Abstract
None of the clinical trials of anti-HIV gels based on conventional polymers or lipid emulsions is successful, suggesting the need of new molecular design of the anti-HIV gels. This paper reports the conversion of anti-HIV prodrugs into self-delivery supramolecular hydrogels. By covalently conjugating reverse transcriptase inhibitors to a versatile self-assembly motif, we obtained the hydrogelators that self-assemble to form supramolecular nanofibers as the matrices of hydrogels in a weak acidic condition. Upon the treatment of prostate acid phosphatase (PAP), the hydrogels exhibit drastically enhanced elasticity. The hydrogelators are biocompatible and able to release the inhibitors under physiological condition. The use of the self-assembly motif as a self-delivery agent containing non-steroid anti-inflammatory drug (NSAID) renders this hydrogel to be both anti-inflammatory and anti-HIV. This work illustrates an unprecedented approach for designing multifunctional supramolecular hydrogels that may serve as potential anti-HIV hydrogels for sustained drug release.
Keywords: Anti-HIV hydrogels, self-delivery, self-assembly, supramolecular interaction, nanotechnology
1. Introduction
This article describes the design, synthesis, and characterization of supramolecular hydrogelators that may lead to potential anti-inflammatory and anti-HIV gels for the sustained release of reverse transcriptase inhibitors (e.g., lamivudine (3TC) and zidovudine (AZT)) against human immunodeficiency virus (HIV). Acquired immunodeficiency syndrome (AIDS), an infectious disease caused by HIV, remains a major public health problem in many parts of the world. While the number of AIDS-related deaths is steadily decreasing worldwide, AIDS-related mortality increases by 60% in the Middle East and North Africa.[1] Because of the difficulty of the development of effective vaccines for preventing the transmission of HIV,[2] there is an urgent need for alternative prevention of HIV acquisition in women. This has led to the development of topical gels of microbicides against HIV. Several type of vaginal gels have reached the stage of clinical trials.[3] Unfortunately, none of them has shown significant clinical benefits, despite some early promises[4] and recent excitements.[5] For example, none of the nonspecific microbiocide-based gels has shown significant benefits in large scale clinical trials.[6] The disappointments led to the shift of strategy by using anti-HIV drugs to produce gels, such as tenofovir gels.[5, 7] Despite the preliminary study reported in 2010 indicated its apparent effectiveness,[5, 8] the tenofovir gel failed in a more recent trial without a clear reason.[9]
The above-mentioned ups and downs in the clinical trials of anti-HIV gels not only underscore the challenges and difficulties, but also highlight the urgent need for exploring new materials and new approaches. One notable candidate is soft nanomaterials made of molecular nanofibers of bioactive molecules. For example, supramolecular nanofibers, made of bioactive small molecules,[10] have become a promising candidate for making hydrogels[11] for a broad range of biomedical applications. Certain small molecules (called hydrogelators) self-assemble in water through non-covalent interactions to form nanofibers as the matrices of the hydrogels. These hydrogels are able to serve as medium for drug delivery or as scaffold for tissue engineering because they exhibit several unique merits, such as synthetic economy, biocompatibility, low immunogenicity to tissues and cells.[12] For example, the nanofibers of peptide amphiphiles can display a high density of epitopes for regulating the differentiation of neuron progenitor cells[13] or guiding cartilage regeneration[14]. The covalent connection of taxol with a motif of self-assembly has led to supramolecular nanofibers of taxol as the delivery vehicle and the drug itself.[15] The hydrogels made of the molecular nanofibers of lanreotide has already received FDA's approval for clinical use in the treatment of acromegaly.[16] These successful cases suggest that the molecular nanofibers/hydrogels of therapeutic agents can perform dual or multiple roles by acting as a new type of sustained, self-delivery drugs.[12g]
Despite the rapid development and promises of molecular nanofibers/hydrogels, the molecular nanofibers of anti-HIV drugs for making gels for sustained self-delivery have yet to be explored. Thus, we choose to develop supramolecular nanofibers of anti-HIV drugs for generating potential anti-HIV gels. In this work, we found that the covalent conjugation of reverse transcriptase inhibitors to a versatile self-assembly motif afford hydrogelators that self-assemble to form supramolecular nanofibers as the matrices of hydrogels in a weak acidic condition that is identical to the physiological environment.[17] Upon the presence of prostatic acid phosphatase (PAP), the hydrogels exhibit drastically enhanced elasticity, which should help to match the change of physiological environment during coitus. In addition, the hydrogelators are biocompatible, and are able to release the inhibitors under physiological condition. When the self-assembly motif in the hydrogelator is an non-steroid anti-inflammatory (NSAID) agent[18] and the other component in the hydrogelator the anti-HIV drug, the hydrogelators are able to address several problems (local inflammation, pH imbalance, lack of specificity, etc.) associated with the previous approaches for anti-HIV gels. This work, thus, illustrates a new and viable approach for creating hydrogels that may serve as potential multifunctional sustained drug-release hydrogels for preventing the transmission of HIV.
2. Results and Discussion
Scheme 1 shows the molecular design of the hydrogelator, which contains anti-HIV and anti-inflammatory drugs and a phosphate group. The inclusion of NSAID is to prevent local inflammation, thus reducing the odds of the viruses to enter T cells.[19] The utilization of the phosphate group has two purposes: (i) it allows hydrogelation at local physiological pH;[17] (ii) it permits PAP to enhance the viscoelasticity of the hydrogels, thus minimizing the dilution of the hydrogels.[20] To demonstrate the concepts, we choose 3TC[21] or AZT[21] as the anti-HIV drugs and naproxen (Nxp)[21] as the NSAID. As an example shown in Scheme 1, the antiviral therapeutic hydrogelator, consisting of an anti-inflammatory gelation motif to carry anti-HIV drugs 3TC (1) by an ester bond, self-assembles in acidic condition (pH 4.2)[17] and produces uniform nanofibers that entangle to form 3D network as the matrices of a hydrogel. The selection of this naproxen based hydrogelator is based on its selectivity towards cyclooxygenase-2 (COX-2).[18] Once PAP catalyzes the cleavage of the phosphate from therapeutic hydrogelator precursor, the resulting hydrogelator allows more extensive interactions among the nanofibers to give a hydrogel composed of a stronger network of nanofibers. The hydrolysis of the ester bond, at pH 7.4 and 37 °C, sustainably releases the anti-HIV drug (e.g., 1) and the anti-inflammatory agent from the hydrogel (e.g., 9b).
Scheme 1.
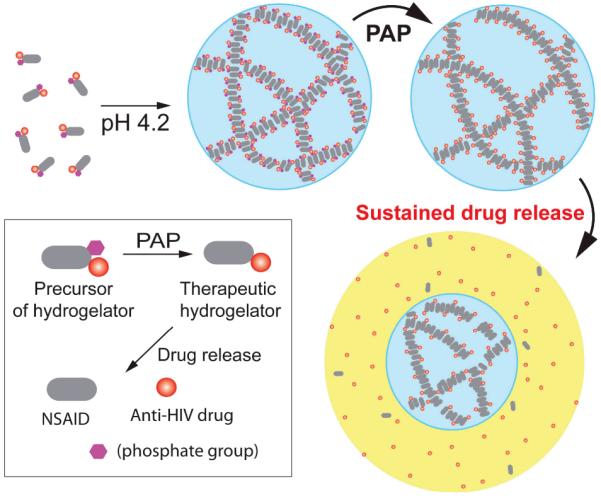
The concept of enzyme responsive anti-HIV hydrogels for sustained release of anti-HIV drugs.
Scheme 2 shows the synthetic process of the phosphate-containing hydrogelators and the corresponding enzymatic reactions. Generally, by conjugating 2-(naphthalen-2-yl)acetic acid (Nap) or (+)-(S)-2-(6-methoxynaphthalen-2-yl)propanoic acid (Nxp) with small peptides Phe-Phe-Lys-Tyr(phosphate) using solid phase synthesis, we obtain two types of motifs, NapFFKY(p) (8a) and Nxpffky(p) (9a) (F = L-phenylalanine and f = D-phenylalanine; K = L-lysine and k = D-lysine; Y = L-tyrosine and y = D-tyrosine; (p) = phosphate). Both of the motifs contain a lysine residue to offer a side chain for the attachment of the anti-HIV drugs and a tyrosine phosphate for forming a more stable hydrogel upon the encounter of phosphatase.[22] In a typical synthesis, the protection of the amine group of 1 affords 2, which reacts with succinic acid anhydride to form 3. After N-hydroxysuccinimide (NHS) activation of 3 to give 4, which couples with the ε-amino group of lysine residue of phosphate-containing motif Nxpffky(p) (9a), the deprotection of 4,4'-dimethoxytrityl chloride (DMT) from the product and purification by HPLC provide 12a in 32% yield. Thus, the anti-HIV drug 1 turns into 3TC containing hydrogelator Nxpffk(3TC)y(p) (12a). The similar approach of the synthesis of 12a also produces other hydrogelators containing either 3TC (1) or AZT (5)—NapFFK(3TC)Y(p) (L-10a), Napffk(3TC)y(p) (D-10a) NapFFK(AZT)Y(p) (L-11a), and Napffk(AZT)y(p) (D-11a)—in 20% to 30% yields.
Scheme 2.
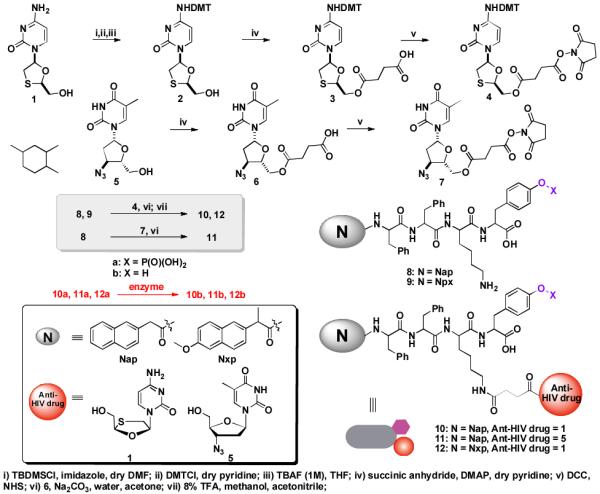
A typical synthetic route of anti-HIV drug containing hydrogelators.
After obtaining the phosphate-containing hydrogelators (10a, 11a, and 12a), we test their gelation abilities in water and determine their critical gelation concentrations (cgc) (Table S1). Typically, 3.0 mg of D-11a and 10.0 mg of L-10a, D-10a, or L-11a each can dissolve in 0.5 mL of water at pH 7.2 to give a clear solution. The addition of 1 U/mL alkaline phosphatase to the solutions turns them into the corresponding hydrogelators, such as L-10b, D-10b, L-11b, or D-11b, to form the respective hydrogels at room temperature overnight. As shown in Figure 1A and 1B, 2.0 wt% of L-10b and 2.0 wt% of L-11b form transparent hydrogels at pH 7.2, which comprise of long and uniform nanofibers with average widths of 8±2 nm and 9±2 nm, respectively. Figure 1C exhibits a slightly opaque hydrogel formed of 2.0 wt% of D-10b, which self-assembles to afford nanofibers with width of 7±2 nm. With lower cgc (0.6 wt%), the molecules of D-11b self-assemble to give uniform long nanofibers with widths of 9±2 nm, which entangle to form the low density network and afford a transparent hydrogel (Figure 1D). We have to increase the concentration of NSAID containing hydrogelator 12a to 2.5 wt% and treat the clear solution with 1 U/mL phosphatase at pH 6.4 for 24 hours to form a stable hydrogel (Figure 1E). At the concentration of 2.5 wt%, 12b forms a slightly opaque hydrogel that comprises of uniform nanofibers with widths of 5±2 nm (Figure 1E). This result indicates that 12a and 12b are less effective hydrogelators than other hydrogelators made from 10b or 11b. Since it is known that the combination of 3TC and AZT at a fixed ratio (mass ratio of 1:2) increases the efficacy and sustainability of the drugs (e.g., in the case of Combivir[23]), we also develop a hydrogel that combines D-10a and D-11a. To keep the mass ratio of 3TC and AZT to 1:2, we dissolve 2 mg of D-10a and 3.6 mg of L-11a (contains 0.4 mg of 3TC and 0.8 mg of AZT) in 0.28 ml of water in pH 7.2. Upon the treatment of 1 U/mL alkaline phosphatase overnight, we obtain the stable combined hydrogel made of Combivir. Although the combivir hydrogel (2.0 wt%) consists of two types of hydrogelators, D-10b and D-11b, it contains nanofibers that have a rather uniform width of 9±2 nm.
Figure 1.
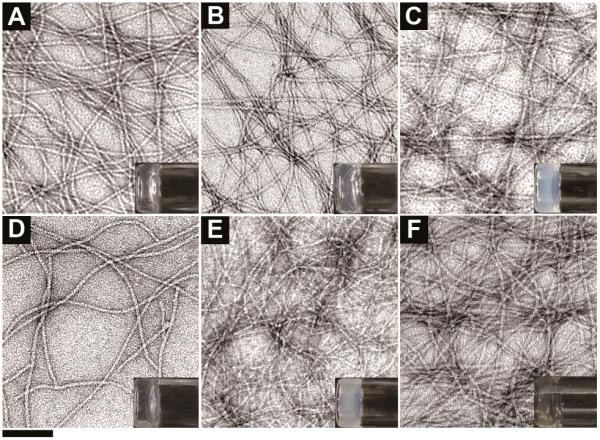
Transmission electron micrograph (TEM) of the hydrogels formed by (A) L-10b (2.0 wt%, pH 7.2), (B) L-11b (2.0 wt%, pH 7.2), (C) D-10b (2.0 wt%, pH 7.2), (D) D-11b (0.6 wt%, pH 7.2), (E) 12b (2.5 wt%, pH 6.4), and (F) combivir hydrogelators of D-10b and D-11b (2.0 wt%, pH7.2) (scale bar = 100 nm, inset: optical images of the hydrogels).
To examine the response of the anti-HIV hydrogel to biological cue (e.g., the presence of PAP during coitus), we use D-10a and 12a to form hydrogels at pH 4.2 then treat them with PAP. After dissolving D-10a in water at pH 7.2 to give 1.5 wt% of clear solution, we adjust the pH value of solution to 4.2, which turns the solution to a slightly opaque soft hydrogel within one hour (Figure 2A). Using the similar method, we prepare a transparent soft gel of 12a from 0.75 wt% of its clear solution (pH 7.2) by adjusting the pH value to 4.2 (Figure 2C). Upon the addition of 20.0 U/mL of PAP into the soft hydrogel of 1.5 wt% of D-10a and 0.75 wt% of 12a for 12 hours, respectively, the formation of new hydrogelators D-10b and 12b produce more stable hydrogels as revealed by the TEM images and viscoelastic properties of the hydrogels. Although the concentration of PAP we used in hydrogel preparation is 20 U/ml, the concentration of PAP in human semen is much higher, which has an average value of 600±357 U/ml and is in the range of 144 to 1510 U/ml. Besides, vaginal mucosa also secretes large amount of acid phosphatase. Therefore, the gelation time of 12a should be greatly shortened in actual application, though further clinical studies are required.[24] As shown in the Figure 2A and 2C, the hydrogels of D-10a and 12a consist of long uniform nanofibers with average width of 8±2 nm and 8±2 nm, respectively, while the corresponding hydrogels of D-10b and of 12b comprise of uniform nanofibers with average width of 10±2nm and 11±2nm (Figure 2B and 2D). As shown in Figure 2E and 2F, all these hydrogels exhibit relatively large values of storage moduli (G'), which are at least five times higher than loss moduli (G”). Together with weak frequency dependence of the elasticity, the results indicate solid-like properties of these hydrogels. Being treated by PAP, although the critical strain of hydrogels have been partially reduced (Table S1), the hydrogel of D-10b exhibits almost 400 times higher value of G' than that of the hydrogel of D-10a, and the hydrogel of 12b exhibits almost 150 times higher value of G' than that of the hydrogel of 12a, which confirm that the elasticity of the hydrogels increase drastically after being treated by PAP. Table S1 summarizes the gelation properties of the hydrogelators and the storage moduli of the corresponding hydrogels.
Figure 2.
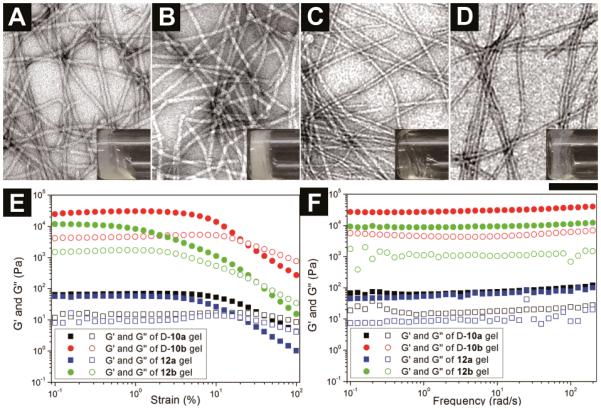
Optical images and transmission electron micrograph (TEM) of the hydrogels of (A) D-10a (1.5 wt%, pH 4.2), (B) D-10b, (1.5 wt%, pH 4.2, 20U/ml PAP) (C) 12a (0.75 wt%, pH 4.2), and (D) 12b, (0.75 wt%, pH 4.2, 20U/ml PAP) (scale bar is 100 nm). The strain (E) and frequency (F) dependence of dynamic storage modulus G' and loss modulus G" of these hydrogels.
For the purpose of evaluating the sustained release of the anti-HIV drugs from the hydrogels, we incubated the hydrogels formed by the treatment of phosphatase in a PBS buffer solution (pH 7.4) at 37 °C for 24 hours. Figure 3A shows the release of 1 from the 3TC-containing hydrogels. After 24 hours, the hydrogels of L-10b and D-10b release 52.7% and 49.6% of 3TC, respectively. The PAP induced hydrogel of D-10b (pH 4.2) releases 3TC at a slower release rate of 38.7% after 24h. The NSAID containing hydrogel of 12b releases only 9.8% of 3TC after 24h. Figure 3B shows release profiles of AZT containing hydrogels. After 24h incubation, the hydrogel of D-11b releases 45.3% of AZT while the hydrogel L-11b releases 34.7% of AZT. We also study the rate of drug release from the combined hydrogel made of combivir and find that, interestingly, the hydrogel of combivir finally releases 24.8% of 3CT and 31.7% of AZT, which has a ratio of release rate of 0.8:1. The different release rates of the anti-HIV drugs from the hydrogels also provide a simple way that uses the ratio of different hydrogelators for tailoring the profiles of the sustained release.
Figure 3.
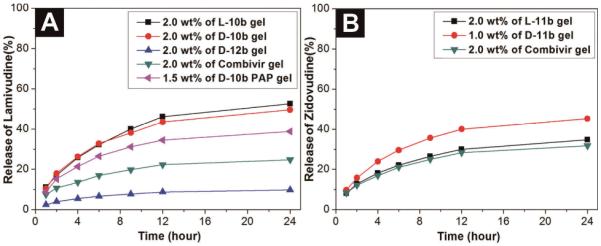
(A) The release profile of 3TC from the hydrogels formed by L-10b (2.0 wt%, pH 7.2), D-10b (2.0 wt%, pH 7.2), D-12b (2.0 wt%, pH 6.4), combivir hydrogel (2.0 wt%, pH 7.2), or D-10b (1.5 wt%, pH 4.2) for 24 hours when incubated with PBS buffer (pH 7.4) at 37 °C; (B) The release profile of AZT from the hydrogels formed by L-11b (2.0 wt%), D-11b (1.0 wt%), or combivir hydrogel (2.0 wt%) at pH 7.2 for 24 hours when incubated with PBS buffer (pH 7.4) at 37 °C.
In the interests of evaluating the efficacies of the NSAID containing hydrogels, we perform in vitro inhibition assays for both COX-1 and COX-2 enzymes. As shown in Figure 4A, naproxen (Nxp) exhibits IC50 values of 4.8 μM and 6.8 μM against COX-1 and COX-2, respectively, and 9b inhibits COX-1 at IC50 of 414.6 μM and COX-2 at IC50 of 30.9 μM. Similarly, 12a exhibits IC50 values of 147.4 μM and 37.3 μM towards COX-1 and COX-2, respectively. Both 9b and 12a exhibit significant lower activity for inhibiting COX-1, but their IC50 values towards COX-2 increase only slightly comparing to that of Nxp. The decrease of inhibition of COX-1 is beneficial because it reduces the adverse gastrointestinal and renal effects.[25] These results show that the NSAID containing hydrogels mainly preserve the inhibition ability of NSAID for COX-2 enzyme (the literature IC50 values of Nxp for COX-2 is 2.0–28.4 μM[26]) and minimize inflammation. Thus, the hydrogel of 12a self-delivers both anti-HIV and anti-inflammatory drugs. We also have measured the cytotoxicity of the anti-HIV drug containing hydrogelators against mammalian cells (e.g., HeLa cells) by MTT assay. As shown in Figure 4B, all of the hydrogelators exhibit low cytotoxicity after 72 hours. The IC50 values of L-10a and L-11a are 328.6 μM and 319.7 μM, respectively. Hydrogelators D-10a, D-11a, and 12a have IC50 values higher than 500 μM. These results suggest that the designed hydrogelators are biocompatible for making self-delivery soft materials, especially for the hydrogelators made of D-amino acids.
Figure 4.
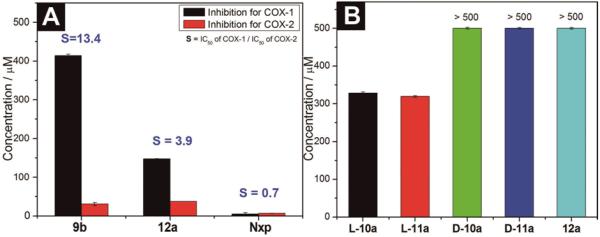
(A) IC50 of COX enzymes inhibition tests of hydrogelator 12a and the control (Nxp). (B) IC50 of the hydrogelators for inhibiting mammalian cells (HeLa).
3. Conclusion
In conclusion, we have designed a new class of hydrogelators that can afford stable hydrogels upon the treatment of prostatic acid phosphatase and self-deliver anti-HIV prodrugs. Furthermore, the use of NSAID containing self-assembly motif that mainly preserve and increase non-steroid anti-inflammatory drug efficacy renders this hydrogel to be both anti-inflammatory and anti-HIV. Moreover, the success of enzymatic formation of the anti-HIV hydrogel at the pH 7.2 (the physiological pH) also extends the applications of these precursors of hydrogels for other organs. This approach should find applications for developing other multifunctional supramolecular hydrogels as new formulations of antiviral therapeutics.
Supplementary Material
Acknowledgements
This work was partially supported by NIH (R01CA14274), a HFSP grant (RGP 0056/2008), and start-up grant from Brandeis University. The TEM images were taken at Brandeis EM facilities.
Footnotes
Supporting Information Supporting Information is available online from the Wiley Online Library or from the author.
References
- [1].WHO . World Health Organization. 2011. [Google Scholar]
- [2].Rappuoli R, Aderem A. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- [3].O'Keefe BR. Future Virol. 2011;6:553–556. [Google Scholar]
- [4].a) Malkovsky M, Newell A, Dalgleish AG. Lancet. 1988;1:645–645. doi: 10.1016/s0140-6736(88)91440-7. [DOI] [PubMed] [Google Scholar]; b) Balzarini J, Naesens L, Verbeken E, Laga M, Van Damme L, Parniak M, Van Mellaert L, Anne J, De Clercq E. Aids. 1998;12:1129–1138. doi: 10.1097/00002030-199810000-00004. [DOI] [PubMed] [Google Scholar]; c) D'Cruz OJ, Uckun FM. Contraception. 2001;64:113–123. doi: 10.1016/s0010-7824(01)00233-5. [DOI] [PubMed] [Google Scholar]
- [5].Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, Grp CT. Science. 2010;329:1168–1174. [Google Scholar]
- [6].Pirrone V, Wigdahl B, Krebs FC. Antivir Res. 2011;90:168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- [7].Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, Masse B, Eshleman SH, Hendrix C, Morrow K, Rooney JF, Soto-Torres L, Team HP. Aids. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- [8].Rohan LC, Moncla BJ, Ayudhya R, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. Plos One. 2010;5 doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anonymous Science. 2011;334:1186–1187. [Google Scholar]
- [10].Yang ZM, Xu B. J. Mater. Chem. 2007;17:2385–2393. [Google Scholar]
- [11].Estroff LA, Hamilton AD. Chem. Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- [12].a) Yang Z, Kuang Y, Li X, Zhou N, Zhang Y, Xu B. Chemical Communications. 2012;48:9257–9259. doi: 10.1039/c2cc34935c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Boekhoven J, Koot M, Wezendonk TA, Eelkema R, van Esch JH. J. Am. Chem. Soc. 2012;134:12908–12911. doi: 10.1021/ja3051876. [DOI] [PubMed] [Google Scholar]; c) Langer R, Tirrell DA. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]; d) Sangeetha NM, Maitra U. Chem. Soc. Rev. 2005;34:821–836. doi: 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]; e) van Bommel KJC, Friggeri A, Shinkai S. Angew. Chem.-Int. Edit. 2003;42:980–999. doi: 10.1002/anie.200390284. [DOI] [PubMed] [Google Scholar]; f) Yoshimura I, Miyahara Y, Kasagi N, Yamane H, Ojida A, Hamachi I. J. Am. Chem. Soc. 2004;126:12204–12205. doi: 10.1021/ja045962a. [DOI] [PubMed] [Google Scholar]; g) Zhao F, Ma ML, Xu B. Chemical Society Reviews. 2009;38:883–891. doi: 10.1039/b806410p. [DOI] [PubMed] [Google Scholar]
- [13].Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- [14].Shah RN, Shah NA, Lim MMD, Hsieh C, Nuber G, Stupp SI. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J Am Chem Soc. 2009;131:13576. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
- [16].Valery C, Paternostre M, Robert B, Gulik-Krzywicki T, Narayanan T, Dedieu JC, Keller G, Torres ML, Cherif-Cheikh R, Calvo P, Artzner F. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10258–10262. doi: 10.1073/pnas.1730609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marques MRC, Loebenberg R, Almukainzi M. Dissolut. Technol. 2011;18:15–28. [Google Scholar]
- [18].Li JY, Kuang Y, Gao Y, Du XW, Shi JF, Xu B. J. Am. Chem. Soc. 2013;2:542–545. doi: 10.1021/ja310019x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mesquita PMM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. J Infect Dis. 2009;200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) supporting information; Lai BE, Geonnotti AR, DeSoto MG, Montefiori DC, Katz DF. Antivir Res. 2010;88:143–151. doi: 10.1016/j.antiviral.2010.08.006.
- [21].Brunton L, Lazo J, Parker K, Buxton I, Blumenthal D. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw Hill; New York: 2006. [Google Scholar]
- [22].Yang ZM, Liang GL, Wang L, Xu B. J. Am. Chem. Soc. 2006;128:3038–3043. doi: 10.1021/ja057412y. [DOI] [PubMed] [Google Scholar]
- [23].Podzamczer D, Ferrer E, Consiglio E, Gatell JM, Perez P, Perez JL, Luna E, Gonzalez A, Pedrol E, Lozano L, Ocana I, Llibre JM, Casiro A, Aranda M, Barrufet P, Martinez-Lacasa J, Miro JM, Badia X, Casado A, Lupo S, Cahn P, Manos M, Estela J, Combine Study T. Antivir. Ther. 2002;7:81–90. [PubMed] [Google Scholar]
- [24].Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Environ. Health Perspect. 2000;108:45. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agarwal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. JAMA-J. Am. Med. Assoc. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- [26].a) Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J, Chan H, Sigal E, Ramesha C. Biochim. Biophys. Acta-Protein Struct. Molec. Enzym. 1994;1209:130–139. doi: 10.1016/0167-4838(94)90148-1. [DOI] [PubMed] [Google Scholar]; b) Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. J. Pharmacol. Exp. Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- [27].Johnston R. Aids Patient Care and Stds. 2002;16:419–430. doi: 10.1089/108729102760330263. [DOI] [PubMed] [Google Scholar]
- [28].Chimalakonda KC, Agarwal HK, Kumar A, Parang K, Mehvar R. Bioconjugate Chem. 2007;18:2097–2108. doi: 10.1021/bc700193d. [DOI] [PubMed] [Google Scholar]
- [29].Giammona G, Cavallaro G, Fontana G, Pitarresi G, Carlisi B. J. Control. Release. 1998;54:321–331. doi: 10.1016/s0168-3659(98)00020-0. [DOI] [PubMed] [Google Scholar]
- [30].a) Cheong E, Ivory K, Doleman J, Parker ML, Rhodes M, Johnson IT. Carcinogenesis. 2004;25:1945–1952. doi: 10.1093/carcin/bgh184. [DOI] [PubMed] [Google Scholar]; b) Cordero JA, Camacho M, Obach R, Domenech J, Vila L. Eur. J. Pharm. Biopharm. 2001;51:135–142. doi: 10.1016/s0939-6411(00)00149-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


