Abstract
This unit discusses a basic method for purification of radiolabeled RNAs using denaturing polyacrylamide gel electrophoresis. The method consists of a number of experimental procedures, including total RNA preparation from yeast cells, isolation of a specific RNA from total yeast RNA, RNA 3' terminal labeling using nucleotide (5’[32P]pCp) addition (via ligation), denaturing (8 M urea) polyacrylamide gel electrophoresis, and RNA extraction from the gel slice. Key points for achieving good electrophoretic separation of RNA are also discussed.
INTRODUCTION
End labeling of RNA is an indispensable technique for nucleic acids research. There are various ways to label RNA. Commonly used materials to label RNA are radioisotopes (3H, 32P, etc.), fluorescent dyes (Cy3, 6-FAM, etc.), and chemicals (Biotin, Digoxigenin (DIG), etc.). Labeling with a radioisotope is the most traditional way, and almost all facilities have a detector for radioactivity. Compared to other labeling methods, radiolabeling has at least two benefits. First, because radioisotopes do not change the chemical properties of a molecule, RNAs containing radioisotopes behave exactly the same way as natural RNAs in virtually all reactions tested. Second, 32P, which is the most commonly used radioisotope in RNA labeling, has high sensitivity for detection and is relatively less expensive than other labeling reagents. Conversely, a disadvantage of radiolabeling is that it may cause health problems. These risks, however, are almost entirely preventable through the proper use of protective clothing and full compliance with radiation safety requirements.
In order to obtain high purity RNA after end labeling, polyacrylamide gel electrophoresis (PAGE) in a denaturing condition followed by elution is widely used. Nucleic acids have a negative charge because of their phosphate backbone and so they migrate to the anode in response to an electric field. Once molecules are in the polyacrylamide gel, their mobility is slowed by the molecular sieving effect of the matrix. RNAs put through a denaturing polyacrylamide gel can be separated based on their molecular weights by this effect. However, RNA often forms secondary structure, which may affect its migration pattern. In order to obtain a stable and correct result, it is important to prevent RNA molecules from forming secondary structures. Urea is the most commonly used denaturant that allows RNA molecules to keep an unstructured form during the gel run.
The following demonstrates a protocol for radiolabeling and gel-purifying a specific RNA derived from cells (for protocols using in vitro synthesized RNAs, see Huang and Yu, 2013).
Basic Protocol 1: RNA extraction and purification from yeast cells
Total cellular RNA can be collected from various species, such as yeast cells, rat livers, or human cell lines (e.g., HeLa, HEK293 etc.). Here we demonstrate a yeast tRNATyr preparation as an example. By using a biotinylated DNA oligonucleotide that is complementary to the target RNA, any RNA could be prepared.
Materials
Yeast (BY4741 strain)
YPD (see recipe)
TRIzol reagent (Invitrogen)
Tris-HCl saturated Phenol/Chloroform/Isoamyl alcohol (PCA) 25:24:1
0.5 mm glass beads (BioSpec Products)
Isopropyl alcohol
Ethanol
Extraction buffer (see recipe) (optional)
10% SDS (optional)
3 M sodium acetate pH 5.2 (optional)
Streptavidin Agarose (Pierce)
Binding buffer (see recipe)
Washing buffer (see recipe)
2.4 M Tetraethylammonium Chloride (TEACl)
Biotinylated oligo DNA (5' biotin-CGAACGCCCGATCTCAAGATTTACAGTCTTGCGCC-3') (Integrated DNA Technologies)
Amicon Ultra-4 10K column (Millipore)
-
Extract tRNAs from yeast cells
- Grow yeast cells in 50 mL of YPD to late-log phase (O.D.600 = higher than 1, which is about 1–2 × 107 cells/mL) at 30°C.Note: If the expression level of target RNA is low, increase the culture volume. For an abundant mRNA use 200 mL, for a low level mRNA up to 1–2 L should be used.
- Spin down the cells at 1,000 × g for 2 minutes.
- Decant the medium from the cell pellet and lyse it in 1 mL of TRIzol Reagent by repetitive pipetting.Note: If the volume of the culture is higher than 200 mL, use a French Press to break the cells; see optional procedure below.
- Disrupt the cells with 300 µL of glass beads in a screw cap tube by Mini-BeadBeater for 20 seconds 4 times.
- Centrifuge the homogenized sample at 12,000 × g at 4°C for 5 minutes.
- Transfer the supernatant (~1 mL) to a new 1.5 mL tube and let it stand at 25°C for 5 minutes.
- Add 0.2 mL of chloroform, shake the tube vigorously by hand for 15 seconds, and incubate them at 25°C for 2 minutes.
- Centrifuge the sample at 12,000 × g at 4°C for 15 minutes.
- Transfer the upper aqueous phase to a new 1.5 mL tube. RNA remains exclusively in the aqueous phase.
- Add 0.5 mL of isopropyl alcohol and incubate it at 25°C for 10 minutes.
- Centrifuge the sample at 12,000 × g at 4°C for 15 minutes.
- Remove the supernatant. (The RNA precipitate forms a white pellet on the bottom of the tube.)
- Wash the RNA pellet with 1 mL of 70% ethanol.
- Centrifuge the sample at 7,500 × g at 4°C for 5 minutes.
- Remove the supernatant and briefly dry the RNA pellet (air-dry for 5–10 minutes). Do not dry the RNA completely since it will cause serious insolubility.
- Dissolve the RNA in 500 µL of 2.4 M TEACl and measure concentration. This total RNA solution can be stored at −80°C for several months.
Optional procedure: lysing cells with French Press (following Basic Protocol 1 (1) step 2)-
1Resuspend the cell pellet in 5 mL (for 200 mL culture) or 10–20 mL (for 1–2 L culture) of Extraction buffer.
-
2Lyse it with French Press at 1,000 psi 3 times.
-
3Centrifuge the homogenized sample at 12,000 × g at 4°C for 15 minutes.
-
4Transfer the supernatant to a new 50 mL tube and add the 1/10 volume of 10% SDS and the equal amount of PCA followed by vortex for 1 minute.
-
5Incubate at 65°C water bath for 5 minutes.
-
6Centrifuge at 12,000 × g at 4°C for 10 minutes.
-
7Transfer the upper aqueous phase to a new clean tube and repeat steps 4 (except that there is no need to add 10% SDS) to 6, total 4 times.Note: If the cloudy interface remains significant after centrifugation, then repeat PCA extraction.
-
8After final extraction, transfer the upper phase to a new clean tube and add the 1/10 volume of 3 M sodium acetate and the equal volume of isopropyl alcohol.
-
9Place it at −20°C or on dry-ice for more than 15 minutes
-
10Centrifuge at 12,000 × g at 4°C for 15 minutes.
-
11Discard the supernatant and add 1 mL of 70% ethanol.
-
12Centrifuge at 12,000 × g at 4°C for 5 minutes.
-
13Discard the supernatant and air-dry the precipitated pellet.
-
14Dissolve the RNA in 500 µL of 2.4 M TEACl and measure concentration.
-
Purify tRNATyr with a biotin conjugated DNA oligonucleotide Streptavidin (SA) resin preparation
-
17Transfer 900 µL of SA beads to a 1.5 mL tube.
-
18Wash beads 3 times with 1 mL of binding buffer; spin down at 1,000 × g for 1 minute and remove supernatant.
-
19Resuspend beads to 90% original volume in binding buffer (~450 µL).
-
20Add 90 µL of a biotinylated DNA oligonucleotide complementary to yeast Tyr-tRNA.Note: The biotinylated DNA oligo can be purchased commercially. The length of DNA depends on its GC%. The Tm value should be 60–65°C. Avoid targeting structured regions of RNA.
-
21Incubate beads with a rotator at 15°C for 10 minutes.
-
22Wash beads 2 times with 1 mL of washing buffer.
-
23Wash beads 3 times with 1 mL of 2.4 M TEACl in order to lower the Tm value of the DNA-RNA duplex.
-
24Resuspend beads in 800 µL of 2.4 M TEACl. Oligonucleotide-bound beads can be stored at 4°C for several months.
Binding of tRNATyr to the oligonucleotide-bound beads-
25Transfer 200 µL of oligo-bound beads to a 1.5 mL tube.
-
26Add 1 mg of total RNA.
-
27Heat the tube at 60°C for 3 minutes to weaken the secondary structure.
-
28Immediately transfer it to 15°C and allow hybridization to proceed for 15 minutes; mix several times by inverting.
-
29Spin down the resin at 1,000 × g for 1 minute.
-
30Transfer the supernatant to a new 1.5 mL tube for another round of purification (see below).
-
31Transfer beads to a new 1.5 mL tube.
-
32Wash beads 3 times with 0.5 mL of 2.4 M TEACl.
-
33Resuspend beads in 300 µL of 2.4 M TEACl.
-
34Immediately place the tube at 60°C for 3 minutes to separate tRNATyr from beads.
-
35Spin down beads at 1,000 × g for 1 minute.
-
36Transfer the supernatant to a new 1.5 mL tube.
-
37Repeat steps 27 through 36 with new oligonucleotide-bound beads and the supernatant saved in step 30.
Desalting and concentration of RNAs-
38When the 2nd round of purification is done, add the supernatant to the same tube in step 36.
-
39Spin at 1,000 × g for 1 minute to pellet residual beads.
-
40Transfer the supernatant to an Amicon Ultra-4 10K column.
-
41Add 2 mL of ddH2O to the column and centrifuge the column at 4,000 × g at 4°C until the volume reaches ~100 µL (usually approximately 1–2 hours; depending on the concentration of RNA).
-
42Add 1 mL of ddH2O to the column and centrifuge again until the volume reaches ~100 µL.
-
43Add 0.5 mL of ddH2O to the column and centrifuge again until the volume reaches ~100 µL.
-
44Set aside the filtrate and collect the sample.
-
17
Basic Protocol 2: Radiolabeling of tRNA at the 3' end
Radiolabeling can be achieved by either 5’ or 3’ end-labeling (Huang and Yu, 2013). Compared to 5’ end-labeling, where RNAs need to be 5' de-phosphorylated (or decapped if the RNAs contain 5’ cap structures) prior to a kinase reaction with a radioisotope, 3’ end-labeling can be used directly for almost all kinds of RNAs. In this protocol, 3’ end-labeling is exploited to confirm the tRNA purity.
Materials
10 mM ATP (Thermo)
5’ [32P]-pCp (3000 Ci/mmol, 10 µCi/µL) (Perkin Elmer)
T4 RNA ligase and 10× buffer (Thermo)
G50 buffer (see recipe)
Ethanol
- Assemble all reaction components below on ice.
- 30 pmol tRNA
- 2 µL 10× buffer for T4 RNA ligase
- 1 µL 10 mM ATP
- 10 µL 5’ [32P]-pCp (3000 Ci/mmol, 10 µCi/µL)
- 10 Units T4 RNA ligase
- Bring up to 20 µL with ddH2O
Note: If a 30-pmol RNA is not possible due to low levels of expression, keep a small amount of RNA; it’s not necessary to proportionally adjust the other reagents. The final yield will be low due to the use of a small amount of RNA, but this should not be a major problem. Incubate at 4°C overnight.
Stop the reaction by adding 230 µL of G50 buffer and 500 µL of PCA followed by vortex for 30 seconds.
Centrifuge the mixture at 12,000 × g at 4°C for 5 minutes.
Transfer the upper aqueous phase to a new 1.5 mL tube and add 600 µL of ethanol.
Place it at −20°C or on dry-ice for more than 15 minutes
Centrifuge the mixture at 12,000 × g at 4°C for 15 minutes.
Discard the supernatant and add 500 µL of 70% ethanol.
Centrifuge the mixture at 12,000 × g at 4°C for 5 minutes.
Discard the supernatant and air-dry the precipitated pellet.
- Resuspend it in 10 µL of ddH2O.Note: After resuspension, the RNA should be stored at −80°C. If the RNA is not used immediately, it can be stored at −80°C in ethanol (in step 6) for more than 3 months.
Basic Protocol 3: Separating and eluting radiolabeled RNA using denaturing gel electrophoresis
Using a longer and thinner gel leads to better resolution, but it also requires more attention to detail with the gel. The apparatus used here has 20 × 30 cm glass plates and 0.42 mm thick spacers.
Although RNase is relatively ineffective when using denaturing conditions, it does not mean that the RNA is undegradable. It is important to wash the whole apparatus, especially the glass plates, carefully. Wiping the plates with 0.2 M NaOH or 10% SDS followed by rinsing with 70% ethanol should be conducted before casting.
To detect radioactivity, a phosphor imaging screen is often used. This screen has several advantages compared to a conventional X-ray film screen: (1) there is no need to deal with the screen in a dark room, (2) the sensitivity is higher, (3) exposing and detecting time is much shorter, and (4) latent images are erased with a white light and the screen can be reused.
Materials
Sigmacote (Sigma)
0.2 M NaOH
Urea
5× TBE (see recipe)
40% Acrylamide (acrylamide: bis acrylamide = 19:1)
10% Ammonium persulfate (APS)
N,N,N’,N’- tetramethylethylenediamine (TEMED)
2× RNA sample buffer (see recipe)
GeneRuler Low Range (size markers) (Thermo)
G50 buffer (see recipe)
Phenol/Chloroform/Isoamyl alcohol (PCA) 25:24:1
Ethanol
- Casting the gel
-
1(Optional) To avoid forming air bubbles when pouring a gel and to reduce the chance of tearing a gel when prying the plates apart after electrophoresis, siliconize one plate with Sigmacote. Apply several drops of Sigmacote and distribute them evenly with a Kimwipe. Air-dry the plate in the hood followed by rinsing it first with ddH2O and then with 100% ethanol.Note: If both plates are siliconized, gel may be slippery when assembling the apparatus. Disassembling the plate could also be difficult. It is better to siliconize only one plate.
-
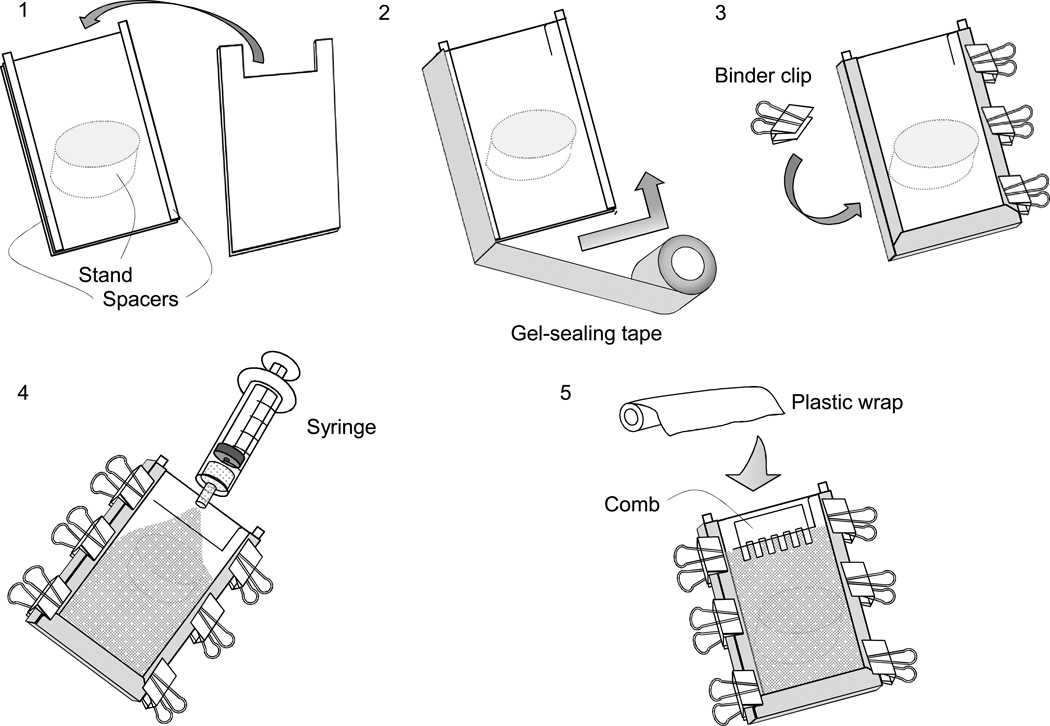
2Lay the longer glass plate on the bench and arrange the spacers along each side of the glass plate (Fig.1-1).
-
3Put the shorter glass plate on top of the longer plate with the spacers. Make sure that the spacers remain in position at the outmost edges of the plates.
-
4Seal the bottom and sides of the plates with a gel-sealing tape (Fig.1-2).
-
5Clamp the plates on both sides with three or four binder clips (Fig. 1-3).
-
1
- Pouring the gel
-
6Prepare a gel solution containing the desired concentration of acrylamide determined by reference to Table 1. Here we use 30 mL of a 6% acrylamide gel. Mix the following:
- 14.41 g Urea
- 3 mL 5× TBE
- 4.5 mL 40% Acrylamide (acrylamide: bis acrylamide = 19:1)
- Bring up to 30 mL with ddH2O
Notes1: Solubilization of urea is an endothermic reaction and it proceeds slowly unless an external source of heat is given. Warm it in a 42°C water bath for a short time (~5 minutes) to help the urea to dissolve. Avoid incubation for too long or in high temperature because it may cause degradation of the urea.Notes2: For convenience, a TBE/Urea/Acrylamide mix can be made on a large scale (e.g. 500 mL) and stored at 4°C for several months. -
7Add 300 µL of freshly made 10% APS and mix the solution gently.
-
8Add 20 µL of TEMED and mix the solution gently.
-
9Fill the solution into a syringe. Holding the gel mold at a ~30° angle to the horizontal (and/or place it against a stand at a similar angle), push the gel solution slowly at a constant flow rate (Fig. 1-4).Note: If bubbles form while pouring the gel, tilt the mold up and tap it gently to let bubbles ascend to the surface of the gel. Small bubbles are sometimes difficult to remove, but they do not have a significant effect if they are near the edge or away from the wells that samples are loaded in.
-
10Insert a comb into the gel and cover the top of the mold with plastic wrap so that less surface is exposed to the air (to minimize evaporation) (Fig. 1-5).
-
11Allow the gel to polymerize at room temperature for at least 20 minutes.
-
6
- Running the gel
-
12After polymerizing the gel, remove the gel-sealing tapes.
-
13Attach the gel mold to the electrophoresis apparatus with binder clips and fill the upper and lower reservoirs with 0.5× TBE.
-
14Remove the comb and flush all wells with 0.5× TBE using a 10 mL syringe.
-
15Pre-run the gel at 20 W (constant power) for 30 minutes.Note: It is better to use the constant power mode because it can keep the temperature of the gel constant. If there is no power supply that has the constant power mode, the constant voltage mode can be used but be careful not to let the temperature go too high, otherwise it may cause a skewed gel or cracked glass plates. The appropriate voltage is ~25 V / cm (gel length).
-
16Mix 1–5 µg of the purified and radiolabeled tRNA with equal volume of 2× RNA sample buffer.
-
17Heat the sample at 95°C for 3 minutes and immediately put it on ice.
-
18Stop pre-running and flush all wells again.
-
19Load the RNA sample into a well. Load size markers in the outside lanes of the gel. Size markers are radiolabeled at their 5’ ends with [γ32P]-ATP by kinase reaction after dephosphorylation (for details see Huang and Yu, 2013, Basic protocol 2).
-
20Carry out electrophoresis at 20 W for about 60 minutes.
-
12
- Detecting and eluting radiolabeled RNA
-
21When the bromophenol blue dye has migrated about 80% down the length of the gel, stop running.
-
22Disassemble the apparatus and gently pry up one corner of the smaller plate (the siliconized one) and slowly remove the plate from the gel.
-
23Put plastic wrap over the gel on the longer glass plate.
-
24Paste radioactive papers with punch holes on the gel in order to position the scanned radioactivity image and gel correctly.Note: Radioactive papers can be made by immersing them in a radioactive solution, such as the electrophoresis running buffer after use, followed by drying completely. Seal the dried marker in Scotch tape.
-
25Place and secure a phosphor imaging screen on top of the gel and keep them touching for a while to expose radioactivity to the filter.Note: Exposure time depends on the intensity of radiation. If it is very hot, it is enough to expose for a few minutes or even seconds.
-
26Scan the recorded image with phosphor imager such as Typhoon or Storm (Molecular Dynamic).
-
27Print out the full-scale image. Cut out the radioactive band in the printed paper.
-
28Place the printed paper on the gel and align the punch holes in the markers and the printed paper. Use a marker pen to label the boundary of the radioactive band from the printed paper.
-
29Cut out the detected band from the gel with a clean scalpel.
-
30Chop up the gel slice into fine particles by forcing the gel through a small bore syringe (10-mL syringe is the best; there is no need to attach a needle) to help the diffusion of the RNA from the gel matrix.
-
31Place the crushed gel in a 1.5 ml tube and add 400 µL of G50 buffer.
-
32Put it in a −80°C freezer for 30 minutes.
-
33Incubate the sample on a rotator overnight at room temperature.Note: To speed-up the experiment, steps 32–33 can be replaced to repeated crushing and soaking; put the sample on dry ice for 15 minutes to freeze and then leave it at room temperature for 15 minutes. Repeat these steps 4 times in total. Bands from higher concentration gels (more than 15% polyacrylamide gel) should be extracted by a repeated crushing/soaking technique.
-
34Remove the polyacrylamide fragments by brief centrifugation or filtration through a 0.2 mm filter.
-
35Extract the RNA with PCA followed by ethanol precipitation as described in Basic Protocol 2, steps 3–10.
-
36Resuspend the air-dried pellet in an appropriate amount of ddH2O.
-
21
Figure 1.
Schematic representation of preparation of a denaturing gel. (Modified from Sambrook and Russell, Molecular Cloning: A Laboratory Manual 3rd Edition, 2001.) 1: Lay the larger glass plate on the bench and arrange the spacers. 2: Seal three sides of the gel plates with gel-sealing tape. 3: Clamp the plates on both sides with three or four binder clips. 4: Pour the gel solution from a syringe. 5: Insert the comb and cover the top of the gel with plastic wrap.
Table 1.
Concentrations of polyacrylamide giving optimum resolution of RNA fragments and migration rates of marker dyes using denaturing PAGE (Adapted from Maniatis et al., 1975.)
| Acrylamide (%) | Fragment sizes separated (bases) |
Migration of bromophenol blue (bases) |
Migration of xylene cyanol (bases) |
|---|---|---|---|
| 4 | 100 to 500 | ~50 | ~230 |
| 5 | 70 to 300 | 35 | 130 |
| 6 | 45 to 70 | 26 | 105 |
| 8 | 35 to 45 | 19 | 75 |
| 10 | 25 to 35 | 12 | 55 |
| 20 | 8 to 25 | 8 | 28 |
REAGENTS AND SOLUTIONS
YPD: 10% Yeast extract, 20% Peptone, 20% Dextrose. Store at room temperature for several months.
Extraction buffer: 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 mM EDTA. Store at 4°C for a year.
Binding buffer: 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA. Store at 4°C for a year.
Washing buffer: 10 mM Tris-HCl (pH 7.5), 1 M NaCl, 1 mM EDTA
Store at 4°C for a year.
G50 buffer: 20 mM Tris-HCl (pH 7.5), 300 mM sodium acetate, 2 mM EDTA, 0.25% SDS. Store at 4°C for a year.
5× TBE: 445 mM Tris base, 445 mM boric acid, 2 mM EDTA. Store at room temperature for a year.
2× RNA sample buffer: 95% formamide, 0.025% SDS, 0.025% bromophenol blue, 0.025% xylene cyanol, 0.5 mM EDTA. Store at −20°C for a year.
Commentary
Background Information
Polyacrylamide gels are made by a chemical reaction known as radical polymerization. The initiators of radical polymerization, APS and TEMED, react with acrylamide and trigger a chained polymerizing response. Although acrylamide forms only straight-chain polymers, branched three-dimensional (3D) structure is formed by adding a divalent cross-linking agent, N, N'-methylenebisacrylamide. The mobility of RNA fragments depends on their lengths and the size of this 3D-network in a gel. The pore size can be adjusted by the concentration of total acrylamide [%T (an abbreviation for Total monomer concentration) = (acrylamide (g) + bisacrylamide (g)) / total volume (mL) × 100] and the ratio of bisacrylamide [%C (an abbreviation for weight percentage of Cross-linker) = (bisacrylamide (g) / (acrylamide (g) + bisacrylamide (g)) × 100]. In general, the common %C utilized for certain types of molecules are: %C = 3.33 (acrylamide : bis = 29 : 1) for proteins or double strand DNAs and %C = 5 (acrylamide : bis = 19 : 1) for RNAs and single strand DNAs. %T can be adjusted according to the molecular weight of target molecules (Table 1). Acrylamide gels that have less than %T=4 are not recommended to use because they are too soft and not easy to handle. For this reason, large RNAs (more than 500 base) are difficult to be analyzed with PAGE. Separation of long RNAs is usually fulfilled with an agarose gel containing formaldehyde. However, elution of RNA with an agarose gel is less commonly used than a polyacrylamide gel. Instead, reducing RNA size by cleavage can be used if applicable. The cleaved RNA that contains a desired region and has an appropriate size can be separated with PAGE (for detailed procedure of RNA cleavage using RNase H, see Huang and Yu, 2013).
In RNA separation, it is important to denature the RNA so that the migration of the RNA depends on its size but not its structures. Urea is known to destabilize secondary structure of RNA by destroying the assembly of solvated water in RNA or breaking up hydrogen bonds between nucleic acid bases. However, urea can only help destabilization and cannot completely denature all structures of molecules. It was reported that 8M urea lowers the melting temperature (Tm) of nucleic acid molecules by ~18°C (Hutton, 1977). RNAs with a high G/C content have more hydrogen bonds than A/T rich sequences and are difficult to denature fully. Some strong structures can retain secondary structure even in the existence of 8 M urea and the percentage of such structures is higher at low temperature. Therefore keeping a gel at a stable high temperature is important in order to obtain fine and reproducible RNA separation.
Critical Parameters and troubleshooting
The key point for getting a good separation and a high resolution is making a gel properly. Glass plates should be completely clean. The gel solution should be filtrated and deaerated before use. APS should be freshly prepared every time; otherwise it may not have the catalytic power to generate free radicals. The amount of TEMED has a considerable effect. A large amount of TEMED ensures that polymerization will occur rapidly, but using too much can cause a scavenging of radical species that are in the middle of elongation and result in elongation arrest of the polymer. A gel containing these short length polymers has a sparse 3D-network, which results in slow mobility and smear bands because of a low electrical resistance. Conversely, too little TEMED is unable to complete polymerization. The amount of TEMED should be adjusted according to the %T of the gel.
As discussed above, the temperature of the gel is also a critical factor. The temperature must be maintained above 40°C during denaturing PAGE. Pre-run should be done in order to warm the gel to an optimal temperature for RNA separation as well as remove remaining APS from the wells. Although higher temperature is preferable, raising the temperature too high (~70°C) causes fuzzy bands, distorted gels, and cracked plates. Adequate temperature for most RNAs is around 50°C. Adjusting a gel’s temperature appropriately may improve results. It can be done by changing not only output power but also buffer concentration. For example, doubling the TBE concentration leads to the gel running at a higher temperature due to an increase in electric current.
Anticipated Results
More than 1 mg of total RNA can be extracted from a 50 mL culture of yeast cells. From 1mg of total RNA, µg-order tRNAs can be purified. For the elution of tRNA from polyacrylamide gels, the efficiency of recovery is expected more than 80%. In general, the elution of longer RNAs has lower efficiency.
By culturing in large scale, the same amount of mRNAs can be obtained. Long mRNAs (more than 1,000 bases) are, however, sometimes difficult to be generated. If it is possible, cleave such long mRNAs into fragments, thus improving the result (see Commentary Background Information section).
Time Considerations
For RNA preparation from yeast cells, it usually takes 4 days to finish. Culturing and harvesting yeast cells takes 2 days if a plate forming single colonies on it is prepared beforehand and is ready to use. (steps (1) 1–2 in Basic Protocol 1; forming colonies on a plate needs additional 1–2 days.) Total RNA extraction takes half a day (steps (1) 3–16 in Basic Protocol 1; Day 2). Purification of a specific RNA from total RNA takes a day (steps (2) 17–44 in Basic Protocol 1; Day 3). Ligation of radiolabeled pCp can be done overnight right after purification (steps 1–2 in Basic Protocol 2). Subsequent PCA extraction and ethanol precipitation takes half a day (steps 3–10 in Basic Protocol 2; Day 4).
For RNA separation with denaturing PAGE and detecting the radiolabeled RNA, it usually takes a day to finish. Casting, pouring, running the gel followed by detecting with a phosphor screen can be done on Day 4 (all steps in Basic Protocol 3 (1)–(3) and steps (4) 21–27).
For eluting RNA, it usually takes 1–2 days to finish. Cutting the detected band and eluting the RNA can be done overnight after denaturing PAGE (steps (4) 28–33 in Basic Protocol 3; Day 4). Recovering RNA can be done the next morning and it takes half a day (steps (4) 34–36 in Basic Protocol 3; Day 5).
Figure 2.
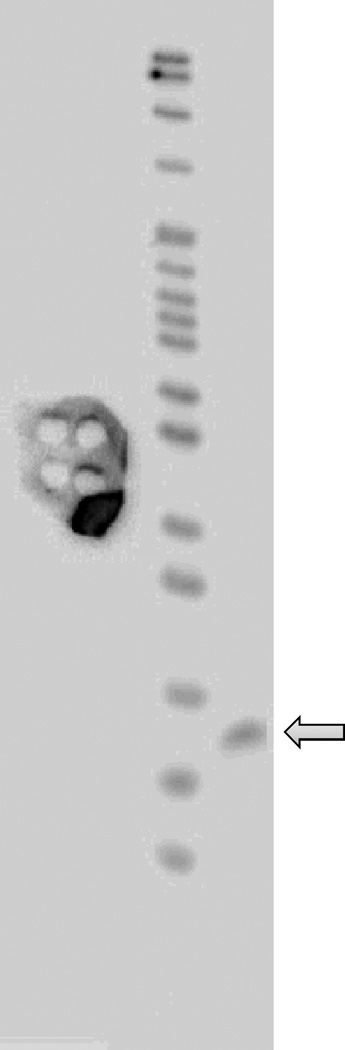
An example of denaturing PAGE of radiolabeled tRNATyr. The arrow indicates tRNATyr (75 bases). A paper with small holes on the side of lane was put to position the gel on the printed image correctly.
ACKNOWLEDGMENTS
The authors thank the members of the Yu laboratory for valuable discussions. This work was supported by grants GM104077 and AG39559 (to Yi-Tao Yu) from the National Institute of Health, and by the University of Rochester CTSA award UL1TR000042 (toYi-Tao Yu) from the National Center for Advancing Translational Sciences of the National Institute of Health.
LITERATURE CITED
- Huang C, Yu YT. Synthesis and Labeling of RNA in vitro. Current Protocols in Molecular Biology. 2013;(Unit 4.15) doi: 10.1002/0471142727.mb0415s102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Jeffrey A, deSande HV. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975;14:3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Hutton JR. Renaturation kinetics and thermal stability of DNA in aqueous solutions of formamide and urea. Nucleic Acids Res. 1977;4(10):3537–3555. doi: 10.1093/nar/4.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Chapter 12. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]