Abstract
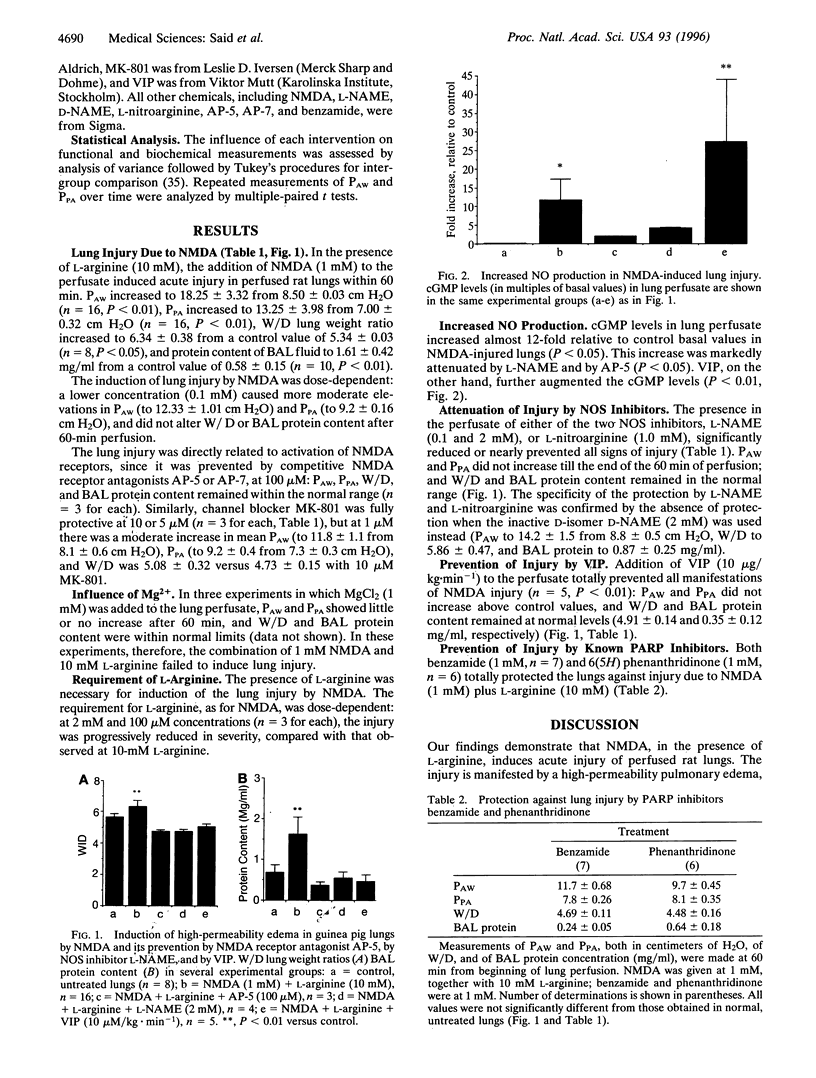
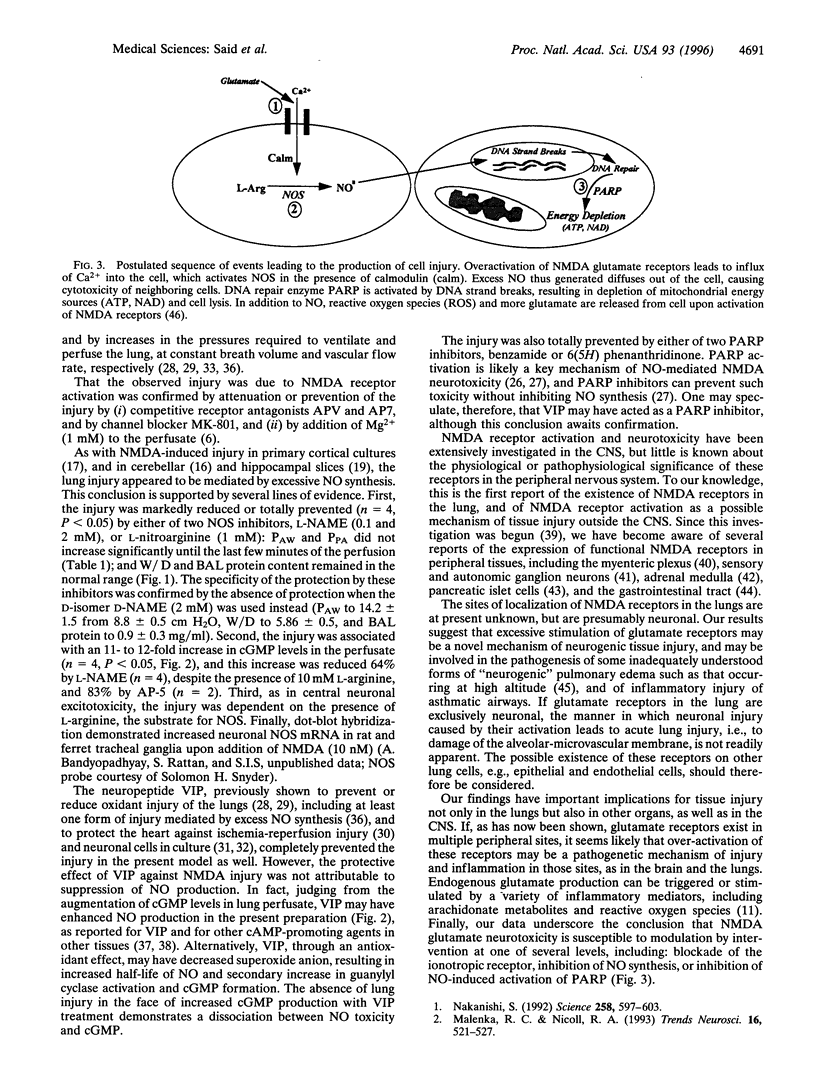
Excitatory amino acid toxicity, resulting from overactivation of N-methyl-D-aspartate (NMDA) glutamate receptors, is a major mechanism of neuronal cell death in acute and chronic neurological diseases. We have investigated whether excitotoxicity may occur in peripheral organs, causing tissue injury, and report that NMDA receptor activation in perfused, ventilated rat lungs triggered acute injury, marked by increased pressures needed to ventilate and perfuse the lung, and by high-permeability edema. The injury was prevented by competitive NMDA receptor antagonists or by channel-blocker MK-801, and was reduced in the presence of Mg2+. As with NMDA toxicity to central neurons, the lung injury was nitric oxide (NO) dependent: it required L-arginine, was associated with increased production of NO, and was attenuated by either of two NO synthase inhibitors. The neuropeptide vasoactive intestinal peptide and inhibitors of poly(ADP-ribose) polymerase also prevented this injury, but without inhibiting NO synthesis, both acting by inhibiting a toxic action of NO that is critical to tissue injury. The findings indicate that: (i) NMDA receptors exist in the lung (and probably elsewhere outside the central nervous system), (ii) excessive activation of these receptors may provoke acute edematous lung injury as seen in the "adult respiratory distress syndrome," and (iii) this injury can be modulated by blockade of one of three critical steps: NMDA receptor binding, inhibition of NO synthesis, or activation of poly(ADP-ribose) polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal M. F. Mechanisms of excitotoxicity in neurologic diseases. FASEB J. 1992 Dec;6(15):3338–3344. [PubMed] [Google Scholar]
- Berisha H. I., Pakbaz H., Absood A., Said S. I. Nitric oxide as a mediator of oxidant lung injury due to paraquat. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7445–7449. doi: 10.1073/pnas.91.16.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha H., Foda H., Sakakibara H., Trotz M., Pakbaz H., Said S. I. Vasoactive intestinal peptide prevents lung injury due to xanthine/xanthine oxidase. Am J Physiol. 1990 Aug;259(2 Pt 1):L151–L155. doi: 10.1152/ajplung.1990.259.2.L151. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985 Aug 5;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Koh J. Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988 Jan;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C., Suzuki H., Milani D., Facci L., Menegazzi M., Vantini G., Kanai Y., Skaper S. D. Poly(ADP-ribose) polymerase: early involvement in glutamate-induced neurotoxicity in cultured cerebellar granule cells. J Neurosci Res. 1994 Sep 1;39(1):38–46. doi: 10.1002/jnr.490390106. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boni U., McLachlan D. R. Controlled induction of paired helical filaments of the Alzheimer type in cultured human neurons, by glutamate and aspartate. J Neurol Sci. 1985 May;68(2-3):105–118. doi: 10.1016/0022-510x(85)90093-0. [DOI] [PubMed] [Google Scholar]
- Demerlé-Pallardy C., Lonchampt M. O., Chabrier P. E., Braquet P. Absence of implication of L-arginine/nitric oxide pathway on neuronal cell injury induced by L-glutamate or hypoxia. Biochem Biophys Res Commun. 1991 Nov 27;181(1):456–464. doi: 10.1016/s0006-291x(05)81441-x. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Neurotoxicity of excitatory amino acid receptor agonists in rat cerebellar slices: dependence on calcium concentration. Neurosci Lett. 1986 May 15;66(2):193–198. doi: 10.1016/0304-3940(86)90189-8. [DOI] [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Hammer B., Parker W. D., Jr, Bennett J. P., Jr NMDA receptors increase OH radicals in vivo by using nitric oxide synthase and protein kinase C. Neuroreport. 1993 Oct 25;5(1):72–74. doi: 10.1097/00001756-199310000-00018. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Imai T., Hirata Y., Kanno K., Marumo F. Induction of nitric oxide synthase by cyclic AMP in rat vascular smooth muscle cells. J Clin Invest. 1994 Feb;93(2):543–549. doi: 10.1172/JCI117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Kuromi H., Gonoi T., Okamoto Y., Ishida H., Seino Y., Kaneko T., Iwanaga T., Seino S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995 May;9(8):686–691. [PubMed] [Google Scholar]
- Izumi Y., Benz A. M., Clifford D. B., Zorumski C. F. Nitric oxide inhibitors attenuate N-methyl-D-aspartate excitotoxicity in rat hippocampal slices. Neurosci Lett. 1992 Feb 3;135(2):227–230. doi: 10.1016/0304-3940(92)90442-a. [DOI] [PubMed] [Google Scholar]
- Kalfin R., Maulik N., Engelman R. M., Cordis G. A., Milenov K., Kasakov L., Das D. K. Protective role of intracoronary vasoactive intestinal peptide in ischemic and reperfused myocardium. J Pharmacol Exp Ther. 1994 Feb;268(2):952–958. [PubMed] [Google Scholar]
- Lafon-Cazal M., Culcasi M., Gaven F., Pietri S., Bockaert J. Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology. 1993 Nov;32(11):1259–1266. doi: 10.1016/0028-3908(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Gendelman H. E. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995 Apr 6;332(14):934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993 Dec;16(12):521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Mayer M. L. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994 Jul;74(3):723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Murthy K. S., Zhang K. M., Jin J. G., Grider J. R., Makhlouf G. M. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993 Oct;265(4 Pt 1):G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Noronha-Dutra A. A., Epperlein M. M., Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett. 1993 Apr 19;321(1):59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Excitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol. 1990;30:47–71. doi: 10.1146/annurev.pa.30.040190.000403. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969 Jul;28(3):455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- Pakbaz H., Foda H. D., Berisha H. I., Trotz M., Said S. I. Paraquat-induced lung injury: prevention by vasoactive intestinal peptide and related peptide helodermin. Am J Physiol. 1993 Oct;265(4 Pt 1):L369–L373. doi: 10.1152/ajplung.1993.265.4.L369. [DOI] [PubMed] [Google Scholar]
- Pincus D. W., DiCicco-Bloom E. M., Black I. B. Vasoactive intestinal peptide regulates mitosis, differentiation and survival of cultured sympathetic neuroblasts. Nature. 1990 Feb 8;343(6258):564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Hastings T. G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995 May;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Hastings T. G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995 May;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S. I., Berisha H. I., Pakbaz H. N-methyl-D-aspartate receptors outside the central nervous system: activation causes acute lung injury that is mediated by nitric oxide synthesis and prevented by vasoactive intestinal peptide. Neuroscience. 1995 Apr;65(4):943–946. doi: 10.1016/0306-4522(95)00021-a. [DOI] [PubMed] [Google Scholar]
- Sata T., Kubota E., Misra H. P., Mojarad M., Pakbaz H., Said S. I. Paraquat-induced lung injury: prevention by N-tert-butyl-alpha-phenylnitrone, a free-radical spin-trapping agent. Am J Physiol. 1992 Feb;262(2 Pt 1):L147–L152. doi: 10.1152/ajplung.1992.262.2.L147. [DOI] [PubMed] [Google Scholar]
- Shannon H. E., Sawyer B. D. Glutamate receptors of the N-methyl-D-aspartate subtype in the myenteric plexus of the guinea pig ileum. J Pharmacol Exp Ther. 1989 Nov;251(2):518–523. [PubMed] [Google Scholar]
- Shigemoto R., Ohishi H., Nakanishi S., Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992 Sep 14;144(1-2):229–232. doi: 10.1016/0304-3940(92)90756-w. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Ogita K. Localization of [3H]glutamate binding sites in rat adrenal medulla. Brain Res. 1986 Sep 24;383(1-2):387–391. doi: 10.1016/0006-8993(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Younkin D. P., Tang C. M., Hardy M., Reddy U. R., Shi Q. Y., Pleasure S. J., Lee V. M., Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zinkand W. C., Stumpo R. J., Thompson C., Patel J., Pullan L. M. Lack of involvement of nitric oxide in NMDA-induced neuronal cell death in cortical culture. Neuroreport. 1993 Nov 18;5(2):148–150. doi: 10.1097/00001756-199311180-00013. [DOI] [PubMed] [Google Scholar]




