63], as well as from the peripheral blood lymphocytes of PV patients [64,65]. Adsorption of pathogenic PV mAbs from PV serum using specific anti-idiotypic antibodies depleted serum pathogenic activity of the PV patient from whom the idiotypic antibodies were cloned [66•], supporting the validity of phage display to capture relevant pathogenic autoantibody repertoires. Additionally, genetic analysis of the cloned anti-Dsg3 mAbs demonstrated restricted heavy chain variable region gene usage. Antibody gene restriction (described in a number of immune and autoimmune states) suggests that a limited number of antibody variable region genes may confer the ability to bind a target antigen. For example, anti-Rh(D) antibodies use heavy chain variable gene segments that encode polypeptides with higher isoelectric points, which may facilitate binding to the negatively charged red cell membrane [67]. Due to the conservation of Dsg antigens among patients, common autoantibody structural features or gene usage may be identified among PV patients and thus may serve as a therapeutic target.
Conclusions
Clinically and scientifically, PV represents a model organ-specific autoimmune disease that is chronic, potentially fatal, and currently without any FDA-indicated drugs for its treatment. Despite its rare disease status, PV has gained increasing interest as a target disease for drug development (summarized in Table 1 and Figure 1). Ultimately, continued preclinical research on the immunology and cell biology of disease, as well as clinical trials to evaluate new PV treatments, should lead to safe and effective FDA-approved treatment options for PV patients.
Acknowledgements
We wish to thank Grant Anhalt, Sergei Grando, Russell Hall, Michael Hertl, Kenneth Katz, David Rubenstein and Vicky Werth for information regarding preclinical and clinical trials for pemphigus.
References
•• of outstanding interest
• of special interest
- 1.Stanley JR. Pemphigus. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in General Medicine. Edition 6 McGraw-Hill; New York, NY, USA: 2003. pp. 558–567. [Google Scholar]
- 2.Ahmed AR, Yunis EJ, Khatri K, Wagner R, Notani G, Awdeh Z, Alper CA. Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA. 1990;87(19):7658–7662. doi: 10.1073/pnas.87.19.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ClinicalTrials.gov: Pemphigus vulgaris. NIH; Bethesda, MD, USA: 2008. http://www.clinicaltrials.gov/ct2/results?term=pemphigus+vulgaris. [Google Scholar]
- 4.Sharma P, Mao X, Payne AS. Beyond steric hindrance: The role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci. 2007;48(1):1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17(1):4–11. doi: 10.1684/ejd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 6.Murrell DF, Dick S, Amagai M, Barnadas MA, Borradori L, Bystryn J-C, Cianchini G, Diaz LA, Fivenson D, Goldsmith LA, Hall R, et al. Consensus statement on definitions of disease endpoints and therapeutic response for pemphigus. J Am Acad Dermatol. 2008 doi: 10.1016/j.jaad.2008.01.012. doi:10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enk AH, Knop J. Treatment of pemphigus vulgaris with mycophenolate mofetil. Lancet. 1997;350(9076):494. doi: 10.1016/S0140-6736(05)63084-X. [DOI] [PubMed] [Google Scholar]
- 8.Enk AH, Knop J. Mycophenolate is effective in the treatment of pemphigus vulgaris. Arch Dermatol. 1999;135(1):54–56. doi: 10.1001/archderm.135.1.54. [DOI] [PubMed] [Google Scholar]
- 9.Mimouni D, Anhalt GJ, Cummins DL, Kouba DJ, Thorne JE, Nousari HC. Treatment of pemphigus vulgaris and pemphigus foliaceus with mycophenolate mofetil. Arch Dermatol. 2003;139(6):739–742. doi: 10.1001/archderm.139.6.739. [DOI] [PubMed] [Google Scholar]
- 10.Beissert S, Werfel T, Frieling U, Böhm M, Sticherling M, Stadler R, Zillikens D, Rzany B, Hunzelmann N, Meurer M, Gollnick H, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of pemphigus. Arch Dermatol. 2006;142(11):1447–1454. doi: 10.1001/archderm.142.11.1447. [DOI] [PubMed] [Google Scholar]
- 11.NCT00140127: CellCept (mycophenolate mofetil) in pemphigus vulgaris. NIH; Bethesda, MD, USA: 2005. http://www.clinicaltrials.gov/ct2/show/NCT00140127?term=NCT00140127&rank=1. [Google Scholar]
- 12.Aspreva Pharmaceuticals Corp Aspreva and Roche receive orphan drug designation from FDA for evaluation of Cellcept in rare autoimmune disease pemphigus vulgaris. Press Release. 2006 Jun;:06. [Google Scholar]
- 13.CellCept prescribing information. Roche Pharmaceuticals; Nutley, NJ, USA: 2007. http://www.rocheusa.com/products/cellcept/pi.pdf. [Google Scholar]
- 14.Chams-Davatchi C, Esmaili N, Daneshpazhooh M, Valikhani M, Balighi K, Hallaji Z, Barzegari M, Akhyani M, Ghodsi SZ, Seirafi H, Nazemi MJ, et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J Am Acad Dermatol. 2007;57(4):622–628. doi: 10.1016/j.jaad.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 15. NCT00626678: Use of prednisone with placebo or azathioprine in pemphigus vulgaris. NIH; Bethesda, MD, USA: 2008. http://www.clinicaltrials.gov/ct2/show/NCT00626678?term=azathiprine&rank=2. [Google Scholar]
- 16.Reuther LO, Vainer B, Sonne J, Larsen N-E. Thiopurine methyltransferase (TPMT) genotype distribution in azathioprine-tolerant and -intolerant patients with various disorders. The impact of TPMT genotyping in predicting toxicity. Eur J Clin Pharmacol. 2004;59(11):797–801. doi: 10.1007/s00228-003-0698-8. [DOI] [PubMed] [Google Scholar]
- 17. NCT00483119: Randomized trial of IVIg with or without cyclosporine in pemphigus. NIH; Bethesda, MD, USA: 2007. http://www.clinicaltrials.gov/ct2/show/NCT00483119?term=NCT00483119&rank=1. [Google Scholar]
- 18.Olszewska M, Kolacinska-Strasz Z, Sulej J, Labecka H, Cwikla J, Natorska U, Blaszczyk M. Efficacy and safety of cyclophosphamide, azathioprine, and cyclosporine (ciclosporin) as adjuvant drugs in pemphigus vulgaris. Am J Clin Dermatol. 2007;8(2):85–92. doi: 10.2165/00128071-200708020-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cummins DL, Mimouni D, Anhalt GJ, Nousari CH. Oral cyclophosphamide for treatment of pemphigus vulgaris and foliaceus. J Am Acad Dermatol. 2003;49(2):276–280. doi: 10.1067/s0190-9622(03)00859-4. [DOI] [PubMed] [Google Scholar]
- 20.Kanwar AJ, Kaur S, Thami GP. Long-term efficacy of dexamethasone-cyclophosphamide pulse therapy in pemphigus. Dermatology. 2002;204(3):228–231. doi: 10.1159/000057886. [DOI] [PubMed] [Google Scholar]
- 21.Hayag MV, Cohen JA, Kerdel FA. Immunoablative high-dose cyclophosphamide without stem cell rescue in a patient with pemphigus vulgaris. J Am Acad Dermatol. 2000;43(6):1065–1069. doi: 10.1067/mjd.2000.110397. [DOI] [PubMed] [Google Scholar]
- 22.Nousari CH, Brodsky R, Anhalt GJ. Evaluating the role of immunoablative high-dose cyclophosphamide therapy in pemphigus vulgaris. J Am Acad Dermatol. 2003;49(1):148–150. doi: 10.1067/mjd.2003.581. [DOI] [PubMed] [Google Scholar]
- 23.Messer G, Sizmann N, Feucht H, Meurer M. High-dose intravenous immunoglobulins for immediate control of severe pemphigus vulgaris. Br J Dermatol. 1995;133(6):1014–1016. doi: 10.1111/j.1365-2133.1995.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 24.Sami N, Qureshi A, Ruocco E, Ahmed AR. Corticosteroid-sparing effect of intravenous immunoglobulin therapy in patients with pemphigus vulgaris. Arch Dermatol. 2002;138(9):1158–1162. doi: 10.1001/archderm.138.9.1158. [DOI] [PubMed] [Google Scholar]
- 25.Arredondo J, Chernyavsky AI, Karaouni A, Grando SA. Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in pemphigus. Am J Pathol. 2005;167(6):1531–1544. doi: 10.1016/S0002-9440(10)61239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bystryn JC, Jiao D. IVIg selectively and rapidly decreases circulating pathogenic autoantibodies in pemphigus vulgaris. Autoimmunity. 2006;39(7):601–607. doi: 10.1080/08916930600972016. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, Roopernian DC, Liu Z. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115(12):3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz KA, Hivnor CM, Geist DE, Shapiro M, Ming ME, Werth VP. Stroke and deep venous thrombosis complicating intravenous immunoglobulin infusions. Arch Dermatol. 2003;139(8):991–993. doi: 10.1001/archderm.139.8.991. [DOI] [PubMed] [Google Scholar]
- 29.Heaphy MR, Albrecht J, Werth VP. Dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch Dermatol. 2005;141(6):699–702. doi: 10.1001/archderm.141.6.699. [DOI] [PubMed] [Google Scholar]
- 30. NCT00429533: Efficacy of dapsone as a steroid sparing agent in pemphigus vulgaris. NIH; Bethesda, MD, USA: 2007. http://www.clinicaltrials.gov/ct2/show/NCT00429533?term=NCT00429533&rank=1. [Google Scholar]
- 31.Werth VP, Fivenson D, Pandya AG, Chen D, Rico MJ, Albrecht J, Jacobus D. Multicenter randomized, double-blind, placebo-controlled, clinical trial of dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch Dermatol. 2008;144(1):25–32. doi: 10.1001/archderm.144.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 33.Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, Gougeon ML, Bedane C, Muller R, Dreno B, Doutre MS, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 34.Goh MS, McCormack C, Dinh HV, Welsh B, Foley P, Prince HM. Rituximab in the adjuvant treatment of pemphigus vulgaris: A prospective open-label pilot study in five patients. Br J Dermatol. 2007;156(5):990–996. doi: 10.1111/j.1365-2133.2007.07800.x. [DOI] [PubMed] [Google Scholar]
- 35.Saito K, Nawata M, Iwata S, Tokunaga M, Tanaka Y. Extremely high titer of anti-human chimeric antibody following re-treatment with rituximab in a patient with active systemic lupus erythematosus. Rheumatology. 2005;44(11):1462–1464. doi: 10.1093/rheumatology/kei075. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ. Preventing antibodies to infliximab in patients with Crohn's disease: Optimize not immunize. Gastroenterology. 2003;124(4):1140–1145. doi: 10.1053/gast.2003.50182. [DOI] [PubMed] [Google Scholar]
- 37.Grando SA, Glukhenky BT, Drannik GN, Epshtein EV, Kostromin AP, Korostash TA. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol. 1989;125(7):925–930. [PubMed] [Google Scholar]
- 38.Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Amerio P, Shivji G, Wang BH, Sauder DN. In vitro and in vivo expression of interleukin-1 α and tumor necrosis factor-α mRNA in pemphigus vulgaris: Interleukin-1 α and tumor necrosis factor-α are involved in acantholysis. J Invest Dermatol. 2000;114(1):71–77. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 39.Pardo J, Mercader P, Mahiques L, Sanchez-Carazo JL, Oliver V, Fortea JM. Infliximab in the management of severe pemphigus vulgaris. Br J Dermatol. 2005;153(1):222–223. doi: 10.1111/j.1365-2133.2005.06672.x. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi A, Schuler G, Hertl M. Rapid control of therapy-refractory pemphigus vulgaris by treatment with the tumour necrosis factor-α inhibitor infliximab. Br J Dermatol. 2005;153(2):448–449. doi: 10.1111/j.1365-2133.2005.06744.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin MH, Hsu CK, Lee JY. Successful treatment of recalcitrant pemphigus vulgaris and pemphigus vegetans with etanercept and carbon dioxide laser. Arch Dermatol. 2005;141(6):680–682. doi: 10.1001/archderm.141.6.680. [DOI] [PubMed] [Google Scholar]
- 42. NCT00135720: Study of etanercept (enbrel) in the treatment of pemphigus vulgaris. NIH; Bethesda, MD, USA: 2007. http://www.clinicaltrials.gov/ct2/show/NCT00135720?term=NCT00135720&rank=1. [Google Scholar]
- 43. NCT00283712: Use of infliximab for the treatment of pemphigus vulgaris. NIH; Bethesda, MD, USA: 2008. http://www.clinicaltrials.gov/ct2/show/NCT00283712?term=NCT00283712&rank=1. [Google Scholar]
- 44.Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, Ahmed AR, Strominger JL. Structural basis for major histocompatibility complex (MHC)-linked susceptibility to autoimmunity: Charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci USA. 1995;92(25):11935–11939. doi: 10.1073/pnas.92.25.11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. NCT00063752: Safety study of PI-0824 to treat pemphigus vulgaris. NIH; Bethesda, MD, USA: 2005. http://www.clinicaltrials.gov/ct2/show/NCT00063752?term=NCT00063752&rank=1. [Google Scholar]
- 46.Anhalt G, Werth V, Strober B, Connolly M, Korman N, Greenstein D, Fantasia J, Kalish R. An open-label phase I clinical study to assess the safety of PI-0824 in patients with pemphigus vulgaris. J Invest Dermatol. 2005;125:Abs023. [Google Scholar]
- 47.Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280(25):23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 48.Berkowitz P, Diaz LA, Hall RP, Rubenstein DS. Induction of p38MAPK and Hsp27 phosphorylation in pemphigus patient skin. J Invest Dermatol. 2007;128(3):738–740. doi: 10.1038/sj.jid.5701080. [DOI] [PubMed] [Google Scholar]
- 49.Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA. 2006;103(34):12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci. 2007;28(6):286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 51.O'Neill LAJ. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5(7):549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 52.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 53. NCT00606749: Use of KC706 for the treatment of pemphigus vulgaris. NIH; Bethesda, MD, USA: 2008. http://www.clinicaltrials.gov/ct2/show/NCT00606749?term=NCT00606749&rank=1. [Google Scholar]
- 54.Liu Z, Li N, Diaz LA. Inhibition of pemphigus vulgaris by targeting of the CD40-CD154 co-stimulatory pathway: A step toward antigen-specific therapy? J Invest Dermatol. 2006;126(1):11–13. doi: 10.1038/sj.jid.5700059. [DOI] [PubMed] [Google Scholar]
- 55.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 56.Aoki-Ota M, Kinoshita M, Ota T, Tsunoda K, Iwasaki T, Tanaka S, Koyasu S, Nishikawa T, Amagai M. Tolerance induction by the blockade of CD40/CD154 interaction in pemphigus vulgaris mouse model. J Invest Dermatol. 2006;126(1):105–113. doi: 10.1038/sj.jid.5700016. [DOI] [PubMed] [Google Scholar]
- 57.Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, Rivitti EA, Santos V, Diaz LA. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. N Engl J Med. 2000;343(1):23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 58.Mehta JN, Martin AG. A case of pemphigus vulgaris improved by cigarette smoking. Arch Dermatol. 2000;136(1):15–17. doi: 10.1001/archderm.136.1.15. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140(3):327–334. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- 60.Iraji F, Yoosefi A. Healing effect of pilocarpine gel 4% on skin lesions of pemphigus vulgaris. Int J Dermatol. 2006;45(6):743–746. doi: 10.1111/j.1365-4632.2006.02766.x. [DOI] [PubMed] [Google Scholar]
- 61.Chernyavsky AI, Arredondo J, Piser T, Karlsson E, Grando SA. Differential coupling of M1 muscarinic and α 7 nicotinic receptors to inhibition of pemphigus acantholysis. J Biol Chem. 2008;283(6):3401–3408. doi: 10.1074/jbc.M704956200. [DOI] [PubMed] [Google Scholar]
- 62.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, Sawe U. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330(12):811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 63.Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105(5):625–631. doi: 10.1172/JCI8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeh SW, Cavacini LA, Bhol KC, Lin MS, Kumar M, Duval M, Posner MR, Ahmed AR. Pathogenic human monoclonal antibody against desmoglein 3. Clin Immunol. 2006;120(1):68–75. doi: 10.1016/j.clim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115(4):888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne AS, Siegel DL, Stanley JR. Targeting pemphigus autoantibodies by their heavy chain variable region genes. J Invest Dermatol. 2007;127(7):1681–1691. doi: 10.1038/sj.jid.5700790. [DOI] [PubMed] [Google Scholar]
- 67.Boucher G, Broly H, Lemieux R. Restricted use of cationic germline VH gene segments in human Rh(D) red cell antibodies. Blood. 1997;89(9):3277–3286. [PubMed] [Google Scholar]
- 68.Szawlowski PW, Al-Safi SA, Dooley T, Maddocks JL. Azathioprine suppresses the mixed lymphocyte reaction of patients with Lesch-Nyhan syndrome. Br J Clin Pharmacol. 1985;20(5):489–491. doi: 10.1111/j.1365-2125.1985.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen VT, Arredondo J, Chernyavsky AI, Kitajima Y, Pittelkow M, Grando SA. Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem. 2004;279(3):2135–2146. doi: 10.1074/jbc.M309000200. [DOI] [PubMed] [Google Scholar]
- 71.Brock N. The history of the oxazaphosphorine cytostatics. Cancer. 1996;78(3):542–547. doi: 10.1002/(SICI)1097-0142(19960801)78:3<542::AID-CNCR23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 72.Wolf R, Tüzün B, Tüzün Y. Dapsone: Unapproved uses or indications. Clin Dermatol. 2000;18(1):37–53. doi: 10.1016/s0738-081x(99)00093-0. [DOI] [PubMed] [Google Scholar]