Abstract
Insulin signaling regulates lifespan, reproduction, metabolic homeostasis, and resistance to stress in the adult organism. In Drosophila, there are seven insulin-like peptides (DILP1–7). Three of these (DILP2, 3 and 5) are produced in median neurosecretory cells of the brain, designated IPCs. Previous work has suggested that production or release of DILPs in IPCs can be regulated by a factor secreted from the fat body as well as by neuronal GABA or short neuropeptide F. There is also evidence that serotonergic neurons may regulate IPCs. Here, we investigated mechanisms by which serotonin may regulate the IPCs. We show that the IPCs in adult flies express the 5-HT1A, but not the 5-HT1B or 5-HT7 receptors, and that processes of serotonergic neurons impinge on the IPC branches. Knockdown of 5-HT1A in IPCs by targeted RNA interference (RNAi) leads to increased sensitivity to heat, prolonged recovery after cold knockdown and decreased resistance to starvation. Lipid metabolism is also affected, but no effect on growth was seen. Furthermore, we show that DILP2-immunolevels in IPCs increase after 5-HT1A knockdown; this is accentuated by starvation. Heterozygous 5-HT1A mutant flies display the same phenotype in all assays, as seen after targeted 5-HT1A RNAi, and flies fed the 5-HT1A antagonist WAY100635 display reduced lifespan at starvation. Our findings suggest that serotonin acts on brain IPCs via the 5-HT1A receptor, thereby affecting their activity and probably insulin signaling. Thus, we have identified a second inhibitory pathway regulating IPC activity in the Drosophila brain.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-011-0789-0) contains supplementary material, which is available to authorized users.
Keywords: 5-hydroxytryptamine, Insulin signaling, G-protein-coupled receptor, Lifespan, Stress resistance
Introduction
Insulin-like peptides regulate growth, reproduction, metabolism and lifespan in both invertebrates and mammals [1–7]. In Drosophila, seven insulin-like peptides (DILPs) and a single insulin receptor have been identified [2, 8–10]. DILP signaling in adult fruitflies is important in the regulation of metabolic homeostasis, resistance to stress of different kinds and regulation of lifespan [3, 11–14]. Three of the DILPs (DILP2, 3 and 5) are produced by a set of median neurosecretory cells in the brain of Drosophila and are thought to be released into the circulation from axon terminals in neurohemal areas in the corpora cardiaca and anterior aorta [2, 15, 16]. In experiments where the insulin-producing cells (IPCs) in the brain were genetically ablated, it was shown that growth was retarded, lifespan extended, levels of carbohydrate increased in the circulation, storage of lipids increased and resistance to various stresses increased [11, 16]. Also, mutations in genes encoding DILP2, 3 and 5 produce these phenotypes, in addition to defects in growth and fertility [9, 14]. Thus, DILP2, 3 and 5 display pleiotropic functions, and experiments to reveal the roles of individual DILPs by targeted mutations suggested partly redundant functions of the three peptides [9, 17]. It is known, however, that the production of the three DILPs in the IPCs can be individually regulated [13, 17, 18].
We are interested in factors regulating the production and release of DILPs from the IPCs in the adult Drosophila brain. In addition to circulating nutritional signals derived from the fat body [19], it has been suggested that a brain-derived short neuropeptide F (sNPF) may stimulate DILP production in IPCs as well as feeding and growth [20, 21]. Another neurotransmitter that seems to stimulate signaling in the IPCs is octopamine [22]. In this study, the IPCs were found to be involved in regulation of sleep-wakefulness, and under stimulatory control of octopamine via the OAMB receptor. Recently, GABA and its metabotropic GABAB receptor were shown to inhibit IPCs and insulin signaling at metabolic stress, but seemed not to affect growth [23]. A fourth neurotransmitter has been implicated in regulation of insulin signaling, the monoamine serotonin [24]. This study demonstrates that the GTPase nucleostemin 3 (NS3) in serotonergic neurons is required for normal growth of Drosophila and that it regulates serotonin levels. Feeding flies the precursor of serotonin, 5-Hydroxytryptophan (5-HTP) mimicks the developmental delay seen in the ns3 mutant flies. Since ns3 mutants feed normally, the developmental effect of NS3 and serotonin was sought in a pathway known to regulate growth, the insulin signaling. It was found that the ns3 mutants have increased levels of DILP2 protein, but not dilp2 transcript and that this may be associated with a decrease of DILP2 release since insulin signaling was reduced [24]. Thus, it was concluded that serotonin-producing neurons, which were found to have axon terminations close to the IPCs, regulate release of DILPs and thus growth. However, the specific receptor type mediating the serotonergic signaling to the IPCs was not determined and a direct action of serotonin on IPCs was not established.
There are four different serotonin receptors in Drosophila, designated 5-HT1ADRO, 5-HT1BDRO, 5-HT2DRO, and 5-HT7DRO, all of which are G-protein-coupled receptors (GPCRs) [25–28]. We henceforth refer to these receptors without the DRO suffix. Of these, 5-HT1A and 5-HT1B are known to inhibit adenylate cyclase and 5-HT7 stimulates it, whereas 5-HT2 activation has not yet been investigated in Drosophila (reviewed in [25, 28]). Specific roles of the 5-HT1A, 5-HT1B and 5HT2 receptors in sleep and circadian activity of Drosophila has been investigated [29–31]. Furthermore, it has been shown that the 5-HT1A and 5-HT2 receptors each modulate aggressive behavior [32] and that 5-HT7 is required for normal courtship behavior [33]. Other studies of serotonergic signaling in Drosophila have not specified the receptor type, but indicate pleiotropic roles of this neurotransmitter both in development, physiological processes and specific behaviors (see [34–39]). Here, we undertook a study to determine which of the serotonin receptors mediate the regulation of insulin signaling from the brain IPCs of Drosophila.
Using a 5-HT1A promoter Gal4 line combined with antiserum to DILP2, we could show that all the brain IPCs in the adult fly express this receptor, whereas Gal4 drivers for the other serotonin receptors did not display expression in any of the IPCs. Using a Dilp2-Gal4 line to drive UAS-mediated RNA interference with 5-HT1A expression in the IPCs, we found that insulin signaling increased with reduced receptor levels. Thus, flies with reduced 5-HT1A in IPCs are less resistant to starvation and heat knockdown, and display delayed recovery from cold coma. Also, the levels of DILP-immunofluoroescence in the IPCs and body lipid are affected by receptor knockdown. Similar results were obtained for 5-HT1A mutant flies, and after feeding flies a 5-HT1A antagonist. Knockdown of 5-HT1A had no clear effect on growth of the flies, however. Our data therefore strongly suggest that the serotonin signaling to the neurosecretory cells producing insulin-like peptides is mediated mainly by the 5-HT1A receptor and that this signaling may convey stress signals. However, it is not clear whether all the effects of 5-HT1A knockdown that are shown can be attributed to increased insulin signaling, or if some effects are caused by other signaling from the IPCs.
Materials and methods
Fly strains
Adult white-eyed flies Drosophila melanogaster (w 1118 strain) were used for some immunocytochemistry and control experiments. For some experiments, early and late third instar larvae were utilized. All flies were kept at 25°C on a 12:12 h light/dark cycle and maintained on a diet of standard Drosophila medium.
The following Gal4 lines were used to drive the expression of green fluorescent protein (GFP) and for crosses to induce RNA interference (RNAi): TRH-Gal4 [34] (a gift from O.V. Alekseyenko and E. Kravitz, Boston, MA), 5-HT 7-Gal4 was generated and characterized as described in [33], 5-HT 1A-Gal4 is described below, 5-HT 1B-Gal4 (a gift from A. Sehgal, Philadelphia, PA, USA), Dilp2-Gal4 (2nd chromosome) [40] (a gift from P. Shen, Athens, GA, USA), Dilp2-Gal4 (3rd chromosome) [18] (a gift from S. Broughton, Univ. Lancaster, UK). OK107-Gal4 [41] and Act5c-Gal4 were from The Bloomington Drosophila Stock Center (BDSC) at Indiana University, Bloomington, IN, USA. Two different UAS-5-HT1A-RNAi lines were used in the experiments: one from the Vienna Drosophila RNAi Center (VDRC) and the other from BDSC. UAS-5-HT7 [42] (a gift from J.A.T. Dow, Glasgow, UK) was used to ectopically express the receptor. UAS-mcd8-gfp or UAS-s65t-gfp flies from (BDSC) were used to visualize Gal4 expression. A 5-HT1A mutant generated by imprecise P element excission was obtained from BDSC (stock number 27640). This mutant was generated and characterized by [30]. The genotype of this mutant is w*; 5-HT1AΔ5kb/CyO, P{ActGFP}JMR1.
Preparation of 5-HT1ADro promoter region
Genomic DNA from adult Canton-S flies was prepared as previously described [33]. To isolate putative 5′ enhancer regions, which are normally contained within the first few kb of genomic DNA upstream of the RNA transcription start site, 5 kb of genomic DNA immediately upstream of the ATG start codon within the 5-HT1ADro locus was amplified from genomic DNA using Platinum Pfx DNA Polymerase (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions (Fig. 1). Primers corresponding to the 5-HT1ADro promoter region containing Not I restriction sites at their 5′ end were ordered from Integrated DNA Technologies (Coralville, IA, USA). Forward primer = 5′-gcggccgcATGGCCCAAGTATCAGGAATCTGC-3′; reverse primer = 5′- gcggccgcAAGATGCGAATGTACGTCCAGTTG-3′; annealing T = 62.0°C, elongation T = 68°C. The amplification product consisted of a single band of 5 kb, which was gel purified using the Wizard SV gel cleanup system (Promega, Madison, WI, USA) following the manufacturer’s instructions.
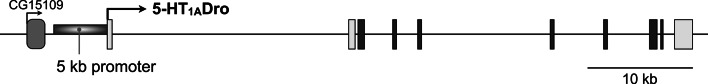
Fig. 1.
The 5-HT1ADro receptor locus and 5 kb region used for Gal4 construct. The genomic region of the 5-HT1ADro locus (CG16720) is on the right arm of the second chromosome (light bars 5′ and 3′ untranslated regions, dark bars coding regions; arrows indicate mRNA transcription start sites). The 5-kb region of genomic DNA used to make the GAL4 construct is indicated immediately upstream of the mRNA start site. The adjacent CG15109 locus is 7 kb upstream of the first exon of the 5-HT1ADro gene. The CG15109 transcript has been reported to be exclusively expressed in the male testis
Generation of the 5-HT1A construct and transgenic flies
Both the purified 5-HT1A PCR product and the pERGP P-element insertion vector [33] were digested with Not I and gel purified. Digested pERGP was dephosphorylated using Apex Heat-Labile Alkaline Phosphatase (Epicentre, Madison, WI, USA) following the manufacturer’s directions. The 5-HT1A promoter fragment was ligated into the Not I site of the pERGP vector using the Fast-Link DNA Ligation Kit (Epicentre) following the manufacturer’s directions. The final construct was verified using a panel of restriction enzymes, as well by sequence analysis of the cloning site junctions. Transgenic lines were generated from this final product using the services of BestGene (Chino Hills, CA, USA).
Antisera and immunocytochemistry
For immunocytochemistry, adult Drosophila heads or central nervous systems (CNS) of third instar larvae were dissected in 0.01 M phosphate-buffered saline with 0.25% Triton X-100, pH 7.2 (PBS-Tx) and fixed in ice-cold 4% paraformaldehyde in 0.1 M sodium phosphate buffer pH 7.4 (PB) for 2–4 h. Following rinsing with 0.1 M PB, adult brains or larval CNS were either dissected out for whole-mount immunocytochemistry or whole heads were incubated overnight in 20% sucrose in 0.1 M PB at 4°C as cryoprotection. Cryostat sections (50 μm thick) of the heads were cut on a cryostat at −23°C.
Incubation with primary antiserum for whole-mount tissues was performed for 72 h, while sections were incubated overnight, both at 4°C. The following primary antisera were used: a rabbit antiserum to Drosophila insulin-like peptide 2 (anti-DILP2) [15], known to recognize Drosophila insulin-producing cells at a dilution of 1:4,000. A monoclonal mouse antibody to serotonin (Clone 5HT-H209; Dako, Copenhagen, Denmark) was used at a dilution of 1:80. An antiserum raised to a peptide (from the third intracellular loop) of the honeybee 5-HT1A receptor [43], kindly donated by M. Thamm and W. Blenau (Potsdam, Germany), was tested at a dilution of 1:1,000. For detection of primary antisera, Cy3-tagged goat anti-rabbit antiserum (Jackson Immuno Research) and Alexa goat anti-mouse 488 were used at a dilution of 1:1,000. Tissues or sections were rinsed thoroughly with PBS-Tx, followed by a final wash in PBS and then mounted in 80% glycerol in PBS. For each experiment, at least 10 adult brains and 5 larval CNS were analyzed.
5-HT1A antagonist
To inactivate the 5-HT1A receptors in the adult flies, we used the 5-HT1A antagonist WAY100635 {N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt; Sigma, St Louis, MO, USA}. We dissolved 5 mg WAY100635 in a solution of 0.25 g agarose and 50 ml Milli-Q water, which yielded 0.18 mM (0.1 g/L). This agarose/antagonist solution was put in glass tubes in 0.5-ml aliquots for starvation tests (see below).
Stress assays
Male flies, 4–6 days old, were used for starvation assays. All flies were kept in an incubator at 25°C with 12:12 h light:dark (LD) conditions, and controlled humidity. For the starvation experiments, flies were placed individually in 2-ml glass vials with 500 μl of 0.5% aqueous agarose. The vials were checked for dead flies every 12 h. These starvation experiments were run in three replicates with at least 40 flies of each genotype per replicate.
For the heat knockdown and chill coma recovery experiments, newly emerged adults were collected, sexed under light CO2 anesthesia, and sorted into groups of 20–30 males in fresh vials with Drosophila medium. Male flies were used at 4–5 days of age, and were not anaesthetized subsequent to the initial sorting. For the heat knockdown experiments, groups of 20–30 males were placed in glass vials (without food), which were completely immersed in a temperature-regulated water bath to induce heat knockdown. Time to knockdown was measured for flies exposed to 39°C at 5-min intervals.
For the chill coma recovery experiments, groups of 20–30 males were placed in glass vials (without food), which were put into a box full of ice inside a refrigerator (2°C) to induce chill coma. The flies were exposed to the environmental stress for 4 h and the recovery at room temperature was recorded in food vials at 5-min intervals.
Lipid measurements
Lipid content was measured in flies of different genotypes after 0, 12 and 24 h of starvation. The lipid content was determined according to the method of Service [44]. Groups of five male flies were weighed on a Mettler MT5 Microbalance (Mettler Toledo, Switzerland) to obtain wet weight and subsequently dried at 65°C for 24 h. Flies were then weighed again to obtain dry weight. Lipids were extracted by placing intact dry flies in glass vials containing diethyl ether for 24 h with gentle agitation at room temperature. The diethyl ether was removed and flies were dried for another 24 h and then weighed to obtain lean dry weight. The difference between dry weight and lean dry weight was considered the total lipid content of the flies. Totals of 40 flies of each genotype were tested in three replicates.
Image analysis
Specimens were imaged with Zeiss LSM 510 META confocal microscope (Jena, Germany) using ×20 or ×40 oil immersion objectives and a Leica TCS-SP2 confocal microscope (Buffalo Grove, IL, USA) using a ×20 dry or ×63 water immersion objectives. Confocal images were obtained at an optical section thickness of 0.2–0.5 μm and were processed with Zeiss LSM or Leica software. Images were edited for contrast and brightness in Adobe Photoshop CS3 Extended version 10.0.
Quantification of immunofluorescence
Immunocytochemistry with DILP2 antiserum was performed on adult brains from starved and fed flies of different genotypes for quantification of immunofluorescence in IPCs. The brains were imaged in a Zeiss LSM 510 confocal microscope with fixed exposure time, using LSM software. The immunofluorescence was quantified in each cell, using Image J 1.40 from NHI, Bethesda, MD, USA (http://rsb.info.nih.gov/ij/).
Statistical analysis
All statistical analyses were performed by using Prism GraphPad 6.0. Survival data were analyzed by Log rank test with Mantel–Cox post-test, for quantification of immunofluorescence, lipid values and body weights we used one-way ANOVA with Tukeys comparison or two-way ANOVA depending on analysis (see Figure legends for details). Data are presented as means ± SEM.
Results
5-HT1A is expressed in brain insulin producing cells
A cluster of 16 insulin-producing cells (IPCs) in pars intercerebralis of the Drosophila brain produce DILP2, 3 and 5 [2, 15, 18]. These IPCs have arborizations in three regions of the brain: branches extending laterally in dorsal protocerebrum, shorter branches along the initial IPC neurites (Fig. 2a), and extensive arborizations in the tritocerebrum. The main IPC axons terminate in neurohemal areas of the corpora cardiaca, in the anterior aorta and in the anterior intestine and crop [15, 23, 45]. To investigate which of the 5-HT receptors is expressed on the IPCs, we utilized different 5-HT receptor Gal4 lines to drive GFP expression in combination with DILP2 immunocytochemistry. An earlier account on 5-HT2 distribution, using Gal4-driven LacZ expression, described no neurons in the median neurosecretory cell group [31].
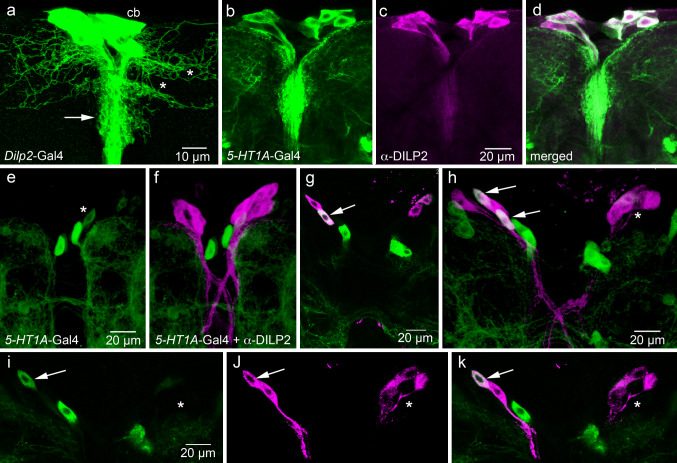
Fig. 2.
Expression of 5-HT1A receptor on IPCs in the Drosophila brain. All images of adult brains are frontal views (dorsal is up) and of larval brain horizontal views (anterior is up). a Anatomy of the IPCs seen with Dilp2-Gal4-driven GFP (projection of optic sections). A cluster of 16 cell bodies (cb) give rise to axons with branches in two regions of the pars intercerebralis: wide dorsal lateral branches (asterisks) and shorter median branches (arrow). b–d In the adult brain, the 5-HT1A-Gal4 driver is expressed in a set of median neurosecretory cells (green), most of which also express DILP2-immunolabeling (magenta). In the merged image (d), it can be seen that all but one of the 5-HT1A-expressing cells display DILP2 immunolabeling (whitish). e, f In the feeding third instar larval brain, there is no 5-HT1A expression in the DILP2-immunolabeled IPCs (projection of several optic sections). The weakly labeled cell body in (e) (asterisk) does not express DILP2. g In the wandering (non-feeding) third instar larva, 5-HT1A expression starts. Here, one IPC (arrow) coexpresses the receptor and DILP2. h In another specimen of the same age, two IPCs on the left side coexpress receptor and DILP2, but no cells on the right (asterisk). This image is a projection of several optic sections. i–k A single optic section of the same specimen showing colocalization of markers in one cell body (arrow) to the left and none on the right (asterisk)
For this study, a 5-HT 1A-Gal4 line was produced as detailed in “Materials and methods”. A promoter fragment consisting of 5 kb of genomic DNA immediately upstream of the ATG start codon within the 5-HT1A locus was used for this construct (Fig. 1). This Gal4 was used for driving UAS-cd8-GFP or UAS-s65t-GFP to display the neuronal expression. Our attempts to raise antisera to a sequence of the Drosophila 5-HT1A protein were unsuccessful, and our tests of antiserum to 5-HT1A of the honey bee [43] did not result in any specific immunolabeling in the fruitfly. Therefore, it was necessary to resort to comparing the distribution of 5-HT 1A-Gal4 expression to that of serotonin. As seen in Supporting material (S Fig. 1), there is a good correlation between 5-HT 1A-Gal4 expression and neuronal processes displaying serotonin immunoreactivity both in the adult and larval CNS.
Significantly, in adult flies, we found the 5-HT 1A-Gal4 to be expressed in median neurosecretory cells (MNCs) similar in location and morphology to the IPCs (Fig. 2b). Application of antiserum against DILP2 to the GFP-labeled brains revealed that most, if not all, of the DILP2-immunolabeled cells also display 5-HT1A-Gal4 expression (Fig. 2b–d). Some additional neurons in the same region express 5-HT1A, but do not display DILP immunoreactivity. These neurons are just adjacent to the IPCs and likely to be other MNCs or interneurons (Fig. 2d). It can be noted that antiserum to the 5-HT1 receptor of the cockroach Periplaneta americana labeled MNCs of the cockroach brain with axons extending to the corpora cardiaca, similar to the Drosophila neurons expressing 5-HT1A and DILPs [46]. In the cockroach, it remains to be determined if these MNCs also express insulin-like peptide.
We also examined 5-HT1A expression in relation to IPCs in larval brains. Surprisingly, we found no 5-HT 1A-Gal4-driven GFP in IPCs of feeding third instar larvae (Fig. 2e, f). Analysis of non-feeding late third instar larvae revealed 5-HT1A expression in one or two of the 16 IPCs in some, but not all, specimens (Fig. 2g–k). Thus, it appears as if the majority of the IPCs start expressing the 5-HT1A receptor only after completion of the larval stages.
Similar analysis of 5-HT1B and 5-HT7, where we used promoter Gal4 drivers combined with anti-DILP2 labeling, revealed that these receptors are not expressed on IPCs in larvae or adults (S Fig. 2). Also, the original reports on 5-HT1B and 5-HT7 distribution show no expression of these receptors in IPCs [29, 33]. Therefore, our findings suggest that only the 5-HT1A is expressed on Drosophila IPCs and that onset of expression occurs late in non-feeding larvae or in pupae.
Processes from serotonergic neurons superimpose IPC branches
In recent papers, it was shown that serotonin-immunoreactive neuron processes impinge on IPC branches in the larval brain [24, 47]. However, these studies provided no information on serotonin distribution in relation to IPCs in the adult brain. Here, we applied a monoclonal antibody to serotonin to brains with IPCs marked by Dilp2-Gal4-driven GFP. In the adult brain, serotonergic neuron branches can be found close to those of the IPCs in the pars intercerebralis (Fig. 3). The superposition between branches is seen in the dorsolateral area as well as in the median region along initial IPC neurites. The individual serotonin-immunoreactive neurons that supply branches to the IPC dendrites were not identified in the adult brain due to the dense packing of neuronal processes. In third instar larvae, serotonin-immunoreactive neuron processes also superimpose branches of the IPCs (not shown; see [24, 47]).
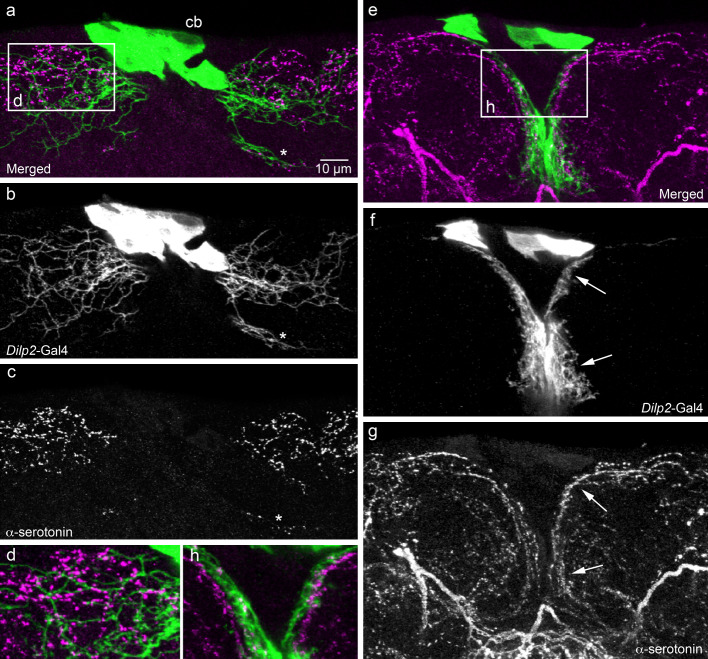
Fig. 3.
Relationships between processes of IPCs and serotonin-immunoreactive neurons in the adult brain. We utilized the Dilp2-Gal4 to drive GFP in IPCs (green) and a mouse monoclonal antibody to serotonin (magenta) to visualize relationships between the two neuron types in the pars intercerebralis (shown in frontal view; dorsal up). a–c IPCs at the level with wide lateral branches dorsally. These IPC branches superimpose those of varicose serotonin immunoreactive ones (even the small set of branches more ventrally, at asterisk). d The framed area in (a) is shown at higher magnification. e–g The IPCs at the level of the median short branches (other specimen). Again, the branches of the two neuron types superimpose (e.g., at arrows). h The framed area in (e) is shown at higher magnification
Knockdown of 5-HT1A causes elevation of DILP2 levels in IPCs
It was shown in an earlier report that manipulation of NS3 in serotonergic neurons affects DILP2 peptide levels in IPCs, but a direct functional connection between serotonergic neurons and IPCs was not demonstrated [24]. We therefore tested whether serotonergic regulation of DILP levels is directly mediated by the 5-HT1A receptor on IPCs. To obtain an estimate of DILP protein levels, we measured the intensity of DILP2 immunofluorecence in the cell bodies of the IPCs after knockdown of 5-HT1A by the transgene Dilp2-Gal4/UAS-5-HT 1A -RNAi. The Dilp2-Gal4 driver used here and in all but one (see S Fig. 3) of the experiments has the insert on the 2nd chromosome [40]. We also tested a loss of function mutation generated previously in the 5-HT1A by imprecise P-element excission [30]; used here as heterozygous mutant flies. The DILP2 levels were measured both in normally fed flies and flies that had been starved for 12 h. It was found that the DILP2-immunolabeling increased in the IPCs of flies with diminished 5-HT1A, both globally and in IPCs only, and that starvation further increased DILP2 (Fig. 4). Since 5-HT1A is likely to act as an inhibitory receptor, the insulin increase in the IPCs after knockdown could be explained by diminished inhibitory signaling to these cells. A very similar increase in DILP2 levels were seen after knockdown of the inhibitory GABAB receptor on IPCs [23] and in the study of the NS3 mutant (reversed by ns3 rescue in serotonergic neurons) [24]. The increase of DILP2 both in fed and starved flies with diminished 5-HT1A, also suggest some regulation of IPCs under non-stress conditions.
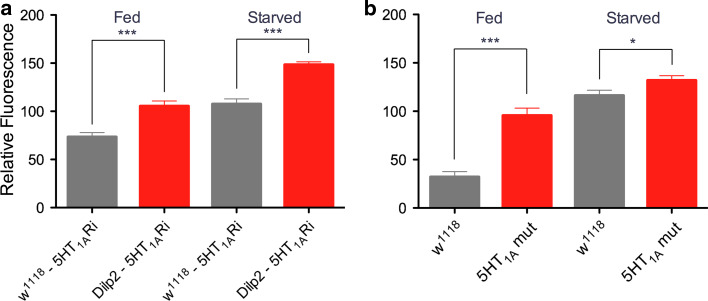
Fig. 4.
Knockdown of 5-HT1A leads to an increase in DILP-immunolabeling in insulin-producing cells (IPCs). a Relative DILP immunofluorescence in IPCs in fed and starved flies with and without 5-HT1A receptor knockdown (5-HT1ARi) in IPCs (Dilp2-Gal4/UAS-5-HT 1A-RNAi). The DILP2 antiserum used is likely to cross react with DILP2, 3 and 5. Control flies (w1118-5-HT1A) display significantly lower levels of DILP-immunofluorescence than the flies with diminished 5-HT1A (Dilp2-5-HT1ARi), both in fed flies (***p < 0.001; One-way ANOVA with Tukey’s comparison) and after starvation (***p < 0.001). Also, the increases of fluorescence in controls (gray bars) and knockdown flies (red bars) when comparing fed and starved flies are significant (p < 0.001 for both). For each genotype and condition, IPCs of 5–7 brains were investigated. b Relative DILP immunofluorescence in IPCs in fed and starved mutant (5-HT1A mut) and wild type (w1118) flies. Wild-type flies display significantly lower levels of DILP-immunofluorescence than the 5-HT1A mutant flies, both in fed flies (***p < 0.001) and starved ones (*p < 0.05). Again, the increases in immunolabeling in fed and starved controls and fed and starved mutants are significant (p < 0.001 for both). IPCs of 7–10 brains of each genotype and condition were investigated
Knockdown of 5-HT1A in IPCs and globally causes decreased resistance to starvation
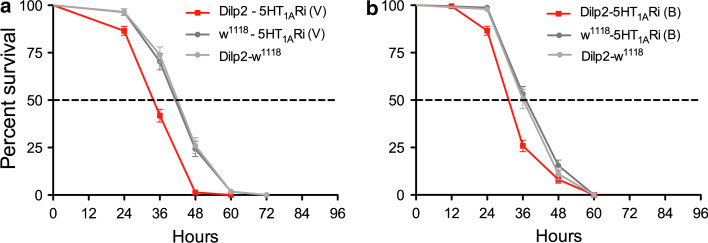
It is known that flies with decreased insulin signaling display an increased resistance to starvation [11], and a recent study demonstrated that knockdown of the inhibitory GABAB receptor on IPCs decreased this resistance [23]. Thus, we tested whether the knockdown of 5-HT1A on IPCs affect survival at starvation. In all experiments described in this paper, we used only adult (4–6 days old) male flies, unless otherwise specified. Statistical data are given in figure legends. Flies were kept on aqueous agarose, and it was found that flies with 5-HT1A-RNAi driven by the Dilp2-Gal4 display a significantly reduced median and maximal lifespan compared to parental controls (Fig. 5). We tested two different 5-HT1A-RNAi lines with the same result (Fig. 5). As a control, we also utilized a different Dilp2-Gal4 driver (insert on 3rd chromosome; [18]) crossed with one of the RNAi lines, and obtained the same phenotype at starvation (S Fig. 3). In all the following experiments, we utilized the Dilp2-Gal4 inserted on the 2nd chromosome.
Fig. 5.
Knockdown of 5-HT1A receptor in IPCs increases sensitivity to starvation. We performed GABAB 5-HT1A receptor knockdown in IPCs with two different RNAi constructs, one from VDRC [5-HT1ARi(V)] (a) and another from Bloomington Stock Center [5-HT1ARi (B)] (b). These flies were crossed to a Dilp2-Gal4 driver [40] used in all experiments, unless other specified. Male flies were kept on aqueous agarose (to induce starvation) and their survival monitored over time. All experiments were run in three replicates. a Using a Dilp2-Gal4 driver to knockdown the 5-HT1A receptor in IPCs [Dilp2-5-HT1ARi (V)], we obtained flies that display significantly reduced survival at starvation (p < 0.0001 to both parental controls, Log rank test, Mantel-Cox; n = 111–215 for each genotype). b Flies obtained from crossing Dilp2-Gal4 with the other strain 5-HT1ARi (B) also displayed significantly reduced survival at starvation (p < 0.0001 and p = 0.0002 to the two controls; n = 159–209 for each genotype). In all subsequent graphs with 5-HT1ARNAi, we used the (V) strain. We also used a different Dilp2-Gal4 driver (on the 3rd chromosome) [18] to drive the UAS-5-HT1ARi(V) and obtained the same significantly reduced life span at starvation (see S. Fig. 3)
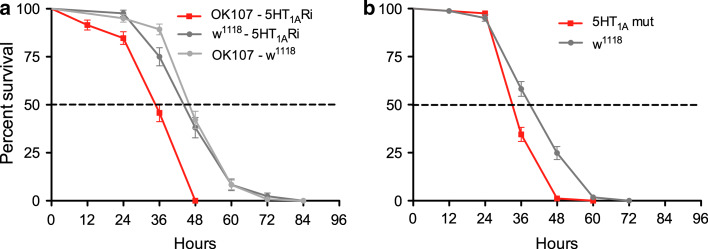
Another Gal4 driver, OK107 [41], also known to be expressed in IPCs [23], was used to drive 5-HT1A-RNAi. This cross resulted in flies with the same abbreviated lifespan at starvation (Fig. 6a). Finally, the heterozygous 5-HT1A mutant flies also displayed reduced resistance to starvation (Fig. 6b). Thus, fly crosses from three different Gal4 drivers and two 5-HT1A-RNAi lines gave the same phenotype at starvation as a 5-HT1A mutant strain. The reduced lifespan at starvation in these experiments indicates an increase in insulin signaling or other activity of IPCs, likely to be caused by loss of inhibition of the IPCs by diminished 5-HT1A expression.
Fig. 6.
Knockdown of 5-HT1A receptor in IPCs or globally increases sensitivity to starvation. Using the same experimental conditions, we tested lifespan at starvation with another Gal4 driver that includes the IPCs and a 5-HT1A mutant. a The enhancer trap Gal4 OK107 includes the IPCs in its expression pattern [23] and was used here to drive UAS-5-HT1ARi. The lifespan of OK107-5-HT1ARi flies is significantly reduced at starvation (p < 0.0001 to both controls, Log rank test, Mantel-Cox; n= 84–121 for each genotype, experiment run in two replicates). b Heterozygous 5-HT1A mutant flies also display significantly reduced survival at starvation compared to wild-type (w1118) and controls (p < 0.0001 to wild-type controls; n = 165 for each genotype, experiment run in three replicates)
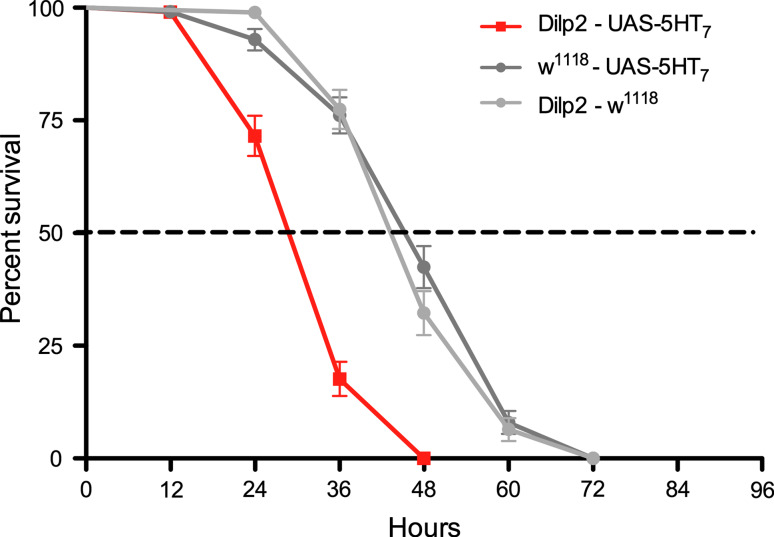
As a further test of serotonin signaling to the IPCs during stress, we ectopically expressed the 5-HT7 receptor on these cells by crossing Dilp2-Gal4 flies to a UAS-5-HT 7 line [42]. Since the 5-HT7 receptor is known to stimulate adenylate cyclase via Gs [25, 26, 33], we expected the ectopic receptor to increase DILP signaling. Indeed, the flies with 5-HT7 expressed on IPCs displayed a significantly reduced lifespan at starvation (Fig. 7). Probably, the ectopic 5-HT7 receptor couples to endogenous Gs and adenylate cyclase. These, and protein kinase A (PKA), are known to be present in the IPCs from a study of octopamine signaling to these cells [22].
Fig. 7.
Targeted expression of 5-HT7 in IPCs decreases lifespan at starvation. The 5-HT7 receptor couples via Gs to stimulate adenylate cyclase. Therefore, we ectopically expressed this receptor by means of the Dilp2 Gal4 driver (Dilp2-UAS-5-HT7) to test the effect on starvation resistance. Indeed, this ectopic expression produced flies with decreased the lifespan at starvation (p < 0.001 to both controls, Log rank test, Mantel-Cox; n = 93–122 for each genotype; experiment in two replicates). The decreased lifespan suggest that the ectopic 5-HT7 couples to Gs and stimulates IPCs and insulin signaling
We finally tested knockdown of the 5-HT1B and 5-HT7 receptors in IPCs by crossing different relevant receptor RNAi lines to the Dilp2-Gal4 driver. Neither of these fly crosses resulted in altered lifespan in response to starvation (S Fig 4). Thus, it seems that of the three tested receptors only 5-HT1A plays a role in regulation of IPCs at starvation.
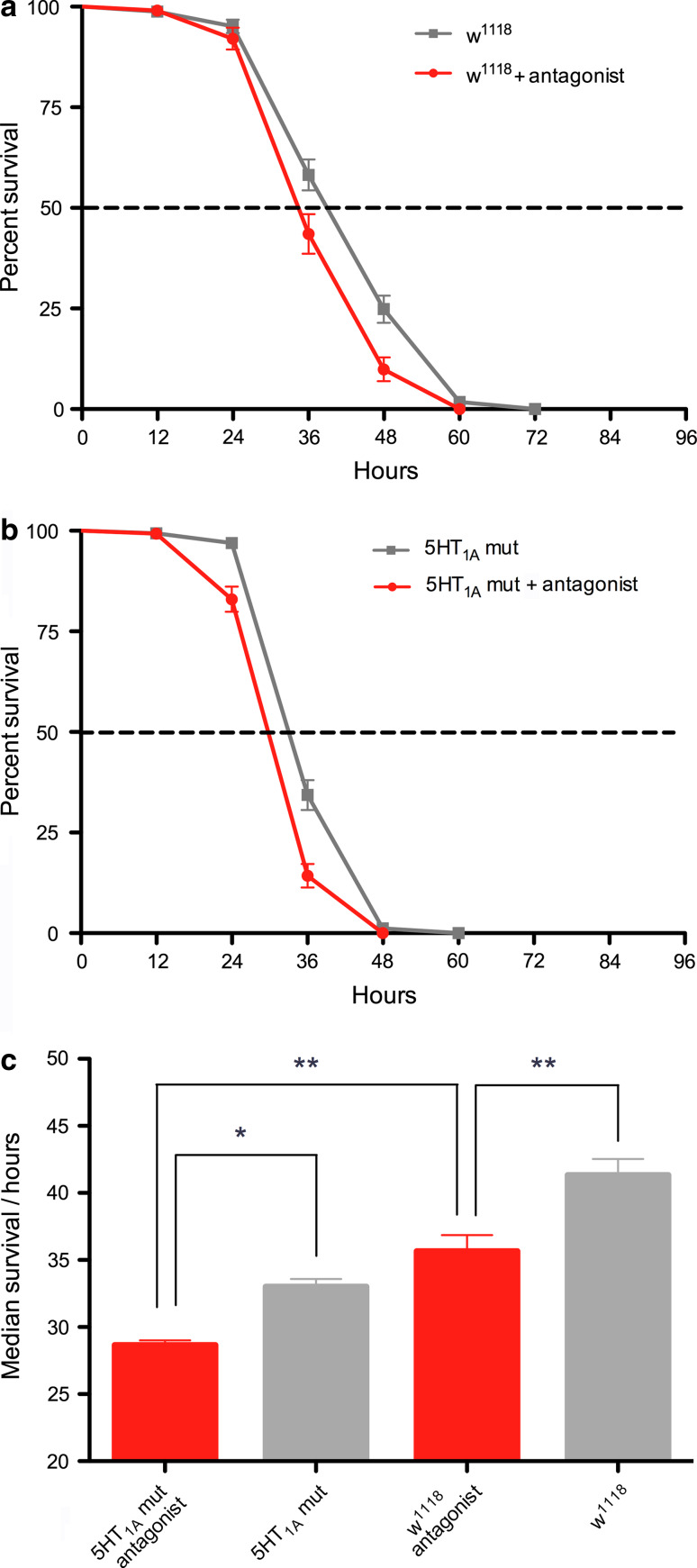
Flies fed a 5-HT1A antagonist display reduced resistance to starvation
To induce conditional interference with 5-HT1A signaling in adults we fed wild-type flies with the 5-HT1A antagonist WAY100635 [32, 48]. This antagonist has also been shown to act as an antagonist at the honeybee 5-HT1A receptor [43] and as an inverse agonist at the 5-HT1 receptor of the cockroach, Periplaneta americana [46]. WAY100635 was dissolved in aqueous agarose that flies were kept on while we monitored lifespan during starvation. Flies fed antagonist displayed significantly reduced lifespan compared to control flies (Fig. 8a). We also fed 5-HT1A mutant flies the antagonist and found that their lifespan was further reduced compared to both wild-type flies and mutants that were not fed the drug (Fig. 8b, c). This additive effect can be explained by the 5-HT1A mutant flies being heterozygous and thus possessing reduced receptor levels, not null levels.
Fig. 8.
Feeding flies a 5-HT1A antagonist increases sensitivity to starvation. The 5-HT1A antagonist WAY100635 was fed to the flies via aqueous agarose at a concentration of 0.18 mM (0.1 g/L) and the flies were kept on this agarose for the duration of the starvation experiment. a Survival of wild-type (w1118) flies kept on agarose with or without the antagonist. Antagonist-fed flies displayed a significant reduction in lifespan (p = 0.001, Log rank test; n = 101 and 165 for the two test groups; run in two replicates). b Survival of heterozygous 5-HT1A mutant (mut) flies fed agarose with or without antagonist. The antagonist further reduces lifespan in the mutant flies (p < 0.0001; n = 166 and 147 for the two test groups; run in two replicates). c Comparison of median survival (lifespan) of the four groups of flies tested in (a) and (b). It can be seen that the antagonist action is additive to the heterozygous mutation of the receptor. Thus, the shortest median life span is seen for mutant flies fed the antagonist, which is significantly shorter than both wild-type flies fed antagonist and mutants fed agarose alone (*p < 0.05, **p < 0.01; one-way Anova with Tukey comparison)
5-HT1A knockdown in IPCs and 5-HT1A mutation affect responses to temperature stress
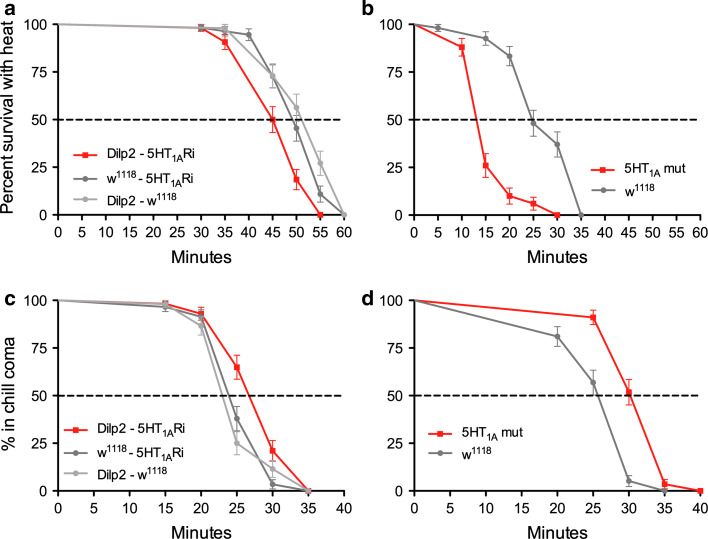
To further investigate 5-HT1A signaling to IPCs in stress responses, we performed tests of heat and cold tolerance (see [11]). For heat tolerance the time to knockdown at increased temperature (39°C) was monitored. Both the 5-HT1A mutant flies and flies with 5-HT1A-RNAi targeted to IPCs displayed a decreased tolerance to heat (Fig. 9a, b). Likewise these flies required increased time to recover from cold coma induced by exposure to 0°C for 4 h (Fig. 9c, d).
Fig. 9.
Responses to temperature stress are influenced by knockdown of 5-HT1A receptor in IPCs or globally. Flies with the 5-HT1A receptor knocked down in IPCs by the transgene Dilp2-Gal4/UAS-5-HT 1A-RNAi or globally in the mutant were tested for responses to temperature stress. All experiments were run in two replicates. a, b Response to heat was tested by exposing flies to 39°C and monitoring time to knockdown (given as percent survival with heat). a The flies with the 5-HT1A receptor diminished in IPCs (Dilp2-5-HT1ARi) displayed a faster knockdown at 39°C (p = 0.0005 and p < 0.0001 to parental controls; Log rank test, Mantel-Cox; n= 48–55 for the three genotypes). b The 5-HT1A mutant (mut) flies displayed a similar increased sensitivity to heat compared to wild-type flies (w1118) (p < 0.0001; n = 50 and 54). c, d Recovery from cold knockdown (coma) was monitored in the same genotypes. Flies were kept at 0°C for 4 h and the time to recovery was monitored (given as percent in chill coma). c The flies with receptor knockdown in IPCs were slower in their recovery from cold (p = 0.0009 and p = 0.0017 to controls; n = 52–58 for the three genotypes). d The receptor mutant flies also display a longer recovery time (p < 0.0001 to wild-type control; n = 56 and 58)
From these stress experiments, it can be suggested that increased DILP signaling, due to diminished 5-HT1A leads to decreased temperature tolerance. These findings are contrary to those in an earlier paper where ablation of IPCs, and presumably reduced insulin signaling, leads to decreased heat and cold tolerance in Drosophila [11]. Our results are, however, in accordance with findings in C. elegans [49].
Knockdown of 5-HT1A alters lipid storage in flies
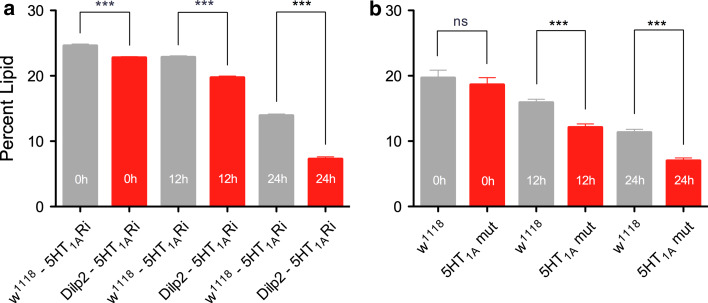
DILP signaling is important in regulation of carbohydrate and lipid metabolism in flies [1, 11, 16]. Ablation of IPCs lead to increased storage of both lipid and carbohydrate [11] and knockdown of the GABAB receptor on IPCs altered the lipid storage profile over time at starvation [23]. We tested flies with 5-HT1A knocked down in IPCs for whole-body lipid levels over 0, 12 and 24 h starvation. Compared to controls, the 5-HT1A-RNAi flies display significantly reduced lipid levels at 12 and 24 h of starvation (Fig. 10a). The heterozygous 5-HT1A mutants also displayed a significant reduction of lipid levels compared to control (wild-type) flies at 12 and 24 h starvation (Fig. 10b). Thus, knockdown of 5-HT1A in IPCs, or globally, produces a lipid storage phenotype consistant with increased insulin signaling, similar to findings in the GABA study [23].
Fig. 10.
Knockdown of 5-HT1A receptor in IPCs or globally affects lipid storage at starvation. Whole-body lipid was measured, as given in “Materials and methods”, in fed flies (0 h) and flies exposed to starvation for 12 and 24 h. a Flies with the 5-HT1A receptor diminished in IPCs (Dilp2-5-HT1ARi) displayed a significantly lower amount of lipid than control flies both in fed and starved flies (***p < 0.001; one-way Anova with Tukey’s comparison; n = 120 for each genotype; experiment run in three replicates). The decrease in lipid over time was also significant for both genotypes (p < 0.001; two-way ANOVA). b In the 5-HT1A mutant flies, the lipid levels were lower than in wild-type flies at 12 and 24 h of starvation. (***p < 0.001, ns not significant; one-way Anova, n = 120 for each genotype; experiment run in three replicates). The decrease in lipid over time was also significant for both genotypes (p < 0.001; two-way ANOVA)
Knockdown of 5-HT1A has no effect on growth
Finally, we tested whether knockdown of 5-HT1A has any effect on growth. Adult flies (4–6 days old) with the receptor knocked down in IPCs by Dilp2-driven 5-HT1A-RNAi or globally in the mutant were weighed. There was no significant difference between controls and flies with receptor knockdown (S Fig. 5), suggesting that 5-HT1A-mediated signaling plays no major role in control of organismal growth as determined by body weight. Similar results were obtained in flies where the GABAB receptor was knocked down in IPCs [23].
Discussion
We have shown here that the inhibitory serotonin 5-HT1A receptor is expressed in IPCs of adult Drosophila and affects IPC activity and probably insulin-like signaling as monitored by different stress responses, DILP levels and lipid storage. On the other hand, we did not obtain evidence for expression or action of the 5-HT1B or 5-HT7 receptors on IPCs, and an earlier report provided no support for 5-HT2 expression in these neurosecretory cells [31]. Thus, it is likely that serotonin signaling to the brain IPCs in adult flies is mainly mediated by the 5-HT1A receptor.
When discussing the role of 5-HT1A in inhibiting IPCs, it should be noted that the IPCs are also known to regulate locomotor activity, sleep-wakefulness and sensitivity to ethanol, and this regulation may be independent of the insulin signaling pathway [22, 50, 51]. Therefore, activation or inhibition of the IPCs could cause actions that are non-insulin mediated. These could be either via other messengers released by the IPCs or by indirect action of DILPs on other circuits or endocrine cells. As an example, the reduced 5-HT1A inhibition of the IPCs could cause an increased starvation-induced hyperlocomotion (see [50]). This could in turn result in increased rates of carbohydrate and lipid consumption, leading to reduced survival at starvation. Thus, it cannot be excluded that some of the phenotypes discussed below are not mediated by typical insulin signaling to the fat body.
Our assays indicated that temperature tolerance and starvation resistance were impaired in flies where the 5-HT1A receptor was knocked down in IPCs as well as in 5-HT1A mutants. Furthermore, the 5-HT1A ablated flies and mutants displayed a higher lipid consumption rate, likely because insulin signaling was increased. However, no clear effect on growth (body weight) was observed after 5-HT1A knockdown, similar to findings in a study of GABAB receptor knockdown on IPCs [23]. Possibly, this lack of effect on growth is due to the absence of 5-HT1A expression on larval IPCs suggested by our expression data. Thus, although we could induce increased insulin signaling in the adult fly by knockdown of the 5-HT1A receptor, our experiments with mutants and targeted RNAi seem not to affect larval IPCs. Other studies have demonstrated effects on growth after ablation of IPCs or interference with DILP signaling in the larva [2, 6, 18, 24, 52]. Also, a paper by Kaplan and coworkers [24] indicated that serotonergic neurons modulate IPC activity and DILP signaling in larvae and that this signaling affects growth. However, that paper did not provide evidence for direct action of serotonin on the IPCs. Certainly, it is possible that one of the other 5-HT receptors is mediating effects on growth and/or that the signaling to the IPCs in growth regulation is indirect.
Since the 5-HT1A knockdown flies display a reduced stress resistance and altered lipid consumption, it is suggestive that DILP release from IPCs increases at stress due to the manipulation. However, the DILP signaling from IPCs is complex, since the different DILPs in the IPCs seem to be individually regulated transcriptionally [13, 17, 18]. If production of DILPs can be individually regulated, it is likely that the ratio of DILP2, 3 and 5 stored in vesicles in the IPC axon terminations also vary. Since the calcium-dependent release of the different DILPs is unlikely to be individually controlled, the release at any given point may result in different ratios of circulating DILPs. Then, if the different DILPs mediate different actions on physiology, as suggested [9], one would expect that the composition of the DILP cocktail could be functionally important.
Several studies have assayed DILP-immunofluorescence levels in cell bodies of IPCs after various manipulations to monitor “insulin signaling” [13, 19, 23, 24]. Our experiments here show increased DILP2 immunolabeling in the IPC cell bodies after 5-HT1A knockdown. This would suggest an increase of DILP production, but it is likely that DILP release is visible only in the axon terminals of the IPCs in the corpora cardiaca and aorta (not monitored here). Thus, the IPC cell bodies are not optimal for monitoring release. Previous studies showed similar changes in DILP2 immunolabeling after knockdown of the inhibitory GABAB receptor on IPCs [23] and in NS3 mutants [24]. It may be that the increase in DILP2 immunolabeling seen in the cell bodies of the IPCs is due to an increased production of DILP as a consequence of increased release into the circulation from axon terminals. A sensitive assay is, however, required for monitoring DILP levels in the hemolymph of Drosophila to obtain a measure of bona fide release.
Due to the extensive arborizations of serotonergic neuron processes in the small and compact brain of Drosophila, we could not identify the individual neurons that innervate the IPCs. In a study of the much larger blowfly brain, two pairs of candidate serotonergic neurons with axon terminations in the pars intercerebralis were shown: one pair with cell bodies in posterior protocerebrum and one pair in the subesohageal ganglion [53]. Also in the larva, in spite of a simpler neuronal organization, these serotonergic neurons could not be individually identified [24, 47]. However, it was suggested that in the larva serotonin-immunoreactive neurons in the subesophageal ganglion may have branches that impinge on the IPCs [47]. Such a location would make sense since the subesophageal ganglion is known to receive chemosensory inputs and contain neurons regulating aspects of feeding and neuroendocrine function [54–57].
The changing expression pattern of 5-HT1A in IPCs during development may indicate that this receptor is involved in a behavioral transition from feeding to non-feeding stage. Neuropeptide F (NPF), an ortholog of NPY in mammals, was shown to be a molecular switch in the transition from feeding to non-feeding (wandering) stage of larvae [56]. These authors showed that NPF is expressed at higher levels in certain brain neurons in feeding third instar larvae and is downregulated in the late third instar non-feeding stage. Maybe the 5-HT1A expression on IPCs starts during the same behavioral transition, and perhaps there is a link between NPF and serotonin receptor expression. There is another relevant example of a change in the larval brain at transition to wandering stage. At this transition, the larvae become positively phototactic, and simultaneously a set of serotonergic neuron branches grow into the larval optic center [37, 58, 59]. Importantly, serotonin signaling, probably mediated by 5-HT1A, is required in the larval photic response [37].
Our findings here that brain IPCs can be directly inactivated by serotonergic signaling suggests that these neurosecretory cells are under complex stimulatory and inhibitory regulation. Production and/or release of DILPs in IPCs is induced by a circulating factor released from the fat body [19] and possibly by the peptide sNPF and the monoamine octopamine, both released by brain neurons [21, 22] and is inhibited by GABA [23] as well as serotonin, as shown here. Such a control by multiple neuronal systems and hormonal factors may serve to fine-tune the activity of the IPCs in the production and release of the physiologically very important DILPs. Similarly, the insulin release from mammalian pancreatic beta cells is under control of circulating glucose levels as well as several neuromediators, such as serotonin, GABA, glucagon-like peptide and other peptides [60–62].
It is not clear whether the two inhibitory signals to the IPCs, GABA and serotonin, are mediated by different core intracellular pathways. The postsynaptic GABAB receptor commonly couples to G-protein-coupled inwardly rectifying potassium channels (GIRKs), but can also inhibit adenylate cyclase via Gαi/Gαo, or even inhibit voltage-dependent Ca2+ channels, all leading to hyperpolarization [63–67]. In Drosophila, knockdown of a putative GIRK subunit in the IPCs phenocopied GABAB receptor knockdown in starvation assays [23], but other pathways were not tested. The 5-HT1A can also act on GIRKs, as well as couple negatively to adenylate cyclase [25, 32]. Interestingly, the stimulatory action of octopamine on IPCs was shown to be mediated by the OAMB receptor through activation of adenylate cyclase, increased cyclic AMP and PKA activation [22]. For the future, it would be interesting to investigate whether octopamine and serotonin act antagonistically on IPCs by converging on adenylate cyclase. Perhaps these biogenic amines play antagonistic roles in tuning insulin signaling during stress, as well as in regulation of sleep wakefulness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
S Fig. 1 The 5-TH1B and 5-HT7 receptors appear not to be expressed in IPCs. Antiserum to DILP2 (magenta) was combined with 5-HT 1B and 5-HT 7-Gal4 driven GFP (green) in adult and larval brains of Drosophila (all images are projections of several optic sections). a. No colocalization of markers is seen in IPCs in the adult brain. Arrow points at a receptor expressing cell body distinct from the IPCs. In ai and aii the separate channels are shown. b. Also in the larval brain there is no colocalization of markers. c. In the adult brain the 5-HT7 is expressed mainly in neurons of the ellipsoid body (EB), including the lateral triangle (LTR), and not in the IPCs. d. The larval IPCs also do not express 5-HT7, although some adjacent cell bodies do so. (TIFF 6995 kb)
S. Fig. 2 The 5-HT1A receptor expression pattern closely resembles that of serotonergic branches. To reveal the relations between the 5-HT1A receptor and neurons processes releasing the ligand, we applied a monoclonal antibody to serotonin (magenta) and 5-HT 1A-Gal4 driven GFP (green). a – c. In the adult brain the match between the receptor and serotonin-immunoreactive processes is close. Note that the 5-HT1A expression may also include non-dendritic portions of the neurons where no receptor protein is located (accounting for part of the mismatch). Especially in the pars intercerebralis (PI) and ellipsoid body (EB) the matching patterns are seen. d. Expression of 5-HT1A (green) and serotonin (magenta) in the larval ventral nerve cord (total projection). Processses from the numerous 5-HT1A expressing neurons superimpose the serotonergic ones in the two columns of synaptic neuropil. The arrow indicates anterior (a) and posterior (p). e - f. Single channels showing 5-HT 1A-Gal4 driven GFP and serotonin-immunolabeling. (TIFF 8746 kb)
S Fig. 3 A different Dilp2 -Gal4 line to drive 5-HT 1A knockdown also renders flies more sensitive to starvation. Another Dilp2-Gal4 line (insertion on 3rd chromosome; [18]) was used for driving 5-HT1A-RNAi in IPCs as a control to establish that the effects seen are not caused by position of insert. As with the other Dilp2-Gal4 driver we observe that 5-HT1A-RNAi in IPCs render flies more sensitive to starvation (p<0.0001 to both controls, Log rank test, Mantel-Cox; n= 180 for each genotype; experiment in three replicates). (TIFF 243 kb)
S Fig. 4 Tests of RNAi for 5-HT 1B and 5-HT 7 receptors in IPCs indicate no effect on sensitivity to starvation. Although we have no evidence for expression of 5-HT1B and 5-HT7 receptors in IPCs of Drosophila we tested driving RNAi for the two receptor with the Dilp2-Gal4 line and exposed the different fly crosses to starvation. a. Attempted knockdown of 5-HT1B in IPCs [Dilp2-5-HT1BRi(V)] did not result in a change in survival at starvation compared to controls (p=0.8140 and p=0.4046 to the two controls, p=0.5431 between controls; n= 142-164 for the three genotypes, experiment in three replicates). This was a UAS-5-HT1BRNAi line from VDRC. b. Attempted knockdown of 5-HT7 in IPCs (Dilp2-5-HT7Ri) did not result in a change in survival at starvation compared to controls. (TIFF 2268 kb)
S Fig. 5 Knockdown of 5-HT 1A in IPCs or globally does not affect growth. Adult flies of the different genotypes were weighed at the age of 4 – 6 d to estimate growth. a. Knockdown of 5-HT1A in IPCs by Dilp2-Gal4 driven RNAi (Dilp2-5-HT1ARi) does not produce a noticable growth phenotype in male flies (ns, p=0.105 and *** p<0.001; one-way ANOVA, Tukey’s comparison; n = 120 for each genotype, experiment in three replicates). b. Weights of wild type (w1118) and 5-HT1A mutant flies (M, males and F, females) also do not differ (ns, p=0.07 for male mutant to wild type, and p=0.162 for female mutant to control; one-way ANOVA; n= 120 for each genotype and sex). (TIFF 344 kb)
Acknowledgments
We thank the persons and organizations listed in “Materials and methods” for flies and reagents. This study was supported by the Swedish Research Council (VR).
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
- BDSC
Bloomington Drosophila stock center
- CNS
Central nervous systems
- DILP
Drosophila insulin-like peptide
- GABA
Gamma-aminobutyric acid
- GFP
Green fluorescent protein
- GIRK
G-protein-coupled inwardly rectifying potassium channel
- GPCR
G-protein-coupled receptor
- IPCs
Insulin- producing cells
- MNCs
Median neurosecretory cells
- NPF
Neuropeptide F
- NS3
Nucleostemin 3
- OAMB
Octopamine receptor (mushroom bodies)
- PCR
Polymerase chain reaction
- PKA
Protein kinase A
- RNAi
RNA interference
- sNPF
Short neuropeptide F
- VDRC
Vienna Drosophila RNAi center
References
- 1.Baker KD, Thummel CS. Diabetic larvae and obese flies—emerging studies of metabolism in Drosophila . Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/S0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 3.Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Géminard G, Arquier N, Layalle S, Bourouis M, Slaidina M, et al. Control of metabolism and growth through insulin-like peptides in Drosophila . Diabetes. 2006;55:S5–S8. doi: 10.2337/db06-S001. [DOI] [Google Scholar]
- 5.Kenyon C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans . Ann N Y Acad Sci. 2010;1204:156–162. doi: 10.1111/j.1749-6632.2010.05640.x. [DOI] [PubMed] [Google Scholar]
- 6.Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila . Biochem J. 2010;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 1995;14:3373–3384. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaidina M, Delanoue R, Grönke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 13.Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, et al. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9:336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Liu J, Li CR, Momen B, Kohanski RA, et al. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc Natl Acad Sci USA. 2009;106:19617–19622. doi: 10.1073/pnas.0905083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001;304:317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- 16.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 17.Broughton S, Alic N, Slack C, Bass T, Ikeya T, et al. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila . Curr Biol. 2002;12:1293–1300. doi: 10.1016/S0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 19.Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila . Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- 21.Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- 22.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enell LE, Kapan N, Söderberg JA, Kahsai L, Nässel DR. Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila . PLoS ONE. 2010;5:e15780. doi: 10.1371/journal.pone.0015780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP. A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev. 2008;22:1877–1893. doi: 10.1101/gad.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 26.Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, et al. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci USA. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blenau W, Thamm M (2011) Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies. Lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct Dev (in press) [DOI] [PubMed]
- 29.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila . Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev Neurobiol. 2007;67:752–763. doi: 10.1002/dneu.20370. [DOI] [PubMed] [Google Scholar]
- 32.Johnson O, Becnel J, Nichols CD. Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster . Neuroscience. 2009;158:1292–1300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becnel J, Johnson O, Luo J, Nässel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE. 2011;6(6):e20800. doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster . PLoS ONE. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez Moncalvo VG, Campos AR. Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci. 2009;10:66. doi: 10.1186/1471-2202-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW. Serotonin modulates olfactory processing in the antennal lobe of Drosophila . J Neurogenet. 2009;23:366–377. doi: 10.3109/01677060903085722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, et al. Serotonin is necessary for place memory in Drosophila . Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila . Proc Natl Acad Sci USA. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerr M, Davies SA, Dow JA. Cell-specific manipulation of second messengers; a toolbox for integrative physiology in Drosophila . Curr Biol. 2004;14:1468–1474. doi: 10.1016/j.cub.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Thamm M, Balfanz S, Scheiner R, Baumann A, Blenau W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell Mol Life Sci. 2010;67:2467–2479. doi: 10.1007/s00018-010-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Service FJ, O’Brien PC, Rizza RA. Measurements of glucose control. Diabetes Care. 1987;10:225–237. doi: 10.2337/diacare.10.2.225. [DOI] [PubMed] [Google Scholar]
- 45.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troppmann B, Balfanz S, Baumann A, Blenau W. Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1 receptor. British J Pharmacol. 2010;159:1450–1462. doi: 10.1111/j.1476-5381.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal N, Padmanabhan N, Hasan G. Inositol 1, 4, 5- trisphosphate receptor function in Drosophila insulin producing cells. PLoS ONE. 2009;4:e6652. doi: 10.1371/journal.pone.0006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, et al. WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther. 1996;278:752–762. [PubMed] [Google Scholar]
- 49.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 50.Mattaliano MD, Montana ES, Parisky KM, Littleton JT, Griffith LC. The Drosophila ARC homolog regulates behavioral responses to starvation. Mol Cell Neurosci. 2007;36:211–221. doi: 10.1016/j.mcn.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corl AB, Rodan AR, Heberlein U. Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster . Nat Neurosci. 2005;8:18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- 52.Walkiewicz MA, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila . PLoS ONE. 2009;4:e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nässel DR. Serotonin and serotonin-immunoreactive neurons in the nervous system of insects. Prog Neurobiol. 1988;30:1–85. doi: 10.1016/0301-0082(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 54.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bader R, Colomb J, Pankratz B, Schrock A, Stocker RF, et al. Genetic dissection of neural circuit anatomy underlying feeding behavior in Drosophila: distinct classes of hugin-expressing neurons. J Comp Neurol. 2007;502:848–856. doi: 10.1002/cne.21342. [DOI] [PubMed] [Google Scholar]
- 56.Wu Q, Wen T, Lee G, Park JH, Cai HN, et al. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/S0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki T, Ito K. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. J Comp Neurol. 2010;518:4147–4181. doi: 10.1002/cne.22433. [DOI] [PubMed] [Google Scholar]
- 58.Campos AR, Lee KJ, Steller H. Establishment of neuronal connectivity during development of the Drosophila larval visual system. J Neurobiol. 1995;28:313–329. doi: 10.1002/neu.480280305. [DOI] [PubMed] [Google Scholar]
- 59.Sawin-McCormack EP, Sokolowski MB, Campos AR. Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J Neurogenet. 1995;10:119–135. doi: 10.3109/01677069509083459. [DOI] [PubMed] [Google Scholar]
- 60.Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adeghate E, Ponery AS, Pallot DJ, Singh J. Distribution of vasoactive intestinal polypeptide, neuropeptide-Y and substance P and their effects on insulin secretion from the in vitro pancreas of normal and diabetic rats. Peptides. 2001;22:99–107. doi: 10.1016/S0196-9781(00)00361-2. [DOI] [PubMed] [Google Scholar]
- 62.Adeghate E, Ponery AS. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell. 2002;34:1–6. doi: 10.1054/tice.2002.0217. [DOI] [PubMed] [Google Scholar]
- 63.Mezler M, Muller T, Raming K. Cloning and functional expression of GABA(B) receptors from Drosophila . Eur J Neurosci. 2001;13:477–486. doi: 10.1046/j.1460-9568.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- 64.Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, et al. Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1998;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 66.Hamasaka Y, Wegener C, Nässel DR. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol. 2005;65:225–240. doi: 10.1002/neu.20184. [DOI] [PubMed] [Google Scholar]
- 67.Kolaj M, Bai D, Renaud LP. GABAB receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol. 2004;92:111–122. doi: 10.1152/jn.00014.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S Fig. 1 The 5-TH1B and 5-HT7 receptors appear not to be expressed in IPCs. Antiserum to DILP2 (magenta) was combined with 5-HT 1B and 5-HT 7-Gal4 driven GFP (green) in adult and larval brains of Drosophila (all images are projections of several optic sections). a. No colocalization of markers is seen in IPCs in the adult brain. Arrow points at a receptor expressing cell body distinct from the IPCs. In ai and aii the separate channels are shown. b. Also in the larval brain there is no colocalization of markers. c. In the adult brain the 5-HT7 is expressed mainly in neurons of the ellipsoid body (EB), including the lateral triangle (LTR), and not in the IPCs. d. The larval IPCs also do not express 5-HT7, although some adjacent cell bodies do so. (TIFF 6995 kb)
S. Fig. 2 The 5-HT1A receptor expression pattern closely resembles that of serotonergic branches. To reveal the relations between the 5-HT1A receptor and neurons processes releasing the ligand, we applied a monoclonal antibody to serotonin (magenta) and 5-HT 1A-Gal4 driven GFP (green). a – c. In the adult brain the match between the receptor and serotonin-immunoreactive processes is close. Note that the 5-HT1A expression may also include non-dendritic portions of the neurons where no receptor protein is located (accounting for part of the mismatch). Especially in the pars intercerebralis (PI) and ellipsoid body (EB) the matching patterns are seen. d. Expression of 5-HT1A (green) and serotonin (magenta) in the larval ventral nerve cord (total projection). Processses from the numerous 5-HT1A expressing neurons superimpose the serotonergic ones in the two columns of synaptic neuropil. The arrow indicates anterior (a) and posterior (p). e - f. Single channels showing 5-HT 1A-Gal4 driven GFP and serotonin-immunolabeling. (TIFF 8746 kb)
S Fig. 3 A different Dilp2 -Gal4 line to drive 5-HT 1A knockdown also renders flies more sensitive to starvation. Another Dilp2-Gal4 line (insertion on 3rd chromosome; [18]) was used for driving 5-HT1A-RNAi in IPCs as a control to establish that the effects seen are not caused by position of insert. As with the other Dilp2-Gal4 driver we observe that 5-HT1A-RNAi in IPCs render flies more sensitive to starvation (p<0.0001 to both controls, Log rank test, Mantel-Cox; n= 180 for each genotype; experiment in three replicates). (TIFF 243 kb)
S Fig. 4 Tests of RNAi for 5-HT 1B and 5-HT 7 receptors in IPCs indicate no effect on sensitivity to starvation. Although we have no evidence for expression of 5-HT1B and 5-HT7 receptors in IPCs of Drosophila we tested driving RNAi for the two receptor with the Dilp2-Gal4 line and exposed the different fly crosses to starvation. a. Attempted knockdown of 5-HT1B in IPCs [Dilp2-5-HT1BRi(V)] did not result in a change in survival at starvation compared to controls (p=0.8140 and p=0.4046 to the two controls, p=0.5431 between controls; n= 142-164 for the three genotypes, experiment in three replicates). This was a UAS-5-HT1BRNAi line from VDRC. b. Attempted knockdown of 5-HT7 in IPCs (Dilp2-5-HT7Ri) did not result in a change in survival at starvation compared to controls. (TIFF 2268 kb)
S Fig. 5 Knockdown of 5-HT 1A in IPCs or globally does not affect growth. Adult flies of the different genotypes were weighed at the age of 4 – 6 d to estimate growth. a. Knockdown of 5-HT1A in IPCs by Dilp2-Gal4 driven RNAi (Dilp2-5-HT1ARi) does not produce a noticable growth phenotype in male flies (ns, p=0.105 and *** p<0.001; one-way ANOVA, Tukey’s comparison; n = 120 for each genotype, experiment in three replicates). b. Weights of wild type (w1118) and 5-HT1A mutant flies (M, males and F, females) also do not differ (ns, p=0.07 for male mutant to wild type, and p=0.162 for female mutant to control; one-way ANOVA; n= 120 for each genotype and sex). (TIFF 344 kb)