Abstract
There has been an upsurge of interest in the adipocyte coincident with the onset of the obesity epidemic and the realization that adipose tissue plays a major role in the regulation of metabolic function. The past few years in particular have seen significant changes in the way we classify adipocytes, and how we view adipose development and differentiation. We have new perspective on the roles played by adipocytes in a variety of homeostatic processes, and the mechanisms used by adipocytes to communicate with other tissues. Finally, there has been significant progress in understanding how these relationships are altered during metabolic disease, and how they might be manipulated to restore metabolic health.
Introduction
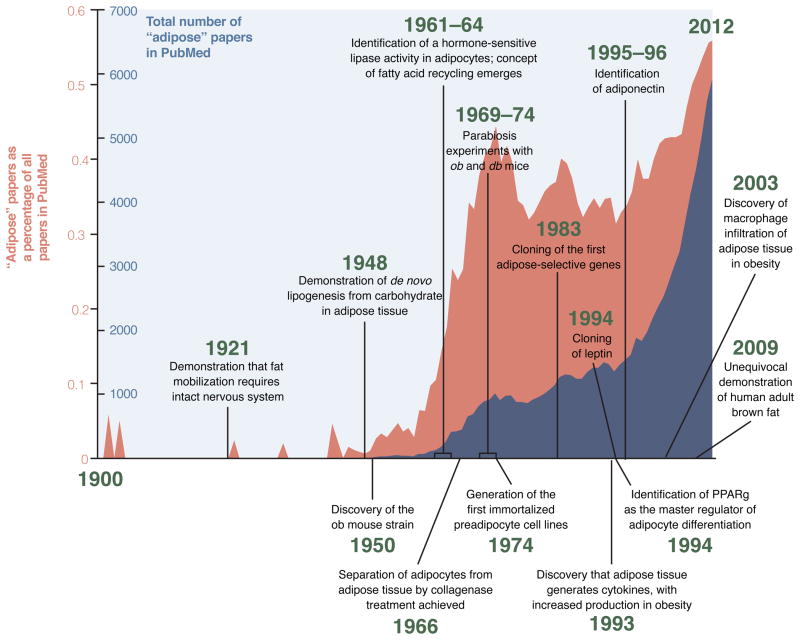
Adipose tissue is a remarkably complex organ with profound effects on physiology and pathophysiology, but it has not always been viewed in this light. Until the late 1940s, adipose tissue was characterized as a form of connective tissue that happened to contain lipid droplets, without linking this fact to the metabolism of the organism in any meaningful way. This gradually began to change with the realization that adipose tissue plays a major role in nutrient homeostasis, serving as the site of calorie storage after feeding and as the source of circulating free fatty acids during fasting. In the late 1980s to mid 1990s came the discovery of adipose-derived serum factors like adipsin, TNF-α and leptin. Suddenly, adipose tissue had to be regarded as an endocrine organ at the center of energy homeostasis. From this point forward, studies on the developmental, functional, and pathophysiological aspects of adipose tissue have expanded markedly. The renewed interest in fat has occurred simultaneously with a tremendous increase in global rates of obesity and Type diabetes; this is not coincidence, of course. We have reached the inflection point at which the global burden of suffering due to overnutrition outpaces that due to undernutrition for the first time in human history, with 1.7 billion people classified as obese (Haslam and James, 2005). Given its central role in energy and glucose homeostasis, interest in ‘solving’ the adipocyte has never been higher, and shows no sign of abatement.
This review will focus on topics in adipose biology that are evolving quickly, and that shed light on areas of particular importance in metabolic health and disease. Such an endeavor can never be truly comprehensive, but our goal is to provide a sense of the ‘state of the field’ for readers both inside and out of the adipose community.
Functions of fat
All eukaryotes from yeast to man are able to store calories in the form of lipid droplets, but only vertebrates have specialized cells that are recognizable as adipocytes (Ottaviani et al., 2011). It is unclear if the lipid storing cells of lower organisms, such as the Drosophila larval fat body or intestinal cells of C. elegans, represent structures that are truly homologous to adipocytes, or simply reflect convergent evolution to solve the problems associated with storing potentially toxic lipid molecules. At the molecular level, one can find orthologous lipid storage genes performing similar functions in worms, flies, and mammals, but there are also many exceptions (Young and Zechner, 2013).
Because of the association with metabolic disease, not to mention the cosmetic and psychological burden of excess body fat, adipocytes are perhaps the most vilified non-malignant cell type in the body. Given that context, it has been easy to overlook the many benefits provided by healthy adipose tissue. Energy homeostasis and reproduction are arguably the two most important biological functions of any organism, and adipose tissue is inextricably entwined with both. The relationship between adiposity and reproduction is quite complex, with fat providing nutrients and hormonal signals that regulate the hypothalamic-pituitary-gonadal axis in both males and females; conversely, blocking reproduction increases adiposity in many species (Michalakis et al., 2013).
Adipose tissue also has important mechanical properties, serving to protect delicate organs (the eye, for example, is surrounded by fat in a manner analogous to the way one might pack a teacup in bubble wrap) and to cushion body parts exposed to high levels of mechanical stress (the heel and toe pads, for example, are filled with fat). Additionally, fat plays an important role in streamlining aquatic mammals and in providing insulation; the role of adipose tissue in the latter may be overblown, however, as arctic and tropical mammals display a similar distribution of subcutaneous and visceral fat (Pond, 1992). Fatty tissues are also used as displays for sexual selection, such as the cheek pads of the male orangutan, and (in some cultures) the human female buttocks (Singleton, 2008).
By far, however, the most important function of adipose tissue is as a master regulator of energy balance and nutritional homeostasis; how these critical processes are coordinated locally and systemically by adipose tissue is a major theme of this review.
Adipocytes are not all the same: white, brown, and beige
Traditionally, adipocytes have been divided into two types: unilocular white adipocytes make up the bulk of fatty tissue in most animals, marbling our steaks and expanding around our midsections. Brown adipocytes, on the other hand, are highly specialized cells that dissipate stored chemical energy in the form of heat. They do this through the actions of uncoupling protein-1 (UCP-1), a brown adipose tissue (BAT)-specific protein located within the mitochondria, which are densely packed in these cells. UCP-1 catalyzes a proton leak across the inner mitochondrial membrane, thus ‘uncoupling’ fuel oxidation from ATP synthesis. While many models have been proposed to explain how UCP-1 works, recent studies suggest that it acts as a long-chain fatty acid/H+ symporter (Fedorenko et al., 2012). Classic brown adipocytes cluster as specific depots located in the interscapular and perirenal regions of rodents, and are richly innervated and vascularized (Bartness et al., 2010b).
Evolutionarily, brown adipocytes appear in eutherian (placental) mammals; all other vertebrates, including marsupials and monotremes, possess only white fat (Hayward and Lisson, 1992). Interestingly, ‘protoendothermic’ mammals, which have body temperatures that track with ambient temperature, also have brown adipose tissue, which enables them to maintain endothermy selectively while pregnant and caring for their young (Oelkrug et al., 2013). Human babies have significant brown fat depots, presumably to provide heat in the cold environment encountered at birth. Adult humans, however, were felt to be largely devoid of brown fat, unless specifically challenged by chronic cold (as experienced by Scandinavian outdoor workers) or by states of catecholaminergic excess (as seen in pheochromocytoma) (English et al., 1973; Huttunen et al., 1981). The existence of significant depots of genuine brown fat in adult humans, however, was recently proven based upon radiological observations of symmetrical [18F]-2-fluoro-D-2-deoxy-D-glucose (FDG) positron emission tomography (PET) positive loci in the supraclavicular and spinal regions of patients getting such scans for cancer diagnosis or staging. These regions were subsequently proven by biopsy to contain bona fide UCP-1+ adipose tissue consistent with brown fat (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009).
In rodents, prolonged cold exposure or adrenergic signaling can provoke the appearance of clusters of UCP-1+ cells with a brown fat-like morphology within white fat depots. For decades, these cells were poorly characterized, and were simply called brown adipocytes. Their abundance varies dramatically between depots, with the highest numbers found in inguinal and retroperitoneal fat and much lower numbers seen in perigonadal fat. There are also significant strain-specific differences in the number of these cells, which correlates positively with resistance to diet-induced obesity (Xue et al., 2007). These inducible cells have been called ‘beige’ or ‘brite’ adipocytes, and have an overlapping but distinct gene expression pattern compared to classic brown adipocytes. Both express a core program of thermogenic and mitochondrial genes, including Ucp1, while murine beige (but not classic brown) cells also express the surface markers Cd137 and Tmem26 (Wu et al., 2012). Other genes, like Zic1, appear to mark classic brown adipocytes but not beige cells (Walden et al., 2012). Are the UCP-1+ cells in humans equivalent to rodent brown adipocytes, or are they more similar to beige cells? Several groups have tackled this issue, and have come to different conclusions based on the relative expression of these and other marker genes. The interscapular brown fat of human infants shares extensive similarity with classic brown fat in rodents (Lidell et al., 2013). In adult humans, the answer may depend on the specific depot sampled, as cells with both brown and beige attributes have been identified, with the brown:beige ratio increasing as one moves deeper within the neck and back (Cypess et al., 2013; Jespersen et al., 2013; Lidell et al., 2013; Sharp et al., 2012; Wu et al., 2012).
We are still early in the process of understanding the similarities and differences between brown and beige adipose cells, and we do not yet have a clear picture of their relative importance in energy homeostasis. Bioenergetic analysis of BAT and WAT that has been rendered more brown by exposure to a β-adrenergic compound suggest that both are truly thermogenic, with a large fraction of their respiration uncoupled. Comparing pure clonal brown and beige cells, it appears that the classical brown fat cells have a higher basal UCP1 expression and elevated uncoupled respiration (relative to white or beige cells) before hormonal stimulation. Beige cells, on the other hand, have low basal UCP1 expression and uncoupled respiration, comparable to white cells. However, stimulation with a β-adrenergic agonist elevates UCP1 to levels seen in brown fat cells. This suggests that beige cells are uniquely programmed to be bifunctional, suited for energy storage in the absence of thermogenic stimuli, but fully capable of turning on heat production when appropriate signals are received (Wu et al., 2012). Interestingly, selective loss of classic brown fat (by ablation of the Type IA BMP receptor) causes compensatory induction of beige fat, restoring both body temperature and resistance to diet-induced obesity, suggesting significant overlap in function (Schulz et al., 2013).
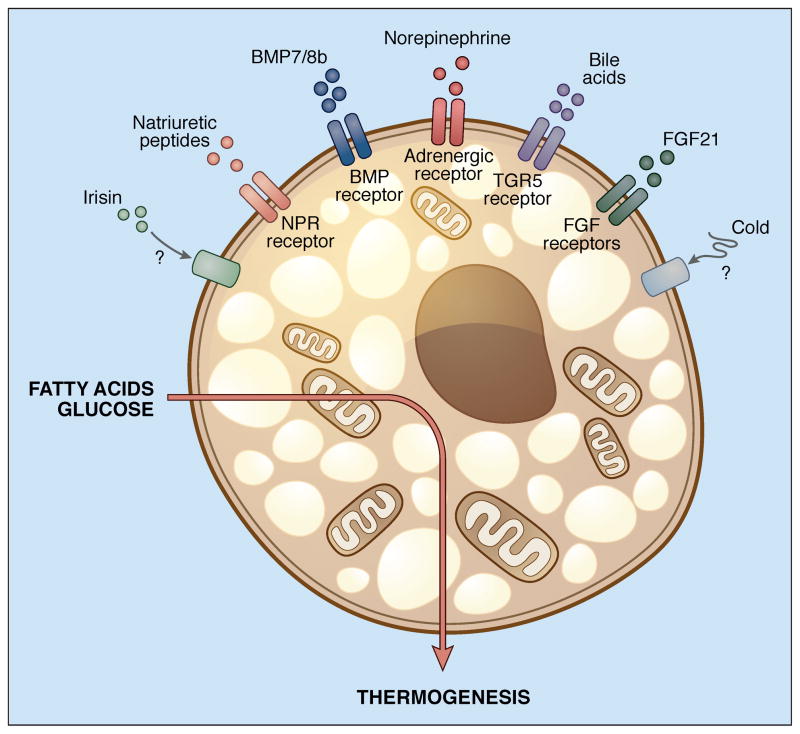
One area of particularly rapid growth concerns the physiological activators of thermogenesis in brown and beige cells. The role of the sympathetic nervous system (SNS) has been long appreciated here, with several hypothalamic and extrahypothalamic areas serving as integrators of the cold response (Chechi et al., 2013). The SNS does not exert a monolithic response to central activation, but rather distributes signals to white or brown adipose depots according to need, so that the effect of food deprivation on SNS input to adipose tissue is qualitatively different than the effect of cold exposure (Brito et al., 2008). Cold may also have effects on BAT function that do not depend on SNS signaling. For example, white and beige (but not brown) adipocytes can directly sense temperature. Mice lacking all β-adrenergic receptors show diminished thermogenic gene induction in interscapular BAT after cold exposure, but still demonstrate browning of white fat. This can be replicated by placing white or beige cells at 30°C in vitro, an effect independent of the traditional cAMP-CREB pathway (Ye et al., 2013). The superficial location of subcutaneous white fat may be ideal for it to serve as a thermal sensor, although the contribution of this pathway to total energy expenditure is still unclear.
Numerous circulating hormones have been implicated in BAT activation in addition to catecholamines, such as triiodothyronine (T3), which is generated from serum thyroxine in large quantities by deiodinase activity within brown and beige adipocytes. Hepatic bile acids and FGF21 have also been shown to enhance browning, as have cardiac hormones like atrial and ventricular natriuretic peptides (ANP and BNP) and cardiotrophin-1 (Villarroya and Vidal-Puig, 2013). Irisin, a hormone produced by skeletal muscle in response to exercise, is also a potent inducer of browning (Bostrom et al., 2012). These agents act through their respective receptors to induce browning by various overlapping mechanisms. Bile acids, for example, activate the TGR5 receptor, which in turn induces the deiodionase enzyme that promotes intracellular T3 formation (Watanabe et al., 2006). Thyroid hormone and catecholamines both induce the local formation of BMP8b, which sensitizes the brown adipocyte to further adrenergic signaling. BMP8b also acts in the brain to direct SNS signaling specifically to BAT (Whittle et al., 2012). Various retinoids have also been implicated in brown adipocyte activation, at least in part through direct transcriptional effects on the Ucp1 gene (Alvarez et al., 1995; Kiefer et al., 2012).
The vast amount of information that has emerged in the past few years on brown and beige fat physiology presents a simple question: Why do so many things cause browning? Browning in response to a thermal challenge seems obvious enough, but why should it have evolved as a response to volume overload of the heart, or exercise? Perhaps the thermogenic response to exercise is a ‘tag-along’ effect, a by-product of the ability to promote thermogenesis in response to nonsynchronous muscle contraction (i.e. shivering) that was neither selected for or against.
Distinctions among white fat depots: location, location, location!
Adipose tissues develop in multiple discrete locations, with larger accumulations recognized as specific depots. The most common classification scheme distinguishes between subcutaneous and visceral fat, in large part because the latter depot has a well-known association with metabolic disease, while the former does not (or may even be inversely correlated with disease risk) (Lee et al., 2013). In fact, the visceral vs. subcutaneous scheme is oversimplified, as there appear to be clear distinctions between nominally visceral depots like the perigonadal, mesenteric, and retroperitoneal fat pads, among others. Importantly, many depots in humans have no precise correlates in mice, and vice versa; for example, a large percentage of visceral fat in humans is contained in the omentum, which is barely present in rodents. Conversely, the large epididymal fat pads of male mice, which are frequently sampled as representative of visceral fat, do not exist in men.
Regardless of these distinctions, there are clearly important regional differences in such aspects of adipocyte behavior as adipokine secretion and rates of lipolysis and triglyceride synthesis (Tchkonia et al., 2013). Two hypotheses have been suggested to explain this phenomenon: either (a) different depots have unique innervation and specific relationships with the circulation (for example, the venous drainage of visceral fat empties into the portal circulation, thus bathing the liver in the by-products of fat metabolism and adipokines), or (b) cell autonomous mechanisms dictate depot-specific differences in adipocyte physiology. These notions are not mutually exclusive, of course, but significant evidence has emerged in support of cell autonomous differences. For example, preadipocytes express gene signatures that are specific for their depot of origin, and they continue to behave distinctly even after isolation and prolonged passage under identical conditions (Macotela et al., 2012; Tchkonia et al., 2013). Transplantation studies have put this to the direct test; placing visceral fat into a subcutaneous position has very little effect, but transplanting subcutaneous fat to the visceral compartment leads to reduced adiposity and improvement in glucose homeostasis (Tran and Kahn, 2010). These results indicate that there are intrinsic differences between depots, and also imply that subcutaneous fat may have beneficial effects on metabolism.
Interestingly, many diseases that affect adipose tissue show depot-specific effects. For example, glucocorticoid excess due either to endogenous overproduction or pharmacological therapy is associated with redistribution of fat to visceral stores with relative wasting of subcutaneous fat. A similar pattern is seen in the acquired lipodystrophy associated with certain HIV treatment regimens. Congenital lipodystrophy can also preferentially affect specific depots, with different patterns of fat loss associated with distinct genetic lesions. Thus, while mutations in BSCL2 cause loss of fat in all depots, mutations in CAV1, AGPAT2, and PTRF are associated with the absence of metabolically active depots but not mechanical sites like the palm, sole, and retroorbital depots, and patients with LMNA mutations lose subcutaneous fat preferentially from the trunk and extremities, but not the face and neck (Garg, 2011). The pathophysiological mechanisms that account for these patterns remain elusive.
The developmental origins of adipose tissue- a bloody mess
The developmental timing of adipose tissue formation varies somewhat between species. In rodents, white adipose tissue appears largely after birth, although using sensitive reporters one can see expression of adipose-specific markers in the subcutaneous region as early as embryonic day 16.5–17.5, and lipid-filled subdermal adipocytes can be detected a day after that (Birsoy et al., 2011; Greenwood and Hirsch, 1974). Visceral fat develops later, becoming visible by postnatal day 7; committed precursor cells are not even found in the nascent epididymal pad until postnatal day 4 (Han et al., 2011). Similarly, zebrafish do not develop adipocytes or discernible precursor cells until after the larval stage (Flynn et al., 2009). In humans, however, one sees obvious white fat development by the 14th week of gestation, although the precise timing may depend to some degree on fetal size, with larger fetuses developing identifiable adipocytes earlier than smaller ones (Poissonnet et al., 1983; Poissonnet et al., 1984). Proliferation tends to diminish late in gestation, and adiposity increases primarily by filling of predetermined cells until age 10 or so, followed by a period of increased cellularity that lasts through adolescence. This period sets the total number of adipocytes that the individual will have as an adult, although new cells are constantly being created and destroyed throughout life (Knittle et al., 1979). In humans, roughly 8% of adipocytes are turned over approximately every year, while in mice, 0.6% of adipocytes are renewed each day (Rigamonti et al., 2011; Spalding et al., 2008).
From a cellular perspective, adipocytes develop from preadipocytes, which themselves derive from precursor cells which carry a bewildering array of names in the burgeoning literature (Cawthorn et al., 2012). In general, the so-called stromal-vascular fraction (SVF) is separated from mature adipocytes by collagenase digestion and low speed centrifugation. When the SVF is cultured ex vivo, blood cells, endothelial cells, and other non-fibroblastic cells do not attach to the dish. What remains can be almost completely differentiated using a hormonal cocktail that typically includes insulin, a glucocorticoid, a phosphodiesterase inhibitor, and often a PPARγ agonist. This does not allow, however, for identification of the specific cell type within the SVF that populates the mature adipocyte fraction in vivo, and this has spurred a number of studies involving selective flow sorting using antibodies against various cell surface markers. Most of these studies have shown that mesenchymal and stem cell markers such as CD34 and Sca-1 strongly enrich for adipogenic precursors (Cawthorn et al., 2012). Additional insight was gained when CD45−;CD31−;Ter119−;CD29+;CD34+;Sca-1+ cells were separated based on their CD24 status. Both CD24+ and CD24− cells could be converted to adipocytes in a dish, but only the former could reconstitute a functional fat pad when transplanted into a lipodystrophic mouse, and only when placed in an appropriate microenvironment (Rodeheffer et al., 2008).
Adipocytes develop from mesenchyme, which is primarily of mesodermal origin. In the cephalic region, however, mesenchyme derives from the neurectoderm, and thus adipocytes in this part of the body are ectodermal (Billon et al., 2007). The earliest recognizable structure that will become a fat pad is a cluster of blood vessels originally called a “primitive organ”; these structures have been identified in creatures as diverse as reptiles, chickens, mice, and humans (Wassermann, 1965). This observation, when combined with ultrastructural data suggesting tight apposition of the vasculature and the developing fat pad, and other studies suggesting a functional link between adipogenesis and angiogenesis (Cinti et al., 1984; Fukumura et al., 2003) led to suspicions that adipocytes might derive from cells associated with blood vessels. Several lineage tracing experiments have strongly supported this idea. For example, early adipose progenitors within the fat pads of young mice express PPARγ; these cells are physically associated with the walls of intra-adipose blood vessels. PDGFRβ marks cells of the mural compartment of the blood vessel, and can be used to enrich for cells with adipogenic potential. In these studies, all adipose progenitors were marked with PDGFRβ, but not all PDGFRβ+ cells had adipogenic potential (Tang et al., 2008). In another study, cells were fluorescently labeled using a Zfp423 driver, a critical transcriptional regulator in adipose lineage commitment. These cells, which have high potential for adipogenic conversion, are also contained within the perivascular compartment (Gupta et al., 2012). Interestingly, this study also suggested that a subpopulation of endothelial cells might also give rise to adipocytes, a notion supported by a separate lineage tracing study using Cre recombinase driven by the VE-cadherin promoter (Tran et al., 2012). It should be pointed out, however, that knocking out PPARγ with a different endothelial Cre line (Tie2-Cre) does not affect adipose development or PPARγ expression within adipocytes (Kanda et al., 2009). Similarly, a recent lineage tracing study failed to detect an adipose progenitor population located within either the endothelial or perivascular compartments; instead, this study identified a common PDGFRα+ precursor for all white adipocytes that was distinct from PDGFRα+ cells found within the vessel wall (Berry and Rodeheffer, 2013).
It has been proposed that some adipocytes derive from hematopoietic precursors. This notion was originally suggested 60 years ago (McCullough, 1944), and has regained currency through the use of sophisticated imaging and bone marrow transfer techniques (Majka et al., 2010). Others, however, have failed to confirm these results (Berry and Rodeheffer, 2013; Koh et al., 2007), and while we cannot rule out the possibility that some adipocytes may derive from hematopoietic origins, it appears that this is not a major pathway for adipocyte development.
Brown fat has a different developmental pattern than white fat. So-called altricial mammals, which have a short gestation period and are born with an immature hypothalamic-pituitary-adrenal (HPA) axis (e.g. mice and rats), stay warm in the extrauterine environment by huddling in the nest, and not by using nonshivering thermogenesis. Thus, although interscapular BAT can be identified during rodent embryogenesis, it does not express significant amounts of UCP-1 until it matures during the postnatal period. In contrast, precocial mammals have a long gestation; such species, which include sheep and humans, are able to rapidly switch on non-shivering thermogenesis at birth. UCP-1 expression peaks at birth, and then slowly diminishes as brown adipocytes are replaced by white fat cells (Symonds, 2013).
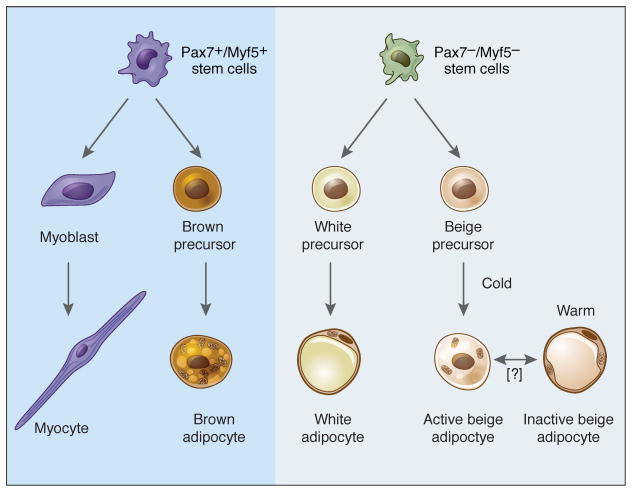
For quite some time it was assumed that brown and white adipocytes share a common precursor, a reasonable conclusion given the numerous similarities between the two cell types. Surprisingly, however, data obtained over the last few years have shown unambiguously that muscle and classical brown fat derive from the same or very similar precursors. This notion took flight with the identification of the transcriptional co-factor PRD1-BF-1-RIZ1 homologous domain-containing protein-16 (PRDM16) as a dominant regulator of the brown fat program (Seale et al., 2007). When PRDM16 is knocked down in primary brown fat cultures, a phenotypic switch to skeletal muscle is seen, while expression of PRDM16 in myoblasts switches them to brown fat (Seale et al., 2008). Additionally, lineage tracing studies using the muscle-selective Myf5-Cre showed that skeletal muscle and classical brown fat share a common precursor (Seale et al., 2008), with the divergence occurring between days 9.5 and 12.5 of mouse gestation (Lepper and Fan, 2010). These findings helped to explain gene expression studies showing that brown preadipocytes express some myogenic genes not seen in white preadipocytes (Timmons et al., 2007). It should be noted that one study has concluded that Myf5+ precursors give rise to some white adipocytes (Sanchez-Gurmaches et al., 2012); generally speaking, however, most evidence suggests that white and brown adipocytes take different developmental paths.
Beige adipocytes do not derive from the same Pax7+Myf5+ precursor cells that give rise to classic interscapular BAT (Seale et al., 2008); indeed, this is the major evidence that these are distinct cell types. Two major theories have been proposed for the origin of these cells. One school of thought holds that these cells derive from trans-differentiation of existing mature white adipocytes. This idea emerges from observations that cold exposure or treatment with a β3-agonist does not induce cellular proliferation in the newly browned fat pad. Furthermore, cells with the morphological appearance of a transition form between white and brown fat can be identified (Himms-Hagen et al., 2000; Vitali et al., 2012). Others, however, have proposed that beige adipocytes derive from unique precursor cells within the white fat pad; such cells can in fact be identified using sorting and/or cloning by limiting dilution (Lee et al., 2012; Schulz et al., 2011; Vegiopoulos et al., 2010; Wu et al., 2012). Two genetic tracing studies have shed light on this issue. In one, the appearance of beige adipocytes upon cold exposure was shown to require new adipogenesis (Wang et al., 2013). The second study used different markers to demonstrate that the beige adipocytes appearing in response to an initial period of cold exposure take on the morphology and gene expression pattern of a typical white adipocyte after reintroduction to warm conditions (Rosenwald et al., 2013). Furthermore, upon placement in the cold for a second time, many of these cells reinduce the thermogenic program. One may thus postulate a unifying model in which a dedicated precursor cell differentiates into a beige adipocyte (without a requirement for proliferation) when conditions require it to do so, followed by conversion back to an energy-storing “white” adipocyte when heat generation is no longer a priority. This subpopulation of white adipocytes then forms a pool of potentially thermogenic cells that can be called upon if environmental conditions change. It should be noted that this is not the first example of such malleability in adipose biology; mammary adipocytes of virgin female mice are converted to secretory epithelial cells during pregnancy and lactation, followed by reconversion to adipocytes during mammary involution (Morroni et al., 2004). Collectively, these studies demonstrate that certain adipose populations show extraordinary plasticity when physiological conditions change.
We are now in a period where new information on the developmental origins of adipose tissue is being accumulated rapidly. At present, it is difficult to reconcile all of the published data into a coherent framework. Some of this is certainly due to experimental variability, with questions arising about the fidelity of different transgenic Cre lines and the specificity of antibodies used for sorting and staining, among other technical issues. We believe, however, that there is likely a strong component of natural variability, with different depots and different mouse strains displaying heterogeneity that underlies the extraordinary plasticity of this cell type.
Additional developments in adipocyte development: epigenomic and transcriptional clues
At the cellular level, adipogenesis can be thought of as occurring in two phases, determination and terminal differentiation. During determination, possible alternate fates of an adipose precursor cell become progressively restricted such that it becomes ‘committed’ to the adipose lineage, and becomes a preadipocyte. Terminal differentiation, on the other hand, describes the process by which the preadipocyte acquires the characteristics of the mature adipocyte. Because most of the cellular models that have been employed to study adipogenesis are already committed to the adipose lineage (e.g. 3T3-L1, 3T3-F442A), we know much more about the process of terminal differentiation than we do about determination. Furthermore, we know very little about mechanisms of adipogenesis in vivo, as the means of studying this are mostly indirect.
Several well-studied signaling pathways help to direct multipotent cells to decide between adipogenic and non-adipogenic fates. Most of these studies have been performed using bone marrow-derived mesenchymal cells, and thus the ‘bone-fat switch’ is the most commonly described fate choice. The Wnt and hedgehog pathways, for example, tend to promote osteogenesis and inhibit adipogenesis in both committed and uncommitted precursor cells (Rosen and MacDougald, 2006). These pathways utilize different signaling intermediates, but both have been reported to converge on the transcription factor COUP-TFII, which inhibits pro-adipogenic transcription factors like PPARγ and C/EBPα (Okamura et al., 2009; Xu et al., 2008). Interestingly, non-canonical signaling via Wnt5b tends to promote adipogenesis, at least in part by blocking β-catenin-mediated signals from classic Wnt signals (Kanazawa et al., 2005). Conversely, IGF/insulin signaling is strongly pro-adipogenic (Garten et al., 2012). For many other pathways, it has been difficult to draw general conclusions because results depend on the specific ligand, cell type, stage of differentiation, or other experimental conditions. The TGFβ/BMP superfamily provides an instructive example. TGFβ and its downstream effector Smad3 have been shown to exert both pro- and anti-adipogenic actions in different in vitro and ex vivo models (Choy et al., 2000; Yadav et al., 2011). Among the BMPs, BMP2 and BMP4 have been shown to increase both osteogenesis and adipogenesis, depending upon other components of the differentiation cocktail, while BMP7 promotes brown adipogenesis specifically (Zamani and Brown, 2011). Still other members of the superfamily, like the activins, have also been reported to have disparate effects on adipogenesis and adiposity (Dani, 2013). Similarly, the fibroblast growth factor (FGF) and Notch signaling pathways have been reported to have complex effects on adipogenesis (Rosen and MacDougald, 2006).
The transcriptional cascade that promotes adipogenesis has also been studied at length, and again, the most detailed information concerns the factors and pathways that promote and repress terminal differentiation. The “master regulator” of fat cell formation is PPARγ, as it is both necessary and sufficient for adipogenesis; PPARγ is so potent an adipogenic factor that it can drive non-adipogenic cells like fibroblasts and myoblasts to become adipocytes (Hu et al., 1995; Tontonoz et al., 1994). Consistent with murine studies, humans with rare loss-of-function mutations in PPARγ have lipodystrophy and severe insulin resistance. The bZIP factors C/EBPα, C/EBPβ, and C/EBPδ are also important inducers of adipogenesis, with C/EBPβ and δ acting early in terminal differentiation. Differentiation is ‘locked in’ by a positive feedback loop between PPARγ and C/EBPα (Rosen et al., 2002; Wu et al., 1999); a second positive feedback loop between PPARγ and C/EBPβ reinforces the decision to differentiate (Park et al., 2012). Many of these factors bind at common genomic ‘hotspots’, with early factors establishing chromatin accessibility at the same locations that will later be bound by downstream factors (Siersbaek et al., 2012). In the years since this core pathway was uncovered, many other transcription factors have been identified that promote or inhibit adipogenesis; most of these exert their actions at least in part by inducing or repressing expression of PPARγ (Cristancho and Lazar, 2011; Rosen and MacDougald, 2006). PPARγ in turn directly binds to and regulates a huge number of genes that control virtually all aspects of adipocyte metabolism. Interestingly, genome-wide localization analysis shows that a surprisingly low number of PPARγ binding sites are conserved between mouse and human; the specific genes and gene sets that are regulated by PPARγ, however, are highly concordant (Mikkelsen et al., 2010; Schmidt et al., 2011; Soccio et al., 2011).
There has been recent progress in identifying transcription factors involved in adipose determination. An expression screen in embryonic fibroblasts with and without adipogenic potential identified Zfp423 as a transcriptional determinant of the adipose lineage (Gupta et al., 2010). Zfp423 induces adipose lineage commitment by amplifying the effects of BMPs via a SMAD-interaction domain. Zfp423 expression in the developing adipocyte is repressed by the highly related factor Zfp521, which promotes osteogenesis and inhibits adipogenesis through interactions with Ebf1, another transcription factor required for early adipose commitment (Festa et al., 2011; Kang et al., 2012). Tcf7l1 also regulates adipogenic lineage commitment, although it acts in a very different manner, by responding to confluency and mediating changes in structural proteins that regulate differentiation (Cristancho et al., 2011).
The core elements of the adipogenic transcriptional cascade appear to be shared by most adipose depots, although details can differ. For example, mice lacking C/EBPα are generally lipodystrophic, but still have mammary fat and brown adipose tissue (Linhart et al., 2001). Similarly, animals lacking Ebf1 are lipodystrophic except in the bone marrow, where the adipocytes are quite hypertrophic (Hesslein et al., 2009). How these specialized depots compensate for the loss of these otherwise critical factors is unknown, but may involve the selective use of related transcription factors like C/EBPβ and Ebf2. Ebf2 seems to be particularly important for brown fat development, as it recruits PPARγ to unique sites that determine brown adipocyte identity (Rajakumari et al., 2013).
Interestingly, much of the specialized function of brown adipocytes is controlled by transcriptional cofactors, which do not bind DNA directly but which determine which targets are bound and activated by transcription factors. The best studied of these is PGC-1α, which is a dominant regulator of mitochondrial biogenesis, oxidative metabolism, and thermogenesis in brown fat (Puigserver and Spiegelman, 2003). PGC-1α exerts its actions on mitochondria and oxidation via interactions with transcription factors like ERRα, Nrf-2, PPARα, and PPARγ (Giguere, 2008; Puigserver and Spiegelman, 2003); the transcription factor partners of PGC-1α that control thermogenesis are still unknown. Interestingly, while ablation of PGC-1α reduces the expression of many thermogenic genes, other brown fat-selective genes remain unaffected. This suggests that other factors might also be important in brown fat identity, which led to the identification of PRDM16, another co-regulator (Seale et al., 2007). PRDM16 binds C/EBPβ (and presumably other transcription factors) and recruits the co-repressor proteins CtBP1 and CtBP2 to prevent gene expression associated with either white fat or muscle (Kajimura et al., 2009; Kajimura et al., 2008). Other co-factors, such as RIP140, SRC-1/2/3, TRIP-Br2, and the pocket proteins pRb and p107, also exert important effects on brown fat development and function (Liew et al., 2013; Seale et al., 2009). TLE3 is a particularly interesting co-factor in that it competes with PRDM16 for PPARγ binding, blocking thermogenesis in favor of genes more indicative of white adipose tissue. Animals that overexpress TLE3 in fat display impaired brown fat function, while adipose-specific knockouts have the opposite phenotype (Villanueva et al., 2013).
Finally, there has been significant attention paid to the role of noncoding RNAs in adipose differentiation. MicroRNA (miRNA) in particular has been studied in this regard; at least 20 miRNA species have now been shown to affect adipogenesis, although some are not specific for fat and appear to be required for mesenchymal cell differentiation generally (Oskowitz et al., 2008). Some miRNAs affecting adipogenesis target transcription factors like PPARγ and C/EBPα directly, while others regulate important signaling pathways like insulin-Akt, TGFβ, and Wnt (Chen et al., 2013b). Other miRNAs have a preferential effect on brown and/or beige adipocyte formation and function, including some that target PRDM16 and C/EBPβ (Trajkovski and Lodish, 2013). Several long noncoding RNAs (lncRNAs) have also been shown to be regulated by PPARγ and C/EBPα and to affect to adipocyte differentiation (Sun et al., 2013b), although the mechanisms must still be worked out.
Adipose tissue expansion in obesity: Go big vs. Go forth and multiply!
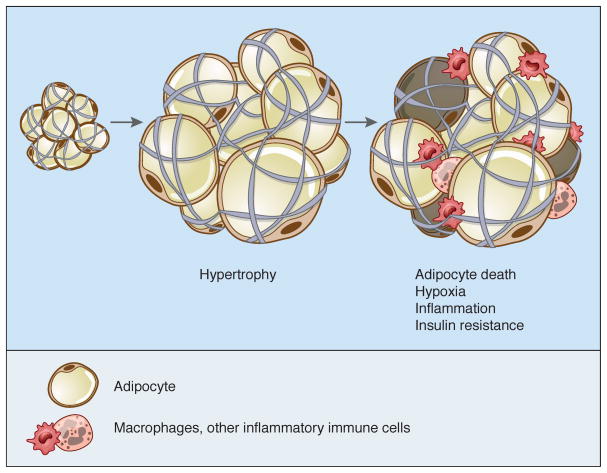
One of the unique attributes of adipose tissue is its incredible capacity to change its dimensions; no other nonneoplastic tissue shares this feature to the same degree. In principle, this can be accomplished by increasing the size of individual cells (hypertrophy) or by recruiting new adipocytes from the resident pool of progenitors (hyperplasia). In the face of overnutrition, adipose depots expand first by hypertrophy until a critical threshold is reached (~0.7–0.8 ug/cell), upon which signals are released that induce the proliferation and/or differentiation of preadipocytes (Krotkiewski et al., 1983). In humans, overfeeding for several months causes increases in cell size but not cell number (Salans et al., 1971); a more recent version of this study suggests that overnutrition induces hypertrophy in upper body subcutaneous fat, but hyperplasia in depots below the waist (Tchoukalova et al., 2010). More recently, stable isotope labeling from mid-century nuclear weapons testing was exploited to suggest that adipocyte number becomes fixed during childhood and early adulthood, with obese people achieving a higher ‘plateau’ (Spalding et al., 2008).
Interestingly, once adipocytes are gained, they are hard to lose, as even significant weight loss is associated with a reduction in adipocyte volume but not overall number (Bjorntorp et al., 1975; Kral et al., 1977). This is not to say that adipocytes never die, as approximately 8% of human subcutaneous adipocytes turn over each year, with birth and death rates matched to result in little change in total cell number (Spalding et al., 2008). Adipocytes may die via necrosis or apoptosis, although the relative contribution of each process is debated (Cinti et al., 2005). Rodent studies suggest that there is a sharp, depot-specific increase in the death rate of adipocytes in obesity, with up to 80% of epididymal adipocytes dying after a few months of high-fat feeding while only 3% of inguinal adipocytes met the same fate (Strissel et al., 2007). This is matched by high proliferation and differentiation rates so that overall fat mass continues to increase as obesity progresses. This adds a layer of complexity to the model discussed above, such that hypertrophy is followed by cell death and finally by the appearance of new adipocytes. This notion is supported by serial analysis of individual Zucker fatty rats, which appear to cycle between hypertrophy and hyperplasia as obesity progresses (MacKellar et al., 2010). Other recent data also support a role for both hyperplasia and hypertrophy upon high fat feeding (Wang et al., 2013). Macrophages play an integral role in this process, with a possible role for both M1 and M2 subtypes (Strissel et al., 2007).
The observation that obesity can be associated with adipocyte hyperplasia, in rodents at least, has contributed to a popular, though false, notion: that adipogenesis per se can cause obesity. This idea has been bolstered by data showing that manipulation of many genes can cause obesity in vivo while also causing increased adipogenesis when tested in vitro. It is important to remember, however, that increased adipogenesis is not the primary driver of obesity in these models. The energy balance equation tells us that overnutrition (or reduced energy expenditure) is the culprit, and that the increase in adipogenesis is driven by the need to store excess calories. Sensibly, the same molecular effectors that provoke increased food intake or reduced energy expenditure also promote the formation of new cells adapted to handling the increased calories safely.
Adipose tissue remodeling during obesity: Are we too fat, or not fat enough?
The ability of the adipose depot to change its size dramatically in response to nutritional demands requires a unique capacity to remodel, the mechanisms of which are now being elucidated. Significant attention has focused on the role of hypoxia, with numerous parallels being drawn to tumor biology, another example of a tissue that expands rapidly. As with cancer, adipose tissue has the potential to outgrow its blood supply. The ability of adipose tissue to promote its own vascularization, and the possibility of exploiting this as a metabolic therapy, is discussed in more detail below. Despite efforts to recruit new blood vessels during adipose tissue expansion, however, hypoxia may develop, although some studies have shown normal or even elevated oxygen tension in fat pads of obese subjects (Trayhurn, 2013). These discrepancies may be based on technical variables and the difficulty of measuring oxygen tension in living tissues. Nonetheless, the oxygen-sensitive transcription factor HIF-1α does become activated in obese adipocytes (Krishnan et al., 2012). Overexpression of HIF-1α in adipose tissue in vivo causes metabolic dysfunction, while adipose-selective ablation of HIF-1α has the opposite effect (Sun et al., 2013a). Several mechanisms have been postulated to account for the actions of HIF-1α, including suppression of βoxidation via transcriptional repression of Sirt1, which deacetylates (and thus activates) PGC-1α (Krishnan et al., 2012), reduction of adiponectin (Jiang et al., 2013), and promotion of fibrosis and inflammation (Halberg et al., 2009).
Fibrosis is an additional key element in determining the health of the fat pad. Adipocytes can be likened to ‘grapes in a mesh bag’, with elements of the extracellular matrix serving as the mesh. Fat cells express a wide variety of matrix proteins as well as the enzymes required to break them down, and the expression of these genes is highly regulated by changes in nutrient availability (Maquoi et al., 2002). Current thinking holds that relaxation of the matrix allows healthy expansion of the fat pad; if the matrix is too rigid, then adipocytes become limited in their ability to store excess nutrients, and this leads to pathological features that include activation of stress-related pathways, inflammation, and ectopic lipid deposition in other tissues (Sun et al., 2013a). Collagen VI, for example, is the predominant form of collagen produced by adipocytes. When the Col6a1 gene is disrupted in leptin deficient ob mice, they develop much larger adipocytes than wild-type littermates (but smaller fat pads overall, for unclear reasons), coupled with reduced inflammation and improved glycemic and lipid parameters (Khan et al., 2009). More recently, fibroblast growth factor 1 (FGF1) was shown to be a critical mediator of adipose remodeling, such that Fgf1−/− mice display dramatically altered adipose morphology upon chronic overfeeding or fasting, accompanied by insulin resistance and dysglycemia (Jonker et al., 2012).
The Col6a1 deficient model, and others with similar features, have been likened to a subgroup of human subjects called the ‘metabolically healthy obese’ (MHO). These individuals tend to have reduced visceral adiposity, increased adiponectin levels, reduced fibrosis and inflammation, and improved glucose and lipid homeostasis relative to other equally obese subjects (Denis and Obin, 2013). Importantly, however, the human MHO population tends to have smaller adipocytes than other obese people (Kloting et al., 2010), suggesting that increased expansibility may not account for the improved metabolic profile of these patients. An alternative hypothesis is that increased adipogenesis, resulting in numerous, smaller adipocytes with excellent glucose uptake and a healthy adipokine profile may account for the improved metabolic health of some obese patients. This is consistent with other lines of evidence demonstrating that thiazolidinedione treatment improves metabolic parameters despite increasing adipocyte cell number and total adiposity (Tang et al., 2011; Yamauchi et al., 2001), and findings that metabolically unhealthy obese patients have a diminished preadipocyte pool (Gustafson et al., 2013). Whether increased expansibility or increased adipogenesis accounts for the phenotype of the MHO individual, it certainly raises the paradox that the health of the obese population might be improved if we made them even more obese. We do not, however, expect this will become a high priority for the pharmaceutical industry.
Adipocyte-immune cell interactions come over to my pad!
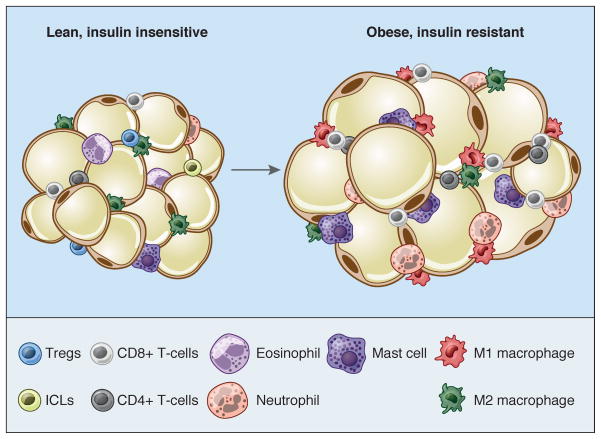
In addition to a matrix of extracellular proteins, adipocytes are surrounded by a wide variety of cells that includes endothelium, immune cells, fibroblasts, preadipocytes, and stem cells. Overall, mature lipid-laden adipocytes are believed to make up only 20–40% or so of the cellular content of a fat pad (although they account for >90% of fat pad volume); every gram of adipose tissue contains 1–2 million adipocytes but 4–6 million stromal-vascular cells, of which more than half are leukocytes (Kanneganti and Dixit, 2012). Immune cells have been known to populate the fat pad for decades (Hellman et al., 1963), but it was not clear until recently that these cells play a central role in adipose biology. This realization began with the observation that adipose tissue is an important source of TNF-α and other cytokines, an effect magnified by overnutrition (Hotamisligil et al., 1993). These proinflammatory cytokines significantly impair the insulin sensitivity of local adipocytes and also liver and muscle. Later work showed that many of these cytokines are produced by macrophages within the fat pad rather than the adipocytes themselves (Weisberg et al., 2003; Xu et al., 2003). These macrophages can be observed histologically as “crown-like structures” surrounding adipocytes, particularly in obese visceral fat; their uneven distribution has been attributed to clustering around dead or dying adipocytes (Cinti et al., 2005). Phenotypically, macrophages exist along a spectrum, the poles of which have been designated M1 (or ‘classically-activated’) and M2 (or ‘alternatively-activated’). M1 macrophages have a pro-inflammatory phenotype; they express the surface marker CD11c and cytokines like TNF-α, IL-6, and IL-1β in response to LPS and IFN-γ. M2 macrophages, on the other hand, express the surface markers CD206 and CD301; they play a role in tissue remodeling and wound healing, and respond to IL-4 and IL-13 by secreting anti-inflammatory cytokines like IL-10 and IL-1 receptor antagonist. In lean animals, M2 macrophages dominate the adipose tissue resident population. As obesity progresses, however, more M1 macrophages infiltrate the fat pad, causing insulin resistance (Oh et al., 2012). It is worth noting that adipose M2 macrophage numbers do not diminish in obesity, and in fact may increase, but there is a major shift in the M1/M2 ratio favoring a pro-inflammatory state (Lumeng et al., 2007a; Lumeng et al., 2007b).
Other innate and adaptive immune cells also play a significant role in setting the inflammatory tome of the obese fat pad; in fact, virtually all known classes of immune cell have been implicated in this process. Neutrophils, mast cells, B lymphocytes, and various classes of T lymphocyte (e.g. CD8+ and CD4+ Th1 cells) all increase in abundance in the obese fat pad, and all exert negative effects on insulin sensitivity (Mathis, 2013). Conversely, eosinophils and innate lymphoid (ILC2) cells act to reduce inflammation, and thus restore insulin sensitivity (Molofsky et al., 2013; Wu et al., 2011). Regulatory T cells (Tregs) are CD4+Foxp3+ immune cells that play a key role in controlling other immune cells, including macrophages. Tregs are enriched in normal rodent visceral fat, but are strongly decreased upon the development of obesity (Feuerer et al., 2009). Furthermore, experimental depletion of adipose Tregs promotes insulin resistance, with enhancement of Treg numbers showing the opposite effect; these attributes are not shared by lymphoid Tregs (Eller et al., 2011; Ilan et al., 2010). The special properties of Tregs from visceral adipose tissue are due to the fact that they express PPARγ (Cipolletta et al., 2012). Interestingly, M2 macrophages also express PPARγ (Odegaard et al., 2007), aligning nicely with long-time reports that PPARγ can promote insulin sensitivity through multiple tissues (Tontonoz and Spiegelman, 2008). NKT cells have also been implicated in insulin resistance and intra-adipose inflammation, but the data from different groups are highly contradictory (Mathis, 2013). In fact, caution must be used in interpreting much of the literature on adipose-immune cell interactions, as many experimental manipulations affect more than one cell type. Furthermore, some approaches affect body weight, which could imply primary actions in other tissues (e.g. the gut or brain) that secondarily affect adipose function.
The precise temporal sequence of inflammatory cell infiltration into adipose tissue is still unclear. Similarly, we do not fully understand the full range and interconnectedness of the initiating events that link overnutrition to inflammation. Adipocyte ‘stress’ due to overnutrition has been linked to oxidative stress, endoplasmic reticulum stress, and toll-like receptor activation due to fatty acids and/or lipopolysaccharide (LPS), which may be elevated in the serum of obese subjects (Cani et al., 2007; Hotamisligil, 2010; Houstis et al., 2006; Shi et al., 2006). Ultimately, these insults cause up-regulation of various chemokines in adipocytes, which recruit immune cells to the fat pad. These chemokines include MCP-1, Ccl5, and others (Ota, 2013). Two recent studies suggest that adipocytes may act as the antigen-presenting cells (APCs) that activate resident T cells shortly after the initiation of high fat diet (Deng et al., 2013; Huh et al., 2013), although others suggest that adipose tissue macrophages are the relevant APCs (Morris et al., 2013). Notably, the specific antigens that signal the overnourished state and trigger T cell activation are still undefined.
Classic inflammation is characterized by rubor (redness), tumor (swelling), dolor (pain), and calor (heat). Clearly, when we overeat or become overweight, our adipose tissue does not become hot and painful, like an inflamed wound or an arthritic joint. It is not entirely clear how inflammation is regulated during overnutrition, so that a chronic, low-grade state of immune cell activation and cytokine elaboration is maintained without causing the full-blown spectrum seen in other inflamed conditions. Some of this may involve the numbers and types of immune cells that inhabit the obese fat pad. For example, M2 macrophages likely keep their M1 counterparts in check in the obese fat pad; loss of the transcription factor IRF4, required for M2 polarization and function, causes worsened inflammation and insulin resistance in the setting of high-fat feeding (Eguchi et al., 2013). Treg cells are another cell type likely to play a ‘braking’ function on inflammation in obesity. Endogenous pathways within the adipocyte and/or adipose-resident immune cells may also play a role, as has been suggested for the transmembrane protein STAMP2, which is induced by feeding and obesity in adipocytes and which suppresses cytokine synthesis and metabolic dysfunction (Wellen et al., 2007).
Finally, one should not assume that the sole role of immune cells in adipose tissue is to cause trouble in the context of obesity. During fasting and weight loss, macrophages become recruited to the fat pad by the products of lipolysis, where they are responsible for taking up the newly available lipids (Granneman et al., 2005; Kosteli et al., 2010). This buffers the animal, and specifically the local adipose microenvironment, from the effects of high levels of free fatty acids. Another intriguing example of adipose-macrophage crosstalk is the newly discovered role of M2 macrophages to promote browning of white adipose tissue. Cold exposure was found to polarize macrophages toward the alternatively activated form in an IL-4-dependent manner, leading to the formation and secretion of catecholamines (Nguyen et al., 2011).
Adipose-cross-talk with other cell types
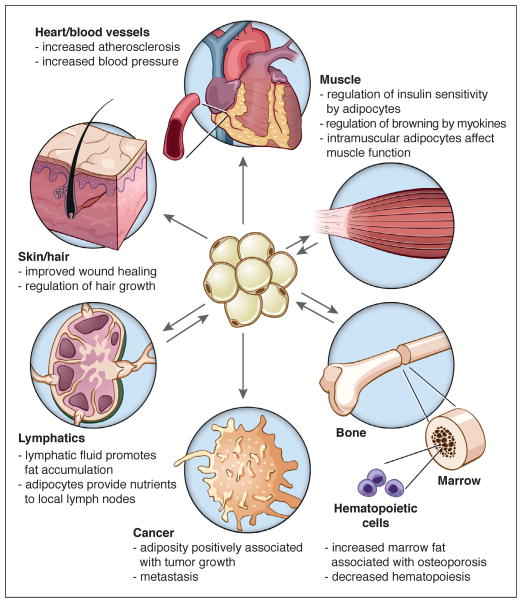
In addition to immune-adipose cross-talk, there is a burgeoning awareness that adipocytes exert a profound influence on neighboring cells and tissues; this appears to be particularly true for some of the smaller and less well-known depots. For example, adipogenesis within the dermis waxes and wanes in concert with the hair cycle. Furthermore, adipocyte progenitor cells (defined as Lin−/CD34+/CD29+/Sca1+) promote hair growth in mice through the elaboration of platelet-derived growth factor α (PDGFα), which induces stem cell activation in the hair follicle (Festa et al., 2011). Subsequent studies have delineated a role for dermal adipocytes in wound healing, although the mechanism is still unclear (Schmidt and Horsley, 2013).
Another oft-overlooked depot is the epicardial fat pad, which may have an outsized effect on cardiomyocyte function and the risk of coronary atherosclerosis, despite representing a relatively small fraction of overall visceral fat. Several studies have identified an anatomic relationship between the specific segments of the coronary vasculature that are prone to plaque formation and the presence of epicardial fat (Cherian et al., 2012).
Adipocytes and skeletal muscle are intertwined in several interesting ways. Both derive from mesenchymal cells, and, as described earlier, there is a shared lineage between skeletal muscle and brown fat through a Myf5+ precursor. Inducible brown precursor cells can be isolated from muscle, and muscle and brown fat share overlapping gene expression patterns (Schulz et al., 2011; Timmons et al., 2007). Interestingly, muscle contains numerous so-called ‘fibro-adipogenic precursor’ (FAP) cells that can differentiate into white adipocytes under certain conditions, such as in muscular dystrophy, obesity, and age-related sarcopenia (Natarajan et al., 2010). These cells can be distinguished from myogenic satellite cells by the expression of PDGFRα, and they arise from a different developmental lineage than the surrounding muscle (Joe et al., 2010; Uezumi et al., 2010). Although intramuscular adipocyte accumulation can disrupt muscle function, undifferentiated FAPs play an important role in normal physiology. When muscle is damaged, FAPs respond to local cytokine production by proliferating, clearing necrotic debris, and supporting myogenesis (Heredia et al., 2013; Joe et al., 2010). Additionally, of course, muscle is a direct and indirect target of several circulating adipokines that regulate metabolism, and conversely, myokines like irisin can affect adipose function.
There is also an interesting and well-established relationship between adipocytes and the lymphatic system (Rosen, 2002). Lymph nodes are invariably encased by fat; interestingly, these depots do not change in size with fasting and feeding, but instead respond to immune stimulation, thus acting as privileged storehouses for the immune system (Pond and Mattacks, 2002). Other complex relationships between adipose tissue and lymph nodes have been described. For example, adipose progenitor cells may contribute to the stroma of the node itself, as cells destined for the adipose lineage can be reprogrammed into lymphoid organizer cells by lymphotoxin-β (Benezech et al., 2012). Additionally, lymphatic fluid itself has a strong pro-adipogenic effect, which can be demonstrated in dramatic fashion by the massive proliferation of adipose tissue seen in chronic lymphedema (Rockson, 2010).
Finally, bone marrow is increasingly being recognized as a unique depot with important local functions. In children, bone marrow is filled largely with osteogenic and hematopoietic precursors, but as we age, the percentage of adipocytes in marrow rises significantly. Marrow adipocytes tend to be smaller than those in other depots and to have somewhat different lipid constituents (Griffith et al., 2009). Interestingly, while marrow adipocytes are fully capable of lipolysis, they do not respond to caloric restriction (Bathija et al., 1979; Devlin et al., 2010), similar to peri-lymphatic adipose depots. In fact, patients with anorexia nervosa often have increased marrow fat in the setting of severe adipose wasting in other depots, an observation corroborated in some, but not all, rodent models (Fazeli et al., 2013). The marrow adipogenic progenitor cell is usually considered to be a type of multipotent mesenchymal stem cell (Pittenger et al., 1999), and there is evidence that some cell surface markers that characterize adipose progenitor cells in other depots (e.g. CD24) are not expressed in marrow stroma (Fazeli et al., 2013). The multipotent nature of the marrow stromal cell has been put forward as a key factor in the pathogenesis of osteoporosis; reduced bone mass is believed to result, in part, from common precursor cells that make the decision to become fat rather than bone. In fact, the relationship between marrow fat and bone density is more complex than encompassed in this simple paradigm. For example, there are situations (such as in human puberty) where one sees increased marrow fat and bone at the same time. Furthermore, PPARγ agonists promote adipogenesis and inhibit osteogenesis in mesenchymal stem cells, and have been associated with increased marrow fat and diminished bone density in some, but not all studies (Fazeli et al., 2013). Does bone marrow fat affect hematopoiesis? One might speculate that the reason we store nutrients in marrow would be to provision this energy-intensive differentiative process. Despite the attractiveness of this idea, marrow fat seems to have a negative impact on hematopoiesis. There is an inverse relationship between the number of fat cells and the number of hematopoietic precursors, and elimination of marrow fat by genetic or pharmacologic means enhances the rate of engraftment following radioablation (Naveiras et al., 2009).
Fat and cancer—dancing with the devil
The ability of adipose tissue to change the behavior of nearby cells is not restricted to normal cell types, as both mature adipocytes and adipose progenitor cells affect the growth and metastasis of cancer cells. There are strong epidemiological associations between fat mass and the incidence (and mortality) of a variety of malignancies, including breast, colon, renal, esophageal, and pancreatic cancer, as well as some lymphomas and leukemias, and obesity is now considered a major modifiable risk factor for cancer (Park et al., 2011). More directly, surgical removal of parametrial fat pads inhibits carcinogenesis in a UVB-irradiation mouse model (Lu et al., 2012).
Several mechanisms have been proposed for this association, including the antiapoptotic effects of obesity-associated hyperinsulinemia, enhanced aromatization of sex steroids (particularly relevant for breast and endometrial cancer) in adipose tissue, and the elaboration of paracrine and endocrine factors that promote either tumorigenesis or angiogenesis directly from adipocytes and stromal cells within fat pads (Khandekar et al., 2011; Park et al., 2011). One such factor is endotrophin, a cleavage product of collagen type VI, which promotes tumorigenesis through matrix/stromal interactions (Park and Scherer, 2012). The enhanced inflammatory milieu of the obese fat pad has also been associated with tumor growth, likely through the secretion of cytokines. Inflammatory factors also promote homing of metastases to adipose depots, which then serve to provision the cancer cells with the massive amounts of lipid required to support rapid cell division (Nieman et al., 2011). Based on these data, there has been significant interest in targeting the adipocyte for cancer prevention as well as treatment.
How do adipocytes communicate with other cell types?
Cross-talk between fat cells and their environment is typically mediated in three ways: nutritional mechanisms, neural pathways, and via the elaboration of autocrine, paracrine, and endocrine agents, collectively termed adipokines. Nutritional mechanisms are the simplest to understand: adipocytes evolved, in large part, to safely store excess calories during periods of nutritional affluence, and to release them during periods of nutritional deprivation. These calories come in the form of free fatty acids, which are liberated by lipolysis during fasting and released into the circulation, where they are utilized by skeletal muscle and other tissues. By enabling these tissues to switch to a lipid-oxidizing economy during fasting, glucose is spared for the central nervous system and red blood cells.
Adipose tissue is richly innervated by both sympathetic and parasympathetic fibers, with the former driving lipolysis during fasting and cold exposure, and latter promoting lipid accumulation after feeding (Bartness et al., 2010a; Kreier et al., 2002). Central neuronal signals also regulate adipose tissue growth and cellularity in a depot-specific manner (Bowers et al., 2004; Foster and Bartness, 2006). Neurally-mediated communication is not all one way, however; adipocytes can communicate information about nutritional status to the brain via afferent nerves. The introduction of UCP-1 into white fat, for example, improves leptin sensitivity in mice, an effect that is lost following denervation (Yamada et al., 2006). Indeed, some actions of the adipokines discussed below may actually be dependent on stimulation of local nerve endings within the adipose depot, and not solely on systemic distribution via the circulation (Murphy et al., 2013).
By and large, however, most of the excitement in this area has come from advances in our understanding of adipokine biology. Early examples of adipose-derived secreted products include immunological proteins like the complement factor adipsin and TNF-α, but the discovery of leptin was an inflection point for the field, serving notice that adipocytes are active endocrine cells, identifying a specific biological cause of obesity (at least in mice), and giving hope for a rational drug therapy. While small amounts may be produced by other tissues in specific contexts (Considine, 2001; Maymo et al., 2011), the lion’s share of leptin comes from adipocytes, and serum levels are tightly associated with fat mass. Many factors regulate leptin expression and secretion, including nutrients, steroid and thyroid hormones, and cytokines (Moon et al., 2013). The transcriptional control of Lep expression is also complex, with C/EBPα and PPARγ playing opposing roles (Hollenberg et al., 1997; Kallen and Lazar, 1996); the transcription factor Fosl2 is also required for the differentiation-dependent expression of Lep in adipocytes (Wrann et al., 2012).
Leptin exerts its effects via specific receptors in the central nervous system and in the periphery. An example of the latter is in the immune system, where leptin promotes inflammation by enhancing cytokine production, macrophage function, and the CD4+ T helper response (Carbone et al., 2012). The effects of leptin to reduce body weight by decreasing food intake and increasing energy expenditure are clearly centrally-mediated, operating through several hypothalamic nuclei (e.g. the arcuate, lateral, dorsomedial, and ventromedial nuclei). In addition, there are leptin receptors in the nucleus of the solitary tract of the hindbrain and in the ventrotegmental area, which may affect dopaminergic reward pathways that affect the hedonic experience of eating and palatability (Leinninger et al., 2009; Myers et al., 2009). It has also been noted that leptin improves glycemic control in lipodystrophic animals, an effect that is also centrally mediated (Asilmaz et al., 2004). The effect of leptin on bone is complex, but it appears to exert anti-osteogenic effects via a hypothalamic relay system (primarily affecting the axial skeleton), while it may have direct pro-osteogenic effects at appendicular sites (Karsenty, 2006).
The other dominant adipokine is adiponectin, both in terms of its serum concentration (2–10 ug/ml) and the number of papers it has engendered since its discovery in the mid-1990’s (>11,000). Adiponectin expression is highly adipose-specific, and is constitutively secreted. It circulates in plasma as trimers, hexamers, and higher order structures; these larger complexes can be difficult to quantify but likely represent the most biologically active forms of the molecule (Turer and Scherer, 2012). Two classes of adiponectin receptor have been identified. The dominant signaling forms are encoded by ADIPOR1 and ADIPOR2, which are 7 transmembrane receptors with the opposite polarity of G-protein-coupled receptors (i.e. the N-terminus is cytoplasmic) (Yamauchi and Kadowaki, 2013). There is also a nonsignaling receptor called T-cadherin, which has nonetheless been shown to be required for some actions of adiponectin (Denzel et al., 2010). One of the more interesting attributes of adiponectin is that its expression and secretion are diminished in visceral obesity despite the increased fat mass (Turer et al., 2011). Although the mechanism underlying this effect is unclear, it has enabled adiponectin to serve as an excellent biomarker for insulin resistance and metabolic dysfunction.
Adiponectin receptors are widespread throughout the body, so it is no surprise that adiponectin affects many tissues and physiological processes. Many of these effects promote metabolic health, including inducing fatty acid oxidation in liver, suppressing hepatic glucose production, improving β-cell function, and enhancing peripheral insulin sensitivity. Cardiac health is enhanced by adiponectin both directly (e.g. through the direct stimulation of cardiomyocyte survival after ischemia/reperfusion injury) and indirectly (e.g. by improving serum dyslipidemia and reducing inflammation) (Goldstein et al., 2009). Interestingly, one might assume that adiponectin might promote weight loss, but this appears not to be the case. In fact, transgenic overexpression of adiponectin causes significant fat accumulation, and when crossed to the ob/ob strain one obtains the most corpulent rodent model seen to date (Kim et al., 2007). This may be due partly to increased insulin sensitivity and partly to direct actions of adiponectin on the hypothalamus to increase food intake (Kubota et al., 2007).
The strength and breadth of adiponectin effects on metabolism makes it an ideal integrator of many different metabolic signals. For example, it has long been known that elevated tissue iron stores reduce peripheral insulin sensitivity. When adipocyte iron levels are high, adiponectin is suppressed, and insulin resistance ensues. Conversely, reductions of tissue or serum iron cause increased adiponectin and improve glucose tolerance (Gabrielsen et al., 2012). A similar situation exists for fibroblast growth factor 21 (FGF21), a secreted protein made by the liver and other tissues (including fat) with a wide range of beneficial effects on metabolic function, including weight loss and improvements in glucose and lipid homeostasis (Iglesias et al., 2012; Potthoff et al., 2012). FGF21 directly increases the production and release of adiponectin by adipose tissue. In the absence of adiponectin, FGF21 can still reduce body weight, but is no longer capable of improving glucose homeostasis, insulin resistance, hypertriglyceridemia, or hepatic steatosis (Holland et al., 2013; Lin et al., 2013). Finally, thiazolidinedione (TZD) agonists of PPARγ used clinically to treat Type 2 diabetes work, at least in part, by promoting the synthesis and secretion of adiponectin (Nawrocki et al., 2006).
Resistin is another small protein identified as an adipokine linking obesity to insulin resistance in rodents. Resistin expression is highly specific for white adipose tissue in mice, and it circulates in higher concentrations in obese animals (Steppan and Lazar, 2004). Elevated resistin causes insulin resistance in vitro and in vivo while reductions have the opposite effect. This relationship has been harder to demonstrate in humans, however, where resistin appears to be secreted primarily by circulating monocytes (Savage et al., 2001).
Another group of adipokines that has garnered attention lately are the lipocalins RBP4 and Lcn2, which are expressed in adipose tissue, circulate at higher levels in obesity, and have been extensively characterized with respect to their effects on glucose homeostasis and insulin action. RBP4 is the major vitamin A transporting protein in serum, where it circulates bound to transthyretin, which extends its serum half-life (Campos-Sandoval et al., 2011). RBP4 is preferentially expressed in visceral adipose tissue, and serum levels are strongly associated with insulin resistance in rodents and humans (Graham and Kahn, 2007). RBP4 has been reported to induce insulin resistance by binding to the receptor Stra6 (Berry et al., 2013). Others have suggested that RBP4 induces insulin resistance by activating inflammatory pathways in macrophages in a retinoid-independent fashion via a receptor that is not Stra6 (Norseen et al., 2012). Lcn2 is an iron trafficking protein produced by a select number of tissues, including white adipose tissue, in response to inflammation. Lcn2 causes insulin resistance in cultured adipocytes and hepatocytes (Yan et al., 2007), but the in vivo data are less clear. Lcn2−/− mice have been reported to be lean and insulin sensitive (Law et al., 2010), obese and insulin resistant (Guo et al., 2010), or to have unaltered adiposity and mild insulin sensitivity (Jun et al., 2011); the source of the discordance is unclear.
Many other adipokines have been identified, with several novel molecules appearing seemingly every year. Chemerin, omentin, vaspin, and others are all produced by adipose tissue and exert metabolic effects (Bremer and Jialal, 2013). Interestingly, the adipocyte fatty acid binding protein aP2 (encoded by the Fabp4 gene), one of the most highly expressed adipocyte genes, has recently been shown to be secreted through an exosomal mechanism. Serum aP2 is elevated in obesity and promotes hepatic insulin resistance and gluconeogenesis (Cao et al., 2013). While most characterized adipokines are peptides, adipose-derived fatty acid derivatives with signaling properties have also been described. The earliest of these was monobutyrin, which was described as a pro-angiogenic factor (Dobson et al., 1990). More recently, palmitoleate was identified as an important ‘lipokine’ secreted by adipocytes following de novo lipogenesis, and which acts on muscle and liver to protect against the adverse consequences of dietary lipid ingestion (Cao et al., 2008). Given the wealth of lipid substrates and modifying enzymes found in adipocytes, it seems likely that additional ‘lipokines’ will be discovered in the near future.
Does BAT have its own set of adipokines? In general, BAT makes the same factors as WAT, although some, like leptin and adiponectin, are produced at lower levels (Villarroya et al., 2013). Conversely, the active form of thyroid hormone, triiodothyronine (T3), is produced in sufficient quantities in BAT to affect systemic levels due to very high expression of type II 5′-deiodinase (encoded by Dio2) (Silva and Larsen, 1985). Speculation that BAT may produce its own unique repertoire of adipokines has rested on two observations. First, ablation of BAT has a much larger effect on systemic metabolism than does deletion of UCP-1, a result not fully consistent with the notion that all benefits of BAT derive from local uncoupling (Enerback et al., 1997; Hamann et al., 1996; Lowell et al., 1993). Second, direct transplantation studies have shown that as little as 100 mg of BAT can improve body weight and glucose homeostasis in obese recipient mice. This latter effect was lost when BAT from Il6−/− animals was used, suggesting that this factor may represent a true ‘BATokine’ (Stanford et al., 2013).
Lipid trafficking in adipocytes: can an old dog teach us new tricks?
Adipocytes are, first and foremost, professional lipid storing cells. Although this aspect of fat has been studied for decades, several recent advances have brought additional insight. While most of the lipid stored in adipocytes comes from the diet, the fat cell is fully capable of synthesizing new lipids from carbohydrates using de novo lipogenesis (DNL). The two major enzymes of DNL, fatty acid synthase and acetyl CoA carboxylase, are abundantly expressed in fat under the control of sterol response element binding protein 1c (SREBP1c) and carbohydrate response element binding protein (ChREBP). While SREBP1c is the dominant regulator of DNL in liver, that role belongs to ChREBP in WAT (Herman et al., 2012; Shimano et al., 1997). Interestingly, DNL is associated with poor metabolic outcomes in liver, but the opposite is true in fat. Several genetic manipulations that increase adipose DNL cause improvements in insulin sensitivity and glycemic control, while loss of ChREBP has the opposite effect; this may involve changes in adipokine secretion, increased adipose browning, or some other mechanism (Herman et al., 2012; Iizuka et al., 2004). Somewhat paradoxically, caloric restriction actually increases adipose DNL, although it is not clear if this mediates the beneficial effects of this intervention (Bruss et al., 2010).
Lipolysis is the process required for fatty acids to be liberated from triglyceride, so that they can be oxidized locally or by other organs. Classically, we think of lipolysis as being driven by β-adrenergic signaling in the adipocyte, but other inducers (such as TNF-α) exist and may have physiological relevance (Ryden and Arner, 2007). The lipolytic machinery consists of at least three major enzymes and associated co-factors. The primary cleavage of triacylglycerol to diacylglycerols is performed by adipose triglyceride lipase (ATGL), a recently discovered enzyme whose existence was inferred when genetic ablation of the well-studied second enzyme in the pathway, hormone-sensitive lipase (HSL), was shown to be dispensable for lipolysis in vivo. HSL is the major diglyceride lipase in adipocytes, while monoglyceride lipase (MGL) completes the process by generating glycerol and free fatty acids. Together, these three enzymes account for over 90% of the lipolytic activity in the adipocyte (Young and Zechner, 2013). ATGL in particular is highly regulated at both the transcriptional and post-transcriptional levels, including multiple phosphorylation events and translocation to the surface of the lipid droplet. It is activated by a protein co-factor called CGI-58, which is normally bound in an inactive state by the lipid droplet protein perilipin-1 (Plin1). PKA-dependent phosphorylation of Plin1 releases CGI-58, allowing it to bind and activate ATGL (Granneman et al., 2009). Conversely, ATGL is inhibited by a protein called G0S2, although its importance in vivo is still unclear (Yang et al., 2010).
Insulin is the major physiological suppressor of lipolysis, a process that becomes impaired in obesity even though insulin levels are high. Insulin acts in several different ways to block lipolysis. First, it activates phosphodiesterase 3b (PDE3b) via Akt-mediated phosphorylation; this has the effect of reducing intracellular cAMP levels and blocking PKA activation (Degerman et al., 1998; Kitamura et al., 1999). More recently, a noncanonical pathway has been described in which insulin blocks activation of PKA selectively on Plin1 through a PI3K-mediated, Akt-independent pathway (Choi et al., 2010b). Over a slightly longer time scale, insulin also represses lipolysis by transcriptionally silencing lipase genes via repression of the transcription factors FoxO1 and IRF4 (Chakrabarti and Kandror, 2009; Eguchi et al., 2011).
Interestingly, lipolysis is required for the generation of endogenous PPARα ligands, as fatty acids imported from the blood or synthesized endogenously can not activate this nuclear receptor until they have undergone a cycle of esterification and hydrolysis (Haemmerle et al., 2011). Thus, animals lacking ATGL in adipocytes show deficient fatty acid oxidation and thermogenesis in BAT, with the acquisition of a WAT-like phenotype (Ahmadian et al., 2011). The key point here is that, by generating specific PPARα ligands, lipolysis is coupled to the downstream oxidation of freshly released fatty acids.
The lipid droplet itself is now recognized as a highly dynamic organelle with extraordinary conservation of its protein composition; over 200 droplet-associated proteins have been identified in adipocytes, most of which are also found associated with droplets in other mammalian tissues as well as in lower organisms (Konige et al., 2013). In addition to the perilipins previously described, other important proteins under active investigation include the CIDE family and various scaffolding proteins such as cavins and caveolins, virtually all of which have been linked to lipid handling, insulin sensitivity, and global energy homeostasis.
Adipose tissue as a therapeutic target
Given its central role in metabolic health and disease, it is no surprise that adipose tissue has become an important therapeutic target. Below, we discuss several strategies by which adipose biology might be exploited for clinical benefit.
TZDs/TZD-like molecules for selective PPARγ activation
TZDs have a variety of beneficial effects in adipose tissue, including insulin sensitization, induction of browning, and anti-inflammation. Unfortunately, the clinical utility of TZDs has been limited by their unfavorable side effect profile, including fluid retention, osteoporosis, and (possibly) increased risk of cardiovascular events (Ahmadian et al., 2013). Interest in PPARγ as a therapeutic target was recently revived, however, by the realization that partial agonists that are not adipogenic can still act as potent insulin sensitizers. This paradox was resolved by the demonstration that PPARγ can be phosphorylated by Cdk5 at Ser273 in rodent and human obesity (Choi et al., 2010a). Phosphorylation at this site changes the pattern of gene expression driven by PPARγ, reducing the expression of adiponectin and several other genes without affecting most direct targets. This suggested that PPARγ might be selectively activated by agents that block Cdk5 phosphorylation without driving ‘classical’ agonism, a prediction that was borne out when such compounds were used to treat animal models of insulin resistance without causing fluid retention (Choi et al., 2010a; Choi et al., 2011). Furthermore, mice lacking the nuclear co-repressor NCoR in adipose tissue have an improved metabolic profile despite obesity, a phenotype consistent with increased PPARγ activity. These animals show reduced phosphorylation at Ser273, suggesting that a major effect of NCoR may be to facilitate the phosphorylation of PPARγ by Cdk5 (Li et al., 2011). To date, selective agonists have not been found that promote browning over other PPARγ-dependent actions. However, the recent finding that deacetylation of PPARγ by SirT1 causes browning suggests that selective modulation of PPARγ to promote thermogenesis may be achievable (Qiang et al., 2012). Other post-translational modifications, such as SUMOylation, are also able to alter PPARγ target selection and function, opening the door for additional therapeutic approaches (Shimizu et al., 2006).
Lipectomy
A somewhat simple-minded approach to weight loss is the surgical removal of excess adipose tissue; perhaps unsurprisingly, liposuction is the most common cosmetic surgical procedure in the world (www.isaps.org). Does cutting out adipose tissue ameliorate the effects of overnutrition? In rodents, the removal of visceral fat does improve metabolic parameters, at least in the short term, while removal of subcutaneous fat has little effect (Gabriely et al., 2002; Shi et al., 2007). This latter result is seen in humans as well, where removal of as much as 20% of total body fat (>40% of subcutaneous fat!) by liposuction does not improve insulin sensitivity or other risk factors for cardiovascular disease (Klein et al., 2004). There is some debate whether the lack of efficacy of liposuction reflects depot-specific differences between visceral and subcutaneous fat, or because the fundamental problem of energy imbalance has not been corrected. Certainly, inducing negative energy balance can improve metabolic dysfunction long before significant weight loss occurs (Henry et al., 1985). Over the long-term, however, it is now clear that eliminating fat stores surgically without correcting energy balance simply causes regrowth of fat mass at either the site of excision or (more commonly) other depots. In rodents, this takes place within weeks to months (Mauer et al., 2001), while in humans the process can last a year (Hernandez et al., 2011).
Brown fat induction
A more elegant approach to reducing body fat might involve the preferential induction of brown or beige adipocytes, a strategy that did not escape the attention of early investigators. Based on the metabolic rate of BAT in mice (~300W/kg, approximately two orders of magnitude higher than any other tissue (Cannon and Nedergaard, 2004), it was calculated that 40–50g of BAT could account for 20% of daily energy expenditure (Rothwell and Stock, 1979), which would be astonishing given current estimates that there may be upwards of 100g of BAT in a normal person (van Marken Lichtenbelt et al., 2009). Unfortunately, two major assumptions underlying this assertion have not been borne out. First, the mouse calculations for BAT were made under conditions of maximal activation, which is almost never the case in thermoneutrality-seeking humans. Second, mammalian energy expenditure is inversely correlated with body size, such that whole body BMR is 1–2 W/kg for humans, compared to 8 W/kg for mice (van Marken Lichtenbelt and Schrauwen, 2011). Even with these caveats, BAT activity has been predicted to account for 2.7–5% of BMR in humans, which could cumulatively promote more than 4 kg of fat loss per year (van Marken Lichtenbelt and Schrauwen, 2011; Virtanen et al., 2009). The potential benefits of BAT activation extend beyond weight loss, as BAT consumes significant amounts of lipid, and to a lesser but still significant extent, glucose (Bartelt et al., 2011; Ouellet et al., 2012).
There has been interest in promoting uncoupling as a weight loss strategy since the 1930’s, with attention focused on chemical agents like dinitrophenol. Experience with this approach has been mixed, with some studies reporting excellent results while others were less sanguine. Side effects, ranging from rash to cataracts to hyperpyrexia have been reported, and there have been fatalities (Colman, 2007; Harper et al., 2001). Presumably, much of the toxicity of these agents is related to their actions in non-BAT tissues, such as muscle or the optic lens, and it is thus unclear if this experience informs us about the safety or utility of inducing the development or function of brown adipocytes, which are adapted to high levels of uncoupling.
How could BAT be induced or activated in a medicinal context? Most of the classical inducers, such as catecholamines and thyroid hormone, cannot be delivered in excess without causing significant morbidity. There was intense interest for some time in developing β3-adrenergic receptor-specific agonists, which increased thermogenesis in some studies. However, absolute β3-selectivity has proven difficult to achieve, the oral bioavailability of such compounds is suboptimal, and published results from early trials have been uninspiring (Arch, 2002). Some investigators have posited that cold itself could be used as therapy, as one can see significant inductions of BAT activity at temperatures that can be achieved without a cold suit (i.e. 15–19°C/59–66.2°F)(Chen et al., 2013a; van der Lans et al., 2013; Yoneshiro et al., 2013). As little as two hours a day at 19°C for six weeks was sufficient to reduce fat mass in young, healthy male subjects (Yoneshiro et al., 2013). Is this feasible in a world where 27% of global warming is predicted to arise from air conditioners by 2050 (Velders et al., 2012)? In this case, the societal costs may well outweigh the benefits to the individual.
Thiazolidinediones have been reported to increase the browning of white fat (Petrovic et al., 2010; Qiang et al., 2012; Teruel et al., 2003), but TZD use causes weight gain, not weight loss (Ahmadian et al., 2013). Similarly, developmental regulators like BMP7 and BMP8b are unlikely pharmaceutical candidates given the pleiotropy of their actions. FGF21 is being actively explored as a therapeutic (Woo et al., 2013), although it’s unclear whether its beneficial actions depend on browning. The natriuretic peptides (or more precisely, inhibitors of neutral endopeptidase, which degrades ANP and BNP) are interesting candidates, as they are being developed for hypertension and heart failure (Nawarskas et al., 2001); unfortunately, blockade of NEP causes weight gain in mice (Becker et al., 2010). Finally, cyclooxygenase (COX)-2 mediates some of the effects of β-adrenergic signaling on brown fat development and function (Vegiopoulos et al., 2010), and COX-2 inhibition is associated with weight gain (Fain et al., 2001). Unfortunately, promoting global prostaglandin synthesis with a COX-2 activator is an unattractive strategy, regardless of any potential effects on body weight. The a priori uncertainty of these approaches makes it imperative that we gain a better understanding of the pathways that promote and inhibit brown/beige adipocyte thermogenesis in humans, so that we can identify an optimal intervention point.
Disruption or promotion of adipose tissue angiogenesis: both or neither?
The profound growth potential of adipose tissue and its ability to foster its own blood supply through the elaboration of vascular growth factors led several investigators to draw analogies to tumor biology, and thus to ask whether angiogenesis inhibitors might promote weight loss. Early studies with these agents in high-fat fed and genetically obese mice looked promising, with significant reductions in body fat achieved by administration of anti-angiogenic agents originally developed for cancer therapy (Brakenhielm et al., 2004; Rupnick et al., 2002). Importantly, these treatments caused the metabolic profiles of the mice to improve, with reduced insulin resistance and hyperlipidemia. This was recapitulated in a more specific fashion by coupling a pro-apoptotic peptide to a peptide ligand for a protein called prohibitin, which is expressed preferentially on intra-adipose endothelial cells. This strategy reduces body weight and improves glucose homeostasis in both rodents and nonhuman primates (Barnhart et al., 2011; Kim et al., 2012; Kolonin et al., 2004).
The picture becomes significantly murkier, however, when VEGF expression is manipulated, with paradoxical results seen in most studies (Cao, 2013). Although difficult to reconcile all the data, it does appear that timing is an important issue, with different results seen when angiogenesis is affected early in overnutrition or after obesity has already been established. Furthermore, these angiogenic agents have effects beyond the vasculature, with effects on brown fat thermogenesis, macrophage polarization, and food intake, all of which can complicate the interpretation of their metabolic actions (Elias et al., 2013; Kim et al., 2010; Sun et al., 2012).
Adipokine-based Therapy
When leptin was first discovered, it was obvious to propose using it to treat obesity in human patients. It was soon discovered that obese people are not, in fact, leptin deficient. Rather, they have elevated levels due to leptin resistance, a complex phenomenon involving altered transport across the blood-brain barrier as well as intracellular mechanisms that reduce signaling efficiency (Myers et al., 2012). In principle, this did not necessarily preclude the successful use of recombinant leptin as an anti-obesity agent; after all, insulin resistant Type 2 diabetics are often treated with supraphysiological doses of insulin. Nevertheless, the results of single agent leptin administration have not been encouraging (Heymsfield et al., 1999; Mittendorfer et al., 2011). More recent trials have focused on co-administration of leptin with other agents that promote weight loss, with the idea that reducing leptin resistance would enhance leptin action. Some of these agents, like amylin, metformin, FGF21, rimonabant, and exendin-4, have shown promise, although their effects have been generally less impressive in humans than in rodents (Boustany-Kari et al., 2011; Kim et al., 2006; Muller et al., 2012; Ravussin et al., 2009; Roth et al., 2008). An emerging idea under study is a role for leptin in maintaining weight loss achieved through other means. The only currently approved indication for leptin is on a compassionate use basis in rare cases of congenital leptin deficiency (Montague et al., 1997), where it is quite efficacious. Patients with congenital lipodystrophy also have access to leptin therapy on the same basis. At present, leptin is not approved for the much larger group of patients with acquired (such as HIV-associated) lipodystrophy, but trials are ongoing. Other indications for leptin are being explored, such as hypothalamic amenorrhea, which is also a hypoleptinemic state (Chou et al., 2011).
The broad beneficial actions of adiponectin make it an attractive target for therapeutic manipulation, although its propensity to cause weight gain when provided in excess needs to be addressed. Unfortunately, direct administration of adiponectin is made difficult by the requirement for proper post-translational modification to enable multimerization, combined with an unusually short serum half-life and high baseline levels. TZDs promote the synthesis and secretion of adiponectin from fat, and other adiponectin secretagogues have been identified and are being assessed for clinical efficacy (Shetty et al., 2009). Finally, small molecule activators of the ADIPOR1/R2 receptors might be of significant utility (Okada-Iwabu et al., 2013).
Blocking the effects of negative adipokines is also a worthwhile strategy. Fenretinide reduces serum RBP4 levels by disrupting its interaction with transthyretin and thus promoting excretion through the urine (Campos-Sandoval et al., 2011). Accordingly, fenretinide was tested for its ability to improve insulin sensitivity and glucose tolerance in obese rodents, and was found to be efficacious (Yang et al., 2005). However, fenretinide achieves this by promoting weight loss, an effect still present in RBP4 null animals (Preitner et al., 2009). Another adipokine that may be amenable to blockade is aP2. A small molecule inhibitor of this fatty acid binding protein was found to ameliorate both insulin resistance and atherosclerosis in rodent models; the fact that these proteins evolved to bind small lipophilic ligands makes them particularly promising drug targets (Furuhashi and Hotamisligil, 2008).
Conclusions
The obesity epidemic has put a spotlight on the adipocyte, and new information emerges with each passing week. We have endeavored to illustrate several key themes:
Adipocytes can be divided into different classes, generally denoted as white, brown, and beige. These types share numerous attributes, but also differ in critical ways that include aspects of their gene expression profile and secretome, their developmental origin, and their therapeutic potential.
The developmental pathways that give rise to mature adipocytes are still being worked out, but likely include a perivascular origin with various transition forms that can be identified using cell surface markers. In some physiological situations there may be interconversion of adipose types or even between adipocytes and nonadipocytes, suggesting remarkable plasticity.
The composition of adipose tissue changes dramatically during overnutrition, involving alterations in adipocyte size and number, immune cell type and number, and extracellular matrix. Ultimately these events predispose to the metabolic dysfunction seen in obesity.
Adipose tissue performs a wide array of functions that depend upon physical location and physiological status.
Communication between adipocytes and extra-adipose tissues can occur via multiple mechanisms, but most commonly through the elaboration of a growing array of secreted factors collectively termed adipokines.
The complexity of adipose tissue presents numerous challenges but also several opportunities for therapeutic intervention.
We have learned an extraordinary amount in a short time, and it seems certain that we will discover much more about this highly complex and relatively unloved cell in the very near future.
Figure 1. Interest in adipose biology has risen over time.
Papers were identified in Pubmed using the term “adipose” for each year from 1900-2012 and expressed as total number of adipose papers (blue) and adipose papers as a percentage of all papers (red). Important events in adipose biology research are indicated by publication date. We are now in the midst of the second surge of interest in adipose biology in the last century.
Figure 2. Different origins for distinct types of adipocyte.
White and beige adipocytes derive from Pax7-/Myf5- cells, via distinct precursor cells. Beige adipocytes differentiate following activation by cold or other inducers. After cold challenge is removed, these cells become inactive, taking on the morphology of a ‘white’ adipocyte. Classic brown fat, in contrast, comes from a Pax7+/Myf5+ lineage shared with skeletal muscle.
Figure 3. Activators of beige/brown fat development and function.
Many inducers of browning and enhanced thermogenesis have been discovered. Some of these agents appear to work primarily by inducing the formation of new beige (e.g. irisin) or brown (e,g. BMP7) adipocytes while others may act on both recruitment and enhancement of thermogenic potential.
Figure 4. Immune cells are integral components of the fat pad in leanness and obesity.
The lean fat depot contains many types of immune cell, dominated by resident M2 macrophages, eosinophils, and Tregs. In the setting of overnutrition, there is accumulation of proinflammatory cells, including M1 macrophages, mast cells, and various T lymphocyte classes.
Figure 5. Adipocyte-matrix interactions play a role in the pathology of obesity.
Adipocytes secrete numerous matrix proteins that maintain the structure of the depot. During overnutrition, adipocytes increase in size until further expansion becomes limited by the matrix, which undergoes fibrotic changes. This triggers changes that include hypoxia, inflammation, and cell death, all of which contribute to insulin resistance.
Figure 6. Adipose cross-talk with other tissues.
Adipocytes store and release calories to the body generally, but numerous examples have emerged demonstrating additional roles of fat in a wide array of biological processes. These examples given here are illustrative, and not exhaustive.
Acknowledgments
We would like to thank members of our laboratories, past and present, for helpful discussions. Leslie Gaffney and Sigrid Knemeyer were of incalculable help with the figures.
Footnotes
with apologies to Raymond Carver
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell metabolism. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nature medicine. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, de Andres J, Yubero P, Vinas O, Mampel T, Iglesias R, Giralt M, Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. The Journal of biological chemistry. 1995;270:5666–5673. doi: 10.1074/jbc.270.10.5666. [DOI] [PubMed] [Google Scholar]
- Arch JR. beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. European journal of pharmacology. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. The Journal of clinical investigation. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart KF, Christianson DR, Hanley PW, Driessen WH, Bernacky BJ, Baze WB, Wen S, Tian M, Ma J, Kolonin MG, et al. A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys. Science translational medicine. 2011;3:108ra112. doi: 10.1126/scitranslmed.3002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology. 2010a;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010b;34(Suppl 1):S36–42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. American journal of hematology. 1979;6:191–198. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- Becker M, Siems WE, Kluge R, Gembardt F, Schultheiss HP, Schirner M, Walther T. New function for an old enzyme: NEP deficient mice develop late-onset obesity. PloS one. 2010;5 doi: 10.1371/journal.pone.0012793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezech C, Mader E, Desanti G, Khan M, Nakamura K, White A, Ware CF, Anderson G, Caamano JH. Lymphotoxin-beta receptor signaling through NF-kappaB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37:721–734. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, Desantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, et al. The STRA6 Receptor Is Essential for Retinol-binding Protein-induced Insulin Resistance but Not for Maintaining Vitamin A Homeostasis in Tissues Other Than the Eye. The Journal of biological chemistry. 2013;288:24528–24539. doi: 10.1074/jbc.M113.484014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nature cell biology. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friedman JM, Rodeheffer MS. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138:4709–4719. doi: 10.1242/dev.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, Carlgren G, Isaksson B, Krotkiewski M, Larsson B, Sjostrom L. Effect of an energy-reduced dietary regimen in relation to adipose tissue cellularity in obese women. The American journal of clinical nutrition. 1975;28:445–452. doi: 10.1093/ajcn/28.5.445. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustany-Kari CM, Jackson VM, Gibbons CP, Swick AG. Leptin potentiates the anti-obesity effects of rimonabant. European journal of pharmacology. 2011;658:270–276. doi: 10.1016/j.ejphar.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R1167–1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circulation research. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. Journal of obesity. 2013;2013:393192. doi: 10.1155/2013/393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R1445–1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. American journal of physiology Endocrinology and metabolism. 2010;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Sandoval JA, Redondo C, Kinsella GK, Pal A, Jones G, Eyre GS, Hirst SC, Findlay JB. Fenretinide derivatives act as disrupters of interactions of serum retinol binding protein (sRBP) with transthyretin and the sRBP receptor. Journal of medicinal chemistry. 2011;54:4378–4387. doi: 10.1021/jm200256g. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell metabolism. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis and Vascular Functions in Modulation of Obesity, Adipose Metabolism, and Insulin Sensitivity. Cell metabolism. 2013 doi: 10.1016/j.cmet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. Journal of lipid research. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. The Journal of biological chemistry. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends in endocrinology and metabolism: TEM. 2013;24:408–420. doi: 10.1016/j.tem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. The Journal of clinical endocrinology and metabolism. 2013a;98:E1218–1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Song J, Cui J, Hou J, Zheng X, Li C, Liu L. microRNAs regulate adipocyte differentiation. Cell biology international. 2013b;37:533–546. doi: 10.1002/cbin.10063. [DOI] [PubMed] [Google Scholar]
- Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. American journal of physiology Endocrinology and metabolism. 2012;303:E937–949. doi: 10.1152/ajpendo.00061.2012. [DOI] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010a;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, et al. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Molecular and cellular biology. 2010b;30:5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. The Journal of cell biology. 2000;149:667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Cigolini M, Bosello O, Bjorntorp P. A morphological study of the adipocyte precursor. Journal of submicroscopic cytology. 1984;16:243–251. [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regulatory toxicology and pharmacology : RTP. 2007;48:115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Considine RV. Regulation of leptin production. Reviews in endocrine & metabolic disorders. 2001;2:357–363. doi: 10.1023/a:1011896331159. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews Molecular cell biology. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Schupp M, Lefterova MI, Cao S, Cohen DM, Chen CS, Steger DJ, Lazar MA. Repressor transcription factor 7-like 1 promotes adipogenic competency in precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16271–16276. doi: 10.1073/pnas.1109409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C. Activins in adipogenesis and obesity. Int J Obes (Lond) 2013;37:163–166. doi: 10.1038/ijo.2012.28. [DOI] [PubMed] [Google Scholar]
- Degerman E, Landstrom TR, Wijkander J, Holst LS, Ahmad F, Belfrage P, Manganiello V. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods. 1998;14:43–53. doi: 10.1006/meth.1997.0564. [DOI] [PubMed] [Google Scholar]
- Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell metabolism. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis GV, Obin MS. 'Metabolically healthy obesity': origins and implications. Molecular aspects of medicine. 2013;34:59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. The Journal of clinical investigation. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson DE, Kambe A, Block E, Dion T, Lu H, Castellot JJ, Jr, Spiegelman BM. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell. 1990;61:223–230. doi: 10.1016/0092-8674(90)90803-m. [DOI] [PubMed] [Google Scholar]
- Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes. 2013 doi: 10.2337/db12-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell metabolism. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Bosch F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte. 2013;2:109–112. doi: 10.4161/adip.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- English JT, Patel SK, Flanagan MJ. Association of pheochromocytomas with brown fat tumors. Radiology. 1973;107:279–281. doi: 10.1148/107.2.279. [DOI] [PubMed] [Google Scholar]
- Fain JN, Ballou LR, Bahouth SW. Obesity is induced in mice heterozygous for cyclooxygenase-2. Prostaglandins & other lipid mediators. 2001;65:199–209. doi: 10.1016/s0090-6980(01)00136-8. [DOI] [PubMed] [Google Scholar]
- Fazeli PK, Horowitz MC, Macdougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow Fat and Bone--New Perspectives. The Journal of clinical endocrinology and metabolism. 2013 doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn EJ, 3rd, Trent CM, Rawls JF. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio) Journal of lipid research. 2009;50:1641–1652. doi: 10.1194/jlr.M800590-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R1630–1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circulation research. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature reviews Drug discovery. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, et al. Adipocyte iron regulates adiponectin and insulin sensitivity. The Journal of clinical investigation. 2012;122:3529–3540. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. The Journal of clinical endocrinology and metabolism. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinology and metabolism clinics of North America. 2012;41:283–295. v–vi. doi: 10.1016/j.ecl.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocrine reviews. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nature clinical practice Cardiovascular medicine. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TE, Kahn BB. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:717–721. doi: 10.1055/s-2007-985879. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. American journal of physiology Endocrinology and metabolism. 2005;289:E608–616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) The Journal of biological chemistry. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. Journal of lipid research. 1974;15:474–483. [PubMed] [Google Scholar]
- Griffith JF, Yeung DK, Ahuja AT, Choy CW, Mei WY, Lam SS, Lam TP, Chen ZY, Leung PC. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44:1092–1096. doi: 10.1016/j.bone.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell metabolism. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Restricted Adipogenesis in Hypertrophic Obesity: The Role of WISP2, WNT, and BMP4. Diabetes. 2013;62:2997–3004. doi: 10.2337/db13-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nature medicine. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hayward JS, Lisson PA. Evolution of brown fat: its absence in marsupials and monotremes. Canadian Journal of Zoology. 1992;70:171–179. [Google Scholar]
- Hellman B, Larsson S, Westman S. Mast cell content and fatty acid metabolism in the epididymal fat pad of obese mice. Acta physiologica Scandinavica. 1963;58:255–262. doi: 10.1111/j.1748-1716.1963.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1985;61:917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, Klein S, Kahn BB. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez TL, Kittelson JM, Law CK, Ketch LL, Stob NR, Lindstrom RC, Scherzinger A, Stamm ER, Eckel RH. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity (Silver Spring) 2011;19:1388–1395. doi: 10.1038/oby.2011.64. [DOI] [PubMed] [Google Scholar]
- Hesslein DG, Fretz JA, Xi Y, Nelson T, Zhou S, Lorenzo JA, Schatz DG, Horowitz MC. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone. 2009;44:537–546. doi: 10.1016/j.bone.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA : the journal of the American Medical Association. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American journal of physiology Cell physiology. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, et al. An FGF21-Adiponectin-Ceramide Axis Controls Energy Expenditure and Insulin Action in Mice. Cell metabolism. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg AN, Susulic VS, Madura JP, Zhang B, Moller DE, Tontonoz P, Sarraf P, Spiegelman BM, Lowell BB. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. The Journal of biological chemistry. 1997;272:5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, et al. A Novel Function of Adipocytes in Lipid Antigen Presentation to iNKT Cells. Molecular and cellular biology. 2013;33:328–339. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. European journal of applied physiology and occupational physiology. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- Iglesias P, Selgas R, Romero S, Diez JJ. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. European journal of endocrinology / European Federation of Endocrine Societies. 2012;167:301–309. doi: 10.1530/EJE-12-0357. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell metabolism. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang C, Kim JH, Li F, Qu A, Gavrilova O, Shah YM, Gonzalez FJ. Hypoxia-inducible factor 1alpha regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. The Journal of biological chemistry. 2013;288:3844–3857. doi: 10.1074/jbc.M112.426338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun LS, Siddall CP, Rosen ED. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. American journal of physiology Endocrinology and metabolism. 2011;301:E825–835. doi: 10.1152/ajpendo.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes & development. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochemical and biophysical research communications. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of clinical investigation. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Akerblad P, Kiviranta R, Gupta RK, Kajimura S, Griffin MJ, Min J, Baron R, Rosen ED. Regulation of early adipose commitment by Zfp521. PLoS biology. 2012;10:e1001433. doi: 10.1371/journal.pbio.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell metabolism. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and cellular biology. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nature reviews Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- Kiefer FW, Vernochet C, O'Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nature medicine. 2012;18:918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sartor MA, Bain JR, Sandoval D, Stevens RD, Medvedovic M, Newgard CB, Woods SC, Seeley RJ. Rapid and weight-independent improvement of glucose tolerance induced by a peptide designed to elicit apoptosis in adipose tissue endothelium. Diabetes. 2012;61:2299–2310. doi: 10.2337/db11-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59:907–915. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. The Journal of clinical investigation. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–724. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, Kotani K, Konishi H, Matsuzaki H, Kikkawa U, Ogawa W, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Molecular and cellular biology. 1999;19:6286–6296. doi: 10.1128/mcb.19.9.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. The New England journal of medicine. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. American journal of physiology Endocrinology and metabolism. 2010;299:E506–515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. The Journal of clinical investigation. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. The Journal of clinical investigation. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nature medicine. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Konige M, Wang H, Sztalryd C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbadis.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral JG, Bjorntorp P, Schersten T, Sjostrom L. Body composition and adipose tissue cellularity before and after jejuno-ileostomy in severely obese subjects. European journal of clinical investigation. 1977;7:413–419. doi: 10.1111/j.1365-2362.1977.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. The Journal of clinical investigation. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes & development. 2012;26:259–270. doi: 10.1101/gad.180406.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. The Journal of clinical investigation. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: mplication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell metabolism. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell metabolism. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Liew CW, Boucher J, Cheong JK, Vernochet C, Koh HJ, Mallol C, Townsend K, Langin D, Kawamori D, Hu J, et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nature medicine. 2013;19:217–226. doi: 10.1038/nm.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, et al. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell metabolism. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, Darlington GJ. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, VSS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Bernard JJ, Peng QY, Li T, Lin Y, Shih WJ, Nghiem P, Shapses S, Wagner GC, et al. Surgical removal of the parametrial fat pads stimulates apoptosis and inhibits UVB-induced carcinogenesis in mice fed a high-fat diet. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9065–9070. doi: 10.1073/pnas.1205810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007b;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- MacKellar J, Cushman SW, Periwal V. Waves of adipose tissue growth in the genetically obese Zucker fatty rat. PloS one. 2010;5:e8197. doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell metabolism. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neuroscience and biobehavioral reviews. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Maymo JL, Perez AP, Gambino Y, Calvo JC, Sanchez-Margalet V, Varone CL. Review: Leptin gene expression in the placenta--regulation of a key hormone in trophoblast proliferation and survival. Placenta. 2011;32(Suppl 2):S146–153. doi: 10.1016/j.placenta.2011.01.004. [DOI] [PubMed] [Google Scholar]
- McCullough AW. Evidence of the macrophagal origin of adipose cells in the white rat as shown by studies on straved animals. Journal of Morphology. 1944;75:193–201. [Google Scholar]
- Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism: clinical and experimental. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, Paruthi J, Mantzoros CS. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocrine reviews. 2013;34:377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose Tissue Macrophages Function As Antigen-Presenting Cells and Regulate Adipose Tissue CD4+ T Cells in Mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni M, Giordano A, Zingaretti MC, Boiani R, De Matteis R, Kahn BB, Nisoli E, Tonello C, Pisoschi C, Luchetti MM, et al. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16801–16806. doi: 10.1073/pnas.0407647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Sullivan LM, Habegger K, Yi CX, Kabra D, Grant E, Ottaway N, Krishna R, Holland J, Hembree J, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of peptide science : an official publication of the European Peptide Society. 2012;18:383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin Sensitive Sensory Nerves Innervate White Fat. American journal of physiology Endocrinology and metabolism. 2013 doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, Tschop MH, Yanovski JA. Challenges and opportunities of defining clinical leptin resistance. Cell metabolism. 2012;15:150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell metabolism. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A, Lemos DR, Rossi FM. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle. 2010;9:2045–2046. doi: 10.4161/cc.9.11.11854. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawarskas J, Rajan V, Frishman WH. Vasopeptidase inhibitors, neutral endopeptidase inhibitors, and dual inhibitors of angiotensin-converting enzyme and neutral endopeptidase. Heart Dis. 2001;3:378–385. doi: 10.1097/00132580-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. The Journal of biological chemistry. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Molecular and cellular biology. 2012;32:2010–2019. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkrug R, Goetze N, Exner C, Lee Y, Ganjam GK, Kutschke M, Muller S, Stohr S, Tschop MH, Crichton PG, et al. Brown fat in a protoendothermic mammal fuels eutherian evolution. Nature communications. 2013;4:2140. doi: 10.1038/ncomms3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami KI, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013 doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskowitz AZ, Lu J, Penfornis P, Ylostalo J, McBride J, Flemington EK, Prockop DJ, Pochampally R. Human multipotent stromal cells from bone marrow and microRNA: regulation of differentiation and leukemia inhibitory factor expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18372–18377. doi: 10.1073/pnas.0809807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T. Chemokine systems link obesity to insulin resistance. Diabetes & metabolism journal. 2013;37:165–172. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: a neglected enigma. General and comparative endocrinology. 2011;174:1–4. doi: 10.1016/j.ygcen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of clinical investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BO, Ahrends R, Teruel MN. Consecutive positive feedback loops create a bistable switch that controls preadipocyte-to-adipocyte conversion. Cell reports. 2012;2:976–990. doi: 10.1016/j.celrep.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocrine reviews. 2011;32:550–570. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. The Journal of clinical investigation. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early human development. 1983;8:1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early human development. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- Pond CM. An evolutionary and functional view of mammalian adipose tissue. The Proceedings of the Nutrition Society. 1992;51:367–377. doi: 10.1079/pns19920050. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine. 2002;17:131–139. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes & development. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. American journal of physiology Endocrinology and metabolism. 2009;297:E1420–1429. doi: 10.1152/ajpendo.00362.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocrine reviews. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell metabolism. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PloS one. 2011;6:e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockson SG. Causes and consequences of lymphatic disease. Annals of the New York Academy of Sciences. 2010;1207(Suppl 1):E2–6. doi: 10.1111/j.1749-6632.2010.05804.x. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rosen ED. The molecular control of adipogenesis, with special reference to lymphatic pathology. Annals of the New York Academy of Sciences. 2002;979:143–158. doi: 10.1111/j.1749-6632.2002.tb04875.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes & development. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nature reviews Molecular cell biology. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nature cell biology. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. Journal of internal medicine. 2007;262:431–438. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- Salans LB, Horton ES, Sims EA. Experimental obesity in man: cellular character of the adipose tissue. The Journal of clinical investigation. 1971;50:1005–1011. doi: 10.1172/JCI106570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell metabolism. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SF, Jorgensen M, Chen Y, Nielsen R, Sandelin A, Mandrup S. Cross species comparison of C/EBPalpha and PPARgamma profiles in mouse and human adipocytes reveals interdependent retention of binding sites. BMC genomics. 2011;12:152. doi: 10.1186/1471-2164-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes & development. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell metabolism. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends in pharmacological sciences. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. American journal of physiology Endocrinology and metabolism. 2007;293:E1012–1020. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS, Horton JD. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. The Journal of clinical investigation. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Yamashita D, Yamaguchi T, Hirose F, Osumi T. Aspects of the regulatory mechanisms of PPAR functions: analysis of a bidirectional response element and regulation by sumoylation. Molecular and cellular biochemistry. 2006;286:33–42. doi: 10.1007/s11010-005-9052-z. [DOI] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends in endocrinology and metabolism: TEM. 2012;23:56–64. doi: 10.1016/j.tem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR. Potential of brown adipose tissue type II thyroxine 5'-deiodinase as a local and systemic source of triiodothyronine in rats. The Journal of clinical investigation. 1985;76:2296–2305. doi: 10.1172/JCI112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AJ. Cultural History of the Buttocks. In: Pitts-Taylor V, editor. Cultural Encyclopedia of the Body. ABC-CLIO; Greenwood: 2008. [Google Scholar]
- Soccio RE, Tuteja G, Everett LJ, Li Z, Lazar MA, Kaestner KH. Species-specific strategies underlying conserved functions of metabolic transcription factors. Mol Endocrinol. 2011;25:694–706. doi: 10.1210/me.2010-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Lazar MA. The current biology of resistin. Journal of internal medicine. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and Adipose Tissue Dysfunction. Cell metabolism. 2013a doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, Sauvageau M, Tazon-Vega B, Kelley DR, Hendrickson DG, et al. Long noncoding RNAs regulate adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013b;110:3387–3392. doi: 10.1073/pnas.1222643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME. Brown adipose tissue growth and development. Scientifica. 2013;2013 doi: 10.1155/2013/305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell metabolism. 2011;14:116–122. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel T, Hernandez R, Benito M, Lorenzo M. Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. The Journal of biological chemistry. 2003;278:263–269. doi: 10.1074/jbc.M207200200. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annual review of biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Lodish H. MicroRNA networks regulate development of brown adipocytes. Trends in endocrinology and metabolism: TEM. 2013;24:442–450. doi: 10.1016/j.tem.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell metabolism. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nature reviews Endocrinology. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiological reviews. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- Turer AT, Khera A, Ayers CR, Turer CB, Grundy SM, Vega GL, Scherer PE. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013 doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R285–296. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Velders GJ, Ravishankara AR, Miller MK, Molina MJ, Alcamo J, Daniel JS, Fahey DW, Montzka SA, Reimann S. Climate change. Preserving Montreal Protocol climate benefits by limiting HFCs. Science. 2012;335:922–923. doi: 10.1126/science.1216414. [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell metabolism. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell metabolism. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? American journal of physiology Endocrinology and metabolism. 2013;305:E567–572. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of lipid research. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. American journal of physiology Endocrinology and metabolism. 2012;302:E19–31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013 doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann F. The Development of Adipose Tissue. In: Renold AE, Cahill GF, editors. Handbook of Physiology: Adipose Tissue. American Physiological Society; 1965. pp. 87–100. [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129:537–548. doi: 10.1016/j.cell.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clinical endocrinology. 2013;78:489–496. doi: 10.1111/cen.12095. [DOI] [PubMed] [Google Scholar]
- Wrann CD, Eguchi J, Bozec A, Xu Z, Mikkelsen T, Gimble J, Nave H, Wagner EF, Ong SE, Rosen ED. FOSL2 promotes leptin gene expression in human and mouse adipocytes. The Journal of clinical investigation. 2012;122:1010–1021. doi: 10.1172/JCI58431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Molecular cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Yu S, Hsu CH, Eguchi J, Rosen ED. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2421–2426. doi: 10.1073/pnas.0707082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. Journal of lipid research. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell metabolism. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell metabolism. 2006;3:223–229. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell metabolism. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. The Journal of biological chemistry. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell metabolism. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of clinical investigation. 2013 doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes & development. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocrine reviews. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]